Abstract
The discovery of nucleotide excision repair in 1964 showed that DNA could be repaired by a mechanism that removed the damaged section of a strand and replaced it accurately by using the remaining intact strand as the template. This result showed that DNA could be actively metabolized in a process that had no precedent. In 1968, experiments describing postreplication repair, a process dependent on homologous recombination, were reported. The authors of these papers were either at Yale University or had prior Yale connections. Here we recount some of the events leading to these discoveries and consider the impact on further research at Yale and elsewhere.
Keywords: DNA repair, homology-dependent repair, leading strand restart, nucleotide excision repair, Yale Radiobiology, recombination, recombinational repair
Introduction
This article describes events related to the first papers published in the 1960s describing nucleotide excision repair (NER) and homology-dependent recombinational repair. Connections of the authors to Yale and particularly the Yale Radiobiology Section will be discussed. I joined the Paul Howard-Flanders lab in the Yale Radiobiology Section in late 1964, several months after publication of the original NER papers. However, I had previous interactions with Paul while doing my PhD studies with Bill Prusoff in the Yale Department of Pharmacology. Paul had given me advice about methodology and provided strains including the original UV-sensitive E. coli strain AB1886. While I was writing up my results, he told me about the exciting discovery that normal wild-type cells were able to cut pyrimidine dimers out of the DNA while the UV-sensitive mutant had lost that ability. Although I was not in the Howard-Flanders lab before the publication of the first NER papers, I subsequently did the experiments first demonstrating homology-dependent recombinational DNA repair and later directed the research in which the bimodal incision mechanism of NER was first described.
Nucleotide Excision Repair (NER)
The three papers first describing NER were published in 1964 [1-3]. Two papers show that in wild type E. coli, pyrimidine dimers are removed from the DNA in the form of oligonucleotides but remain in high molecular weight DNA in UV-sensitive mutants [1,2]. The third paper describes repair-replication in UV-irradiated cells [3]. Taken together, these papers demonstrate the removal of a section of DNA containing the damage and the accurate replacement by copying the intact template strand. The significance of these papers is that they changed the way people thought about DNA. Cutting and replacing sections of the DNA backbone had not been anticipated. The use of UV-sensitive mutant strains (E. coli K12, Boyce and Howard-Flanders [2] and E. coli B, Setlow and Carrier [1]) were important elements in both 1964 papers first describing NER.
While only the Boyce and Howard-Flanders paper has Yale listed as the originating site, Yale was important in the earlier careers of Setlow and Hanawalt, the senior authors on the other two papers, as well. In 1964, Boyce and Howard-Flanders were at Yale, while Dick Setlow was at the Oak Ridge National Laboratory (ORNL) and Phil Hanawalt was at Stanford. (Prior to moving to ORNL in 1961, Setlow had been in Yale Biophysics and was the PhD thesis advisor for both Hanawalt and Boyce. An additional point of interest is that Jane Setlow, after finishing her PhD in Yale Biophysics, went to the Howard-Flanders lab as a postdoc. After the Setlows moved to ORNL, Dick Boyce joined the Howard-Flanders lab as a postdoc.)
Separate Threads
Two separate but complementary threads were critically important in the discovery of nucleotide excision repair. One was the biophysical and chemical studies on UV and pyrimidine dimers, and the second was the genetic study of UV-sensitivity which led to the availability of UV-sensitive mutants. Dick Setlow’s longstanding research background was in the biophysical aspects of UV effects, while Howard-Flanders recognized the value of UV-sensitive mutant strains and employed the E. coli K12 system for the isolation and characterization of repair-defective strains.
Yale Biophysics and ORNL: Dick Setlow and the Biophysical Approach
Before going to ORNL in 1961, Dick Setlow was a Yale Biophysics faculty member during the late 1950s. This group included several scientists (Pollard, Forro, Hutchinson, Morowitz, and others) who used physical methods to investigate biological effects. Setlow’s publications in the years before 1964 used methods such as action spectra to learn about UV effects on DNA and cells. His work demonstrated that thymine dimers were the main products responsible for the biological action of UV [4,5]. He first used a UV-sensitive strain (the Hill E. coli Bs strain) in a paper published in late 1963. In that publication, the discussion is clear about repair taking place and that it was not due to the direct reversal of thymine dimers to monomeric thymines as was known to occur during photoreactivation [6].
Yale Radiobiology: Paul Howard-Flanders and the Genetic Approach
Events in Yale Radiobiology during the 1960s are discussed here. In 1959, Paul Howard-Flanders was recruited to the Department of Radiology at Yale to form the Radiobiology Section. He came with a background as a physicist interested in radiation instrumentation for therapy and in the oxygen effect on the radiosensitivity of cells and biological materials [7,8]. Shortly after his arrival at Yale, he learned of mutants in E. coli B that were sensitive to ultraviolet isolated by Ruth Hill [9,10]. He describes meeting her in 1961 at a Brookhaven Symposium in which he recognized that such mutants would be useful tools to study the effects of radiation on cells [11]. This meeting changed the primary direction of his research activities and resulted in an emphasis on DNA Repair in the Yale Radiobiology Section that continues to the present. At Yale, Paul learned from Ed Adelberg about the methods developed for the genetic analysis of E. coli K12 and recognized that K12 was far superior to E. coli B for genetic studies. He developed a positive selection (using host cell reactivation of UV-irradiated bacteriophage) for isolating UV sensitive mutants [12]. This selection was used to isolate and identify the genes designated uvrA, uvrB and uvrC [13,14]. Three publications from the Howard-Flanders lab in 1962 show the rapid development of the genetic approach. The first paper uses the Hill E. coli Bs strain [15], while the other two papers describe the selection method and its successful application for isolating sensitive mutants in E. coli K12 [12,13]. It is noteworthy that the discussion in these papers clearly articulates the concept that host cell reactivation of UV-irradiated bacteriophage is due to repair and that the wild type parents have an enzymatic process to repair damage that is missing in the sensitive mutant derivatives.
Dual Incision During Nucleotide Excision Repair: Identification, Purification, and Characterization of UvrABC
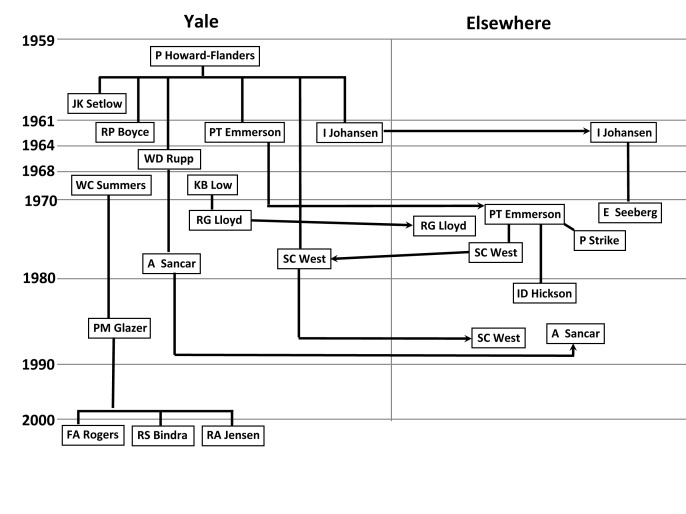
Although the general features of NER were described in 1964, the cell-free assay and biochemical purification of the activity proved particularly difficult and was not accomplished until 1976 when it was done successfully by Erling Seeberg and Peter Strike, two researchers with second generation ties to Yale Radiobiology [16,17]. Ivar Johansen from Norway (Seeberg’s mentor) and Peter Emmerson from Newcastle (Strike’s mentor) had both been postdocs with Howard-Flanders in the early 1960s when NER was first described (Figure 1).
Figure 1.
Abbreviated lineage of several researchers in the Radiobiology Section at Yale University during the first decade. Paul Howard-Flanders formed the Radiobiology Section when he came to Yale in 1959. Postdoctoral associates who were in his lab before or during the discovery of NER in 1964 included Jane Setlow, Boyce, Emmerson, and Johansen. Rupp joined the Howard-Flanders lab as a postdoc a few months after the discovery of NER. In 1968, Summers and Low came to Yale as junior faculty members with independent labs. Solid lines (without arrows) indicate that one individual worked in another lab. Lines with arrows indicate movement either to or from Yale. Although not shown, Seeberg (1 year) and Strike (6 months) were both in the Rupp lab and later collaborated with each other as described in the text. Another interaction not shown in the figure is between West and Lloyd, who did important studies on the resolution of Holliday junctions. Ian Hickson, who did his predoctoral work in Emmerson’s lab, is the only person in this diagram who did not work at Yale.
In my laboratory, we used gene cloning methodology, which at that time was a novel and rapidly developing field. Fortunately, Aziz Sancar, who had previously cloned the phr gene, joined my lab and completed the cloning of the uvrA, uvrB, and uvrC genes. A new procedure, the “maxicell” method, was developed to specifically label the products from cloned genes [18]. Transposons were inserted to identify the three uvr gene products and to orient the cloned genes [19-21]. The UvrA, UvrB and UvrC proteins were purified and were active in the assay for incision of UV-irradiated DNA [22,23]. Gel analyses of the reaction products demonstrated that incisions occurred on both sides of a DNA lesion, a finding that was unexpected. It is now recognized that this bimodal incision is a property of NER in all species.
Homologous Recombination and Repair are Related
In the early 1960s, investigators were not aware that repair and recombination were related. John Clark describes the original isolation of a recA mutant in E. coli K12 and relates how he learned in a seminar from Delbruck that the Howard-Flanders uvr excision-defective mutant strain might be defective in recombination [24,25]. Clark tested the newly-isolated Rec- strain and found it to have an increased sensitivity to UV. Clark, however, after studying the data in the Howard-Flanders 1962 paper [13], concluded that the UV-sensitive mutants isolated at Yale were proficient in recombination. Independently, Howard-Flanders had suggested that there might be overlapping steps between excision repair and recombination because breakage-reunion models of recombination were then being advanced by workers in the field [26]. Clark contacted Howard-Flanders to find out whether any of the unpublished UV-sensitive mutants isolated in the Yale lab were defective in recombination and learned that there were none. He brought his Rec- strain to the Howard-Flanders lab at Yale for further characterization, and together they determined that even though UV-sensitive, it was proficient for the excision repair of pyrimidine dimers [27]. (In addition they discovered that unlike the Yale excision-repair mutants, the Clark-Margulies Rec- mutant was highly sensitive to ionizing radiation, a property that was subsequently used to screen for additional sensitive mutants that generated mutants in a number of additional interesting genes, including recB, recC, lex, and lon [28-31].)
Repair is Dependent on Homologous Recombination
The E. coli K12 genetic system facilitated the construction and comparison of various mutant combinations. The combination of two separate uvr mutations resulted in a strain with the same sensitivity as the original single mutant strain. In marked contrast, combination of uvrA and recA generated an extremely UV-sensitive strain that was much more sensitive than either of the single mutants [29]. Although Howard-Flanders had earlier suggested the possibility of common steps in nucleotide excision repair and recombination [26], the supersensitivity of the double mutant indicated the existence of two separate pathways. At about that time, Paul attended a meeting on genetic recombination at Lake Arrowhead and learned of the increased frequency of recombination near the ends of DNA molecules. Shortly after that, he came up with the idea that there might be a recombination-dependent postreplication repair process initiated by the free ends at gaps opposite pyrimidine dimers. At that point, he suggested that I should look for recombinant exchanges between old and new strands in irradiated cells, presumably because I had prior experience using CsCl density gradient centrifugation during my thesis work [32]. This approach was more problematic than anticipated but eventually did yield significant positive results that demonstrated exchanges between the newly synthesized DNA and the old pre-existing template strands [33]. In the meantime, a different approach gave striking results much sooner. At a meeting in early 1966, I had heard a presentation by McGrath describing the lysis and sedimentation of labeled bacterial DNA in alkali and invited him to Yale to teach me his procedure before publication later that year [34]. I was soon able to show that the DNA synthesized after UV-irradiation in an excision-defective strain was present in short pieces whose size was similar to the distance between pyrimidine dimers in the template and that on further incubation, these short pieces were converted to normal high molecular weight DNA [35]. (In this case, Paul’s intuition was incorrect as he assumed that any short pieces would be repaired in a time frame too short to be identified by this method.)
Postreplication Repair (Recombinational Repair, Homology-Dependent Recombinational Repair)
In summary, our interpretation was that replication proceeds past many template strand lesions generating gaps in both newly-synthesized strands and that these short strands are joined together in a recombination-dependent process. We called this “postreplication repair,” but in some cases also referred to the process as “recombinational repair” and used that as a descriptive term in the body of several papers [33,36].
“Recombinational repair” was first used in a title of a paper [37] where we suggested this mode of repair need not be limited to products of replication but could apply to any structure with DNA damage in both strands that would make it unable to be repaired by NER. Resnick used our results as the starting rationale for proposing a double strand break repair model [38] depending on recombinational exchange and further suggested that the Holliday junction is a likely intermediate.
Restart Leading Strand?
At the time of our 1968 paper, little was known about the details of DNA replication. Subsequent studies led to the generally accepted model that while the lagging strand is synthesized discontinuously to generate Okazaki fragments, the leading strand must always be synthesized continuously with no interruptions. This led to various models to explain how a replication fork deals with polymerase blocking lesions in the leading strand template. One particularly common version is that the replication complex stops until a different polymerase carries out a direct bypass of the lesion, at which time replication continues with the leading strand always remaining continuous. Although a variety of such polymerases are known, this direct bypass is inconsistent with our original observation that all DNA synthesized in UV-irradiated E. coli is present in short pieces. We concluded that if our data are correct, it follows that the leading strand must frequently be restarted [39,40]. Recent data from Yeeles and Marians [41] using purified components in a cell-free DNA replication reaction and from the Fuchs lab [42], in whole E. coli cells, now support this idea that the leading strand can frequently be restarted.
Multiple Facets of RecA
The recA gene and RecA protein have many interesting and diverse features, including roles in UV-induced mutagenesis and control of rec-lex regulated genes that are induced by DNA damage, that are too wide ranging for consideration here. However, I will make a slight digression to relate how the lex gene was named. It was found by Lee Theriot during a screen for X-ray sensitive mutants and was known in the Howard-Flanders lab as Lee’s X-ray sensitive mutant. However, in order to sound more scientific at publication, the name was said to be an abbreviation of “locus for X-ray sensitivity” [29].
Influence of Howard-Flanders Shifts Emphasis of Future Research to DNA Repair with a Molecular Biological Approach
Although the first Yale Radiobiology postdoctoral fellows recruited in the early 1960s had traditional physics/chemistry backgrounds typical for investigators in the field of radiation research, their time at Yale with Howard-Flanders resulted in a shift of emphasis to a molecular biological approach to the study of DNA repair. Peter Emmerson arrived at Yale after completing his PhD studies with J. Weiss at Newcastle, whose lab studied the interaction of radiation radicals with biological materials. At Yale, Emmerson isolated the first mutants in the recC gene, and after his return to Newcastle, several of his students made significant contributions to the DNA repair field. Peter Strike’s participation in developing the assay for UvrABC has been mentioned earlier. Steve West has made a series of important contributions. He identified RecA while doing his doctoral research with Emmerson in Newcastle and then went to the Howard-Flanders lab at Yale, where he did further experiments with RecA. After his return to Britain, he has continued to do exceptional work with many of the proteins involved in recombination. Ian Hickson, who also trained in Emmerson’s lab, has made important contributions in elucidating the role of recombination in Bloom’s Syndrome and other human diseases.
Ivar Johansen had been trained in Oslo, Norway, and after 2 years with Howard-Flanders at Yale, returned to Norway where he studied DNA damage and repair in microorganisms. He mentored Erling Seeberg, who carried out significant studies in both NER and subsequently in base excision repair.
After doing outstanding work in my lab and moving to North Carolina, Aziz Sancar has continued to make important discoveries in multiple areas of the DNA repair field.
In 1968, Brooks Low and Bill Summers joined the Yale Radiobiology Section. Brooks and Bill could both be classified as molecular biologists in contrast to the more traditional physics/chemistry backgrounds of Johansen and Emmerson, who had worked with Howard-Flanders a few years earlier. Bob Lloyd worked with Brooks and went on to Nottingham, where he has made important contributions studying various genes participating in recombination. Another “second generation” trainee is Peter Glazer, who did his thesis work with Bill Summers and is now Chair of Therapeutic Radiology. Peter in turn has trained R. Bindra, R. Jensen, and F. Rogers, who are now on the Yale Therapeutic Radiology faculty.
Conclusion and Outlook
In 1961, Howard-Flanders recognized that UV-sensitive mutants would be useful to study DNA repair mechanisms and that the E. coli K12 background offered the best opportunity for detailed genetic analysis. While this decision shifted the research direction for Howard-Flanders, it also set the future course for Yale Radiobiology and influenced many of his colleagues and trainees to pursue research careers studying the molecular biology of DNA repair.
The discovery of NER was obviously groundbreaking, but the realization in the mid-1960s that recombination and repair were related opened up vast new areas for investigation that remain fertile today. It is mindboggling that there are thousands of papers on the BRCA genes (which are components of the homology-dependent repair system) and that BRCA has even reached the US Supreme Court.
Abbreviations
- NER
nucleotide excision repair
- ORNL
Oak Ridge National Laboratory
References
- Setlow RB, Carrier WL. The disappearance of thymine dimers from DNA: An error-correcting mechanism. Proc Natl Acad Sci USA. 1964;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce RP, Howard-Flanders P. Release of ultraviolet light-induced thymine dimers from DNA in E. coli K-12. Proc Natl Acad Sci USA. 1964;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D, Hanawalt P. Evidence for repair-replication of ultraviolet damaged DNA in bacteria. J Mol Biol. 1964;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Setlow JK. Evidence that ultraviolet-induced thymine dimers in DNA cause biological damage. Proc Natl Acad Sci USA. 1962;48:1250–1257. doi: 10.1073/pnas.48.7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow JK, Setlow RB. Nature of photoreactivable ultra-violet lesion in deoxyribonucleic acid. Nature. 1963;197:560–562. [Google Scholar]
- Setlow RB, Swenson PA, Carrier WL. Thymine dimers and inhibition of DNA synthesis by ultraviolet irradiation of cells. Science. 1963;142(3598):1464–1466. doi: 10.1126/science.142.3598.1464. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. The development of the linear accelerator as a clinical instrument. Acta Radiol Suppl. 1954;116:649–655. [PubMed] [Google Scholar]
- Howard-Flanders P, Alper T. The sensitivity of microorganisms to irradiation under controlled gas conditions. Radiat Res. 1957;7(5):518–540. [PubMed] [Google Scholar]
- Hill RF. A radiation-sensitive mutant of Escherichia coli. Biochim Biophys Acta. 1958;30(3):636–637. doi: 10.1016/0006-3002(58)90112-4. [DOI] [PubMed] [Google Scholar]
- Hill RF, Simson E. A study of radiosensitive and radioresistant mutants of Escherichia coli strain B. J Gen Microbiol. 1961;24:1–14. doi: 10.1099/00221287-24-1-1. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. In: DNA repair mechanisms. Hanawalt PC, Friedberg EC, Fox CF, editors. New York: Academic Press; 1978. Historical Perspectives and Keynotes on DNA Repair; pp. 105–111. [Google Scholar]
- Howard-Flanders P, Theriot L. A method for selecting radiation-sensitive mutants of Escherichia coli. Genetics. 1962;47:1219–1224. doi: 10.1093/genetics/47.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P, Boyce RP, Simson E, Theriot L. A genetic locus in E. coli K12 that controls the reactivation of UV-photoproducts associated with thymine in DNA. Proc Natl Acad Sci USA. 1962;48:2109–2115. doi: 10.1073/pnas.48.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P, Boyce RP, Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P, Boyce RP, Theriot L. Mechanism of sensitization to ultra-violet light of T1 bacteriophage by the incorporation of 5-bromodeoxyuridine or by pre-irradiation of the host cell. Nature. 1962;195:51–54. doi: 10.1038/195051a0. [DOI] [PubMed] [Google Scholar]
- Seeberg E, Strike P. Excision repair of ultraviolet-irradiated deoxyribonucleic acid in plasmolyzed cells of Escherichia coli. J Bacteriol. 1976;125(3):787–795. doi: 10.1128/jb.125.3.787-795.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E, Nissen-Meyer J, Strike P. Incision of ultraviolet-irradiated DNA by extracts of E. coli requires three different gene products. Nature. 1976;263(5577):524–526. doi: 10.1038/263524a0. [DOI] [PubMed] [Google Scholar]
- Sancar A, Hack AM, Rupp WD. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Wharton RP, Seltzer S, Kacinski BM, Clarke ND, Rupp WD. Identification of the uvrA gene product. J Mol Biol. 1981;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sancar A, Clarke ND, Griswold J, Kennedy WJ, Rupp WD. Identification of the uvrB gene product. J Mol Biol. 1981;148(1):63–76. doi: 10.1016/0022-2836(81)90235-7. [DOI] [PubMed] [Google Scholar]
- Sancar A, Kacinski BM, Mott DL, Rupp WD. Identification of the uvrB gene product. Proc Natl Acad Sci USA. 1981;78(9):5450–5454. doi: 10.1073/pnas.78.9.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacinski BM, Sancar A, Rupp WD. A general approach for purifying proteins encoded by cloned genes without using a functional assay: isolation of the uvrA gene product from radiolabeled maxicells. Nucleic Acids Res. 1981;9(18):4495–4508. doi: 10.1093/nar/9.18.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Rupp WD. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Clark AJ, Margulies AD. Isolation and characterization of recombination-deficient mutants of Escherichia coli K12. Proc Natl Acad Sci USA. 1965;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ. recA mutants of E. coli K12: a personal turning point. Bioessays. 1996;18(9):767–772. doi: 10.1002/bies.950180912. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P, Boyce RP. Biochemical pathway in repair of DNA after uv-irradiation that may have steps in common with those of genetic recombination by breakage-reunion. Genetics. 1964;50(2):256–257. [Google Scholar]
- Clark AJ, Chamberlin M, Boyce RP, Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P, Simson E, Theriot L. A locus that controls filament formation and sensitivity to radiation in Escherichia coli K-12. Genetics. 1964;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P, Boyce RP. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;Suppl 6:156–184. [PubMed] [Google Scholar]
- Emmerson PT, Howard-Flanders P. Cotransduction with thy of a gene required for genetic recombination in Escherichia coli. J Bacteriol. 1967;93(5):1729–1731. doi: 10.1128/jb.93.5.1729-1731.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson ID, Emmerson PT. Identification of the Escherichia coli recB and recC gene products. Nature. 1981;294(5841):578–580. doi: 10.1038/294578a0. [DOI] [PubMed] [Google Scholar]
- Rupp WD, Prusoff WH. Incorporation of 5-iodo-2'-deoxyuridine into bacteriophage T1 as related to ultra-violet sensitization or protection. Nature. 1964;202:1288–1290. doi: 10.1038/2021288a0. [DOI] [PubMed] [Google Scholar]
- Rupp WD, Wilde CE 3rd, Reno DL, Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- McGrath RA, Williams RW. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Rupp WD, Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp WD, Zipser E, von Essen C, Reno DL, Prosnitz L, Howard-Flanders P. In: Time and dose relationships in radiation biology as applied to radiotherapy. Bond VP, Suit HD, Marcial V, editors. Upton, NY: Brookhaven National Laboratory; 1969. Repair and reconstruction of chromosomal DNA after replication; pp. 1–13. [Google Scholar]
- Howard-Flanders P, Rupp WD. Recombinational repair in UV-irradiated Escherichia coli. Johns Hopkins Med J. 1972;1:212–225. [PubMed] [Google Scholar]
- Resnick MA. The repair of double-strand breaks in DNA; a model involving recombination. J Theor Biol. 1976;59(1):97–106. doi: 10.1016/s0022-5193(76)80025-2. [DOI] [PubMed] [Google Scholar]
- Rupp WD. Uncoupling of DnaB helicase and Pol III holoenzyme can account for replication past bulky lesions. J Cell Biochem. 1992;Suppl 16B:108. [Google Scholar]
- Rupp WD. In: Escherichia coli and Salmonella, 2nd ed. Neidhardt F, et al., editors. Washington, DC: ASM; 1996. DNA Repair Mechanisms; pp. 2277–2294. [Google Scholar]
- Yeeles JTP, Marians KJ. The Escherichia coli Replisome Is Inherently DNA Damage Tolerant. Science. 2011;334(6053):235–238. doi: 10.1126/science.1209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages V, Mazon G, Naiman K, Philippin G, Fuchs RP. Monitoring bypass of single replication-blocking lesions by damage avoidance in the Escherichia coli chromosome. Nucleic Acids Res. 2012;40(18):9036–9043. doi: 10.1093/nar/gks675. [DOI] [PMC free article] [PubMed] [Google Scholar]