Abstract
By interrogating nature at the length scale of important biological molecules (proteins, DNA), nanotechnology offers great promise to biomedicine. We review here our recent work on nanofilm biomaterials: “nanoscopically” thin, functional, polymer-based films serving as biocompatible interfaces. In one thrust, films containing carbon nanotubes are shown to be highly antimicrobial and, thus, to be promising as biomedical device materials inherently resistive to microbial infection. In another thrust, strategies are developed toward films of independently controllable bioactivity and mechanical rigidity — two key variables governing typical biological responses.
Keywords: nanotechnology, nanofilm, biomaterial, layer-by-layer, polyelectrolyte, carbon nanotube
Introduction
Nanotechnology concerns materials/devices with functionally important features from 1 to 100 nanometers (nm), where 1 nm is one billionth of a meter. For perspective, normal human scalp hair has a width of 100,000 nm and grows at a rate of 5 nm per second. The “nano” length scale is generally larger than the size of small molecules (e.g., molecular oxygen is 0.36 nm in length), yet small enough where material properties begin to deviate significantly from bulk values. For example, at the nm length scale, system sizes begin to approach their de Broglie wavelengths, leading to the appearance of quantum behavior. Also at this length scale, surface effects become important, as an appreciable fraction of atoms exist at or near the surface. Of particular importance to biomedicine, the nm length scale is where the smallest synthetic material features approach the dimensions of biology’s fundamental building blocks: proteins. Indeed, nanotechnology may interrogate nature at its smallest natural length scale.
Carbon nanotubes (CNT) are an important nanomaterial [1]. Essentially, a single layer of sp2 carbon in a cylindrical geometry, these ca. 1 nm diameter materials possess many amazing properties: CNT exhibit the highest aspect ratio, strength, and stiffness of any material ever measured [2] and can superconduct electricity at comparatively high temperatures (to about 12 K) [3]. Despite their promise, CNT applications to date are largely limited to sporting equipment (tennis rackets, golf clubs, baseball bats), where high strength-to-weight ratio offers performance advantages, and to automotive plastics, where high strength may be uniquely combined with electrical conductivity. However, many future applications are envisioned in biomedicine [4]. For example, CNT are proving to be excellent cell contacting materials, promoting strong cellular adhesion owing to CNT nanoscale roughness and high rigidity [5]. CNT are also being explored as gene delivery vehicles, where high aspect ratio enables enhanced transport of genetic material across the cell membrane [6-8]. Finally, CNT are highly antimicrobial and so represent an interesting route toward biomaterials inherently resistive to microbial infection [9-13].
Nanoscale polymer films also offer great promise in biomedicine. These ca. 10 to 100 nm coatings serve as the interface between biological objects (ranging from biomolecules to cells to tissues) and materials (e.g., plastics, metals). Layer-by-layer (LbL) assembly of oppositely charged macromolecular species [14] offers a facile route toward nanofilms for a variety of applications (Figure 1) [15,16]. LbL films are easy to fabricate on a variety of flat or irregularly shaped objects (only simple solution exposures are required) and are amenable to fine control over physicochemical properties (through choice of polymers, solution conditions, and post-formation steps). Bioactivity may be conferred through the facile incorporation of biomolecular species, making LbL films excellent candidates for biomaterial applications, e.g., cell culture, tissue engineering, biomedical implants [17-20].
Figure 1.
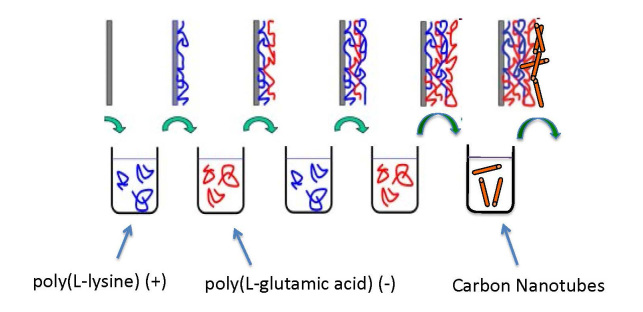
Schematic of the layer-by-layer (LbL) thin film assembly system, showing the alternate adsorption of positively and negatively charged polymers from aqueous solution. Addition of a carbon nanotube layer is also shown.
In this article, we review our recent work on nanotechnology for medicine. The focus is on nanofilm biomaterials, i.e., materials of nanoscale thickness designed to interface with biology. In one line of inquiry, we seek CNT-containing films that are inherently antimicrobial and hence resistive of intracorporeal infection [21-23]. In other work, we seek films in which the two key film properties affecting the biological response ― mechanical rigidity and bioactivity — are independently controllable [24,25]. We follow with a discussion on some of the most promising application areas for these technologies and some of the challenges likely to be faced in their implementation.
Carbon Nanotube (CNT)-Based Nanofilm Biomaterials
About half of all Americans will host a biomedical implant at some point during their life, and about 1 million of these each year will become infected, resulting in 60,000 to 90,000 deaths and $17 to $29 billion in associated costs [26]. With the future rate of medical implantation likely to rise, biomedical device materials resistive of microbial infection represent a key public health priority.
Current strategies toward antimicrobial materials are generally based on i) release of anti-infective agent or ii) grafted antimicrobial polymers [27-31]. In the first approach, anti-infective agents such as antibiotics are embedded within a material and are released by diffusion and/or material degradation. While effective in some cases, disadvantages include difficult-to-control release rate, potential toxicity to human cells, eventual agent depletion, and the possibility of resistance development. In the second approach, polymers containing highly charged, quaternary amine groups are chemically grafted to the material surface and potentially provide a more permanent antimicrobial effect. However, the grafting chemistry can be quite intricate and even inapplicable to certain materials, and long-term stability of the grafts remains a challenge.
Nanotechnology offers great promise toward inactivating microbes [32,33]. In particular, nanoparticles may release antimicrobial agents, or inactivate microbes, through physical disruption of the cell wall. CNT have recently been shown to be highly antimicrobial [9-13]. Owing to their high stability and compatibility with a variety of base materials, CNT offer many potential advantages to other antimicrobial approaches. We are developing thin polymer film approaches employing CNT as a minority component. In one approach, we employ the well-established hydrolytically degradable biomedical polymer poly(lactic-co-glycolic acid) (PLGA), together with CNT, in a spin-coated thin film formulation [21]. As shown in Figure 2, the degree of E. coli inactivation (at 1 h) increases from about 10 percent for pure PLGA and 1/7000 w-CNT to w-PLGA systems to about 80 percent for 1/700 and 1/70 w-CNT to w-PLGA systems. A marked decrease in inactivation degree upon increasing average CNT length at constant CNT content suggests tube ends play an important role in the antimicrobial mechanism.
Figure 2.
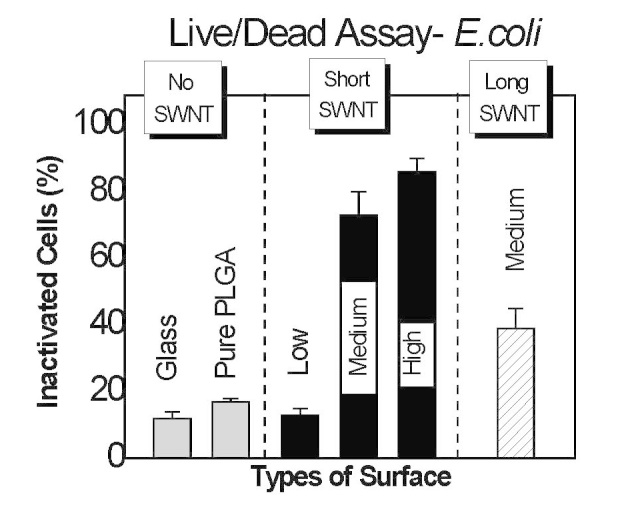
Percent E. coli inactivated following 1 h exposure to glass, poly(lactic-co-glycolic acid) (PLGA), and PLGA containing carbon nanotubes (CNT) at concentrations (w CNT / w-PLGA) 1/7000 (low), 1/700 (medium), and 1/70 (high). CNT of length ca. 300 nm (short) and > 1 (long) are considered. Reproduced with permission from The Royal Society of Chemistry [21].
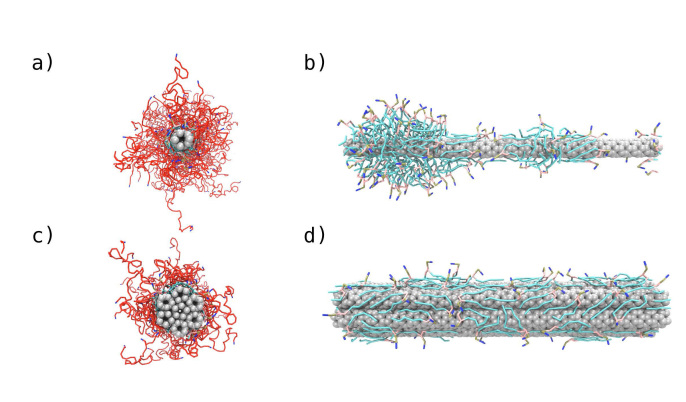
In another approach, we are developing an aqueous-based, layer-by-layer strategy toward films composed of CNT and charged polymers (Figure 1) [22,23]. Aqueous-based approaches are appealing on economic and environmental grounds, but an important consideration is the solubility of the (generally very hydrophobic) CNT. We employ here an amphiphilic polymer, PL-PEG, consisting of a phosphoethanolamine-based lipid with a grafted poly(ethylene glycol) chain. The lipid assembles around the CNT, and the PEG chain acts to repel the coated CNT from one another via entropic stabilization. Aqueous CNT dispersions are created through a sonication process, with the time of sonication serving to control the degree of CNT bundling. For example, at 5 min sonication at 60 W, CNT bundles of length 1200 nm result, whereas at 60 minutes sonication at 60 W, (nearly) isolated CNT of length 400 nm are apparent. Interestingly, the degree of bundling greatly impacts the layer-by-layer assembly process. As shown in Figure 3, for a film composed of CNT and the polypeptides poly(L-lysine) (PLL) and poly(L-glutamic acid) (PGA), layers of bundled CNT are about twice as thick as layers of isolated CNT (30 vs 15 nm). Molecular simulations performed by Matta and Sammalkorpi reveal the molecular mechanism behind the thick layers associated with bundled CNT [23]. The diameter of isolated CNT is less than that of the lipid assemblies, so the lipid tends to form (only weakly perturbed) micelles around the CNT (see Figure 4). However, the CNT bundles are too large to allow for lipid micelle formation, so instead, lipid adsorbs in a relatively flat and sparse monolayer. These different interfacial morphologies result in very different CNT-CNT interactions: the isolated CNT repel one another to a much greater degree, owing to the greater density and extension of the PEG chains, and thus form much thinner adsorbed layers. Films formed via isolated and bundled CNT interact with microbes in very different ways: E. coli rest on top of films formed by isolated CNT, but become engulfed by films composed of bundled CNT (Figure 5). Although both films inactivate about 90 percent of E. coli after 24 h, only the bundled CNT film achieves this level of performance after 1 h and thus has the advantage of being “fast acting.” (As a comparison, the isolated CNT film inactivates only 20 percent of E. coli at 1 h. Results based on the standard LIVE/DEAD® assay, Invitrogen.)
Figure 3.
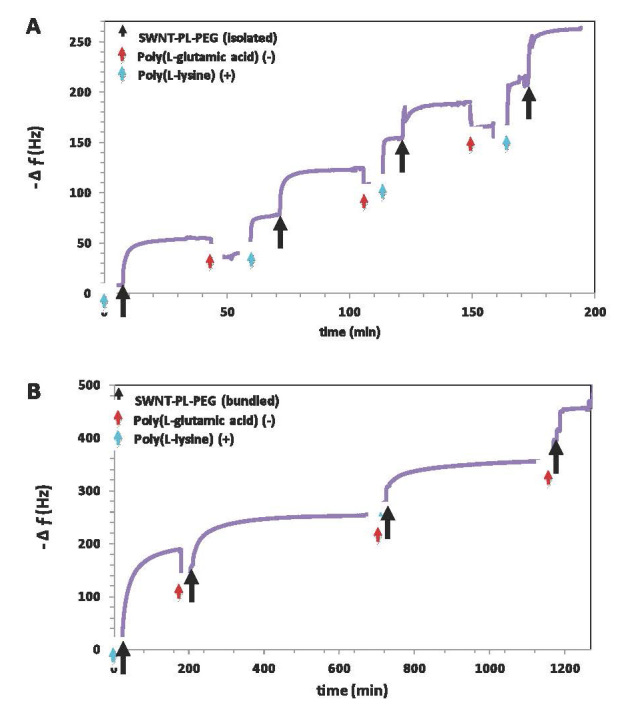
Quartz crystal microgravimetry frequency shift versus time demonstrating the layer-by-layer assembly of poly(L-lysine), poly(L-glutamic acid), and phospholipid coated carbon nanotubes (CNT). Corresponding estimates of layer thickness are 15 and 30 nm for isolated and bundled CNT, respectively. Reproduced with permission from The Royal Society of Chemistry [23].
Figure 4.
Molecular computer simulation snapshots of poly(ethylene glycol) modified phospholipid (PL-PEG) assembly around isolated (a and b) and bundled (c and d) carbon nanotubes (CNT). Greater PEG-chain extension is observed on isolated CNT, leading to greater steric repulsion and thinner adsorbed layers. Reproduced with permission from The Royal Society of Chemistry [23].
Figure 5.
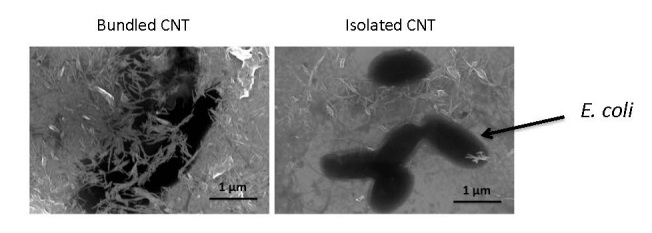
Scanning electron micrographs of E. coli seeded onto layer-by-layer films formed with charged polymers and either bundled or isolated carbon nanotubes (CNT). Reproduced with permission from The Royal Society of Chemistry [23].
Nanofilm Biomaterials of Controllable Bioactivity and Mechanical Rigidity
Biomaterials are “nonviable materials used in a medical device, intended to interact with biological systems” [34]. Controlling the cellular response is perhaps the grandest challenge in biomaterials science. A number of material properties are known to influence contacting cells: charge, hydrophobicity, topography, and mechanical rigidity [35,36]. In addition, cells may react to bioactive elements presented by a material [37,38]. Ideally, each of these properties would be independently tunable, so that an optimal material could be designed toward a desired cellular response. In practice, bioactivity and mechanical rigidity are often difficult to decouple. An important example comes from nanofilm biomaterials formed via the LbL method, where rigidity is generally controlled through post-assembly chemical cross-linking of the polymer network [39-42], and bioactivity is generally conferred through film-embedded or surface-adsorbed biomolecules [43-47]. As shown in Figure 6, when film cross-linking follows film bioactivation, embedded biomolecules may become inaccessible to contacting cells [43,44], and when cross-linking precedes bioactivation, biomolecular loading tends to be limited to the film surface (Figure 6B) [46,47]. In summary, for current approaches, LbL film mechanical rigidity and bioactivity ― two key features governing cell behavior — tend to be strongly (often inversely) coupled.
Figure 6.
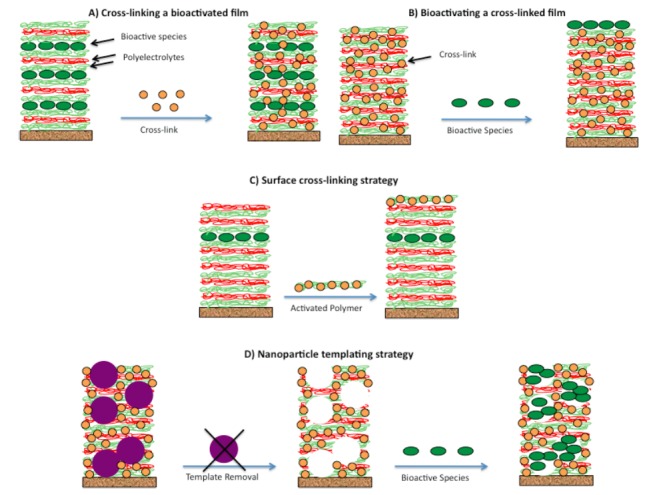
Schematic of standard approaches (A and B) and our approaches (C and D) toward nanofilm biomaterials of independently controllable mechanical rigidity and bioactivity. A) Film is constructed via the layer-by-layer assembly of charged polymers and bioactive agents (see Figure 1), and subsequently chemically cross-linked to increase rigidity, often at the expense of bioactivity. B) Bioactive agents are added following film cross-linking, with loading typically limited to the film surface. C) The surface cross-linking strategy, where cross-links form between an activated polymer and previously deposited polymers, and are thus confined to the film surface, away from the bioactive species, resulting in a rigid outer skin and high biomolecular accessibility. D) The nanoparticle templating strategy, where film assembly and cross-linking occurs in the presence of nanoparticle templates, whose removal via dissolution results in a pore space that may be subsequently filled with biomolecules. Film rigidity is controllable by the extent of cross-link formation and bioactivity by the extent of biomolecular loading.
We seek to develop approaches toward nanofilm biomaterials with independently tunable mechanical rigidity and bioactivity. One strategy involves “surface cross-linking,” where cross-link formation is confined to the surface region of the polymer film, so as not to perturb any bioactive species within the film interior (Figure 6C) [24]. Another strategy is “nanoparticle (NP) templating,” involving film formation in the presence of spherical latex NP, chemical cross-linking to increase film rigidity and “lock in” a porous morphology, and removal of NP via dissolution (Figure 6D) [25]. The idea is to create a porous film where the polymer portion may be “hardened” via standard chemical cross-linking methods, and the pore space then filled with bioactive species, both to independently controllable extents.
Within each strategy, key questions involve the degree to which the film is penetrated by macromolecular species. In the surface cross-linking approach, cross-linking agents are bound to a polymer, such that the polymer will adsorb to but not penetrate the film and allow cross-link formation to occur with previously adsorbed polymers near the film surface. The important question is whether cross-link formation occurs prior to polymer film penetration. Using laser scanning confocal microscopy and fluorescently labeled polymers, we verify the formation of a truly surface cross-linked layer (Figure 7). In the nanoparticle templating approach, the pore space is intended to be filled by bioactive species, but pore filling requires the bioactive species to penetrate into the film interior. Again using laser scanning confocal microscopy, we establish that a bioactive species proxy ― fluorescently labeled bovine serum albumin — penetrates a porous film but not a control film without pores (Figure 7). Based on the known bulk concentration, the concentration of albumin in the porous film could be estimated to be about 0.1 g/mL, or about 10 percent of the film mass.
Figure 7.
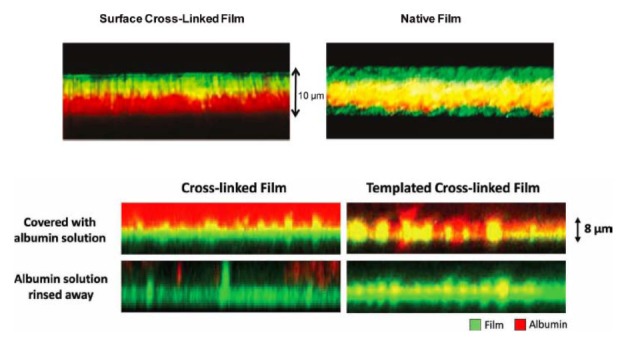
Laser scanning confocal microscopy cross-sectional images of nanofilm biomaterials formed by the layer-by-layer assembly of charged polymers. Top) A 60-layer red fluorescing film terminated with a green fluorescing activated polymer (i.e., capable of forming chemical cross-links, left) and a green fluorescing standard polymer (right). Green confined to the surface of the film to the left (but not right) suggests cross-links to occur in the surface region. Bottom) Red fluorescing albumin is added to a cross-linked (i.e., non porous) and a templated cross-linked (i.e., porous) film. The albumin penetrates only the porous film, as evidenced by the red and yellow color throughout. Reproduced with permission from The American Chemical Society (top) [24] and John Wiley and Sons (bottom) [25].
Do the strategies depicted in Figure 6 affect film properties beyond mechanical rigidity and bioactivity? An analysis of surface topography via atomic force microscopy (AFM) shows a standard cross-linked film and a nanparticle templated film to possess quite similar surface roughness values (7 nm vs. 10 nm) and similar domain sizes distributions (of order 20-50 nm) [25]. However, subtle structural (and perhaps other) differences could still be imparted through the templating process and possibly contribute to the eventual cell response.
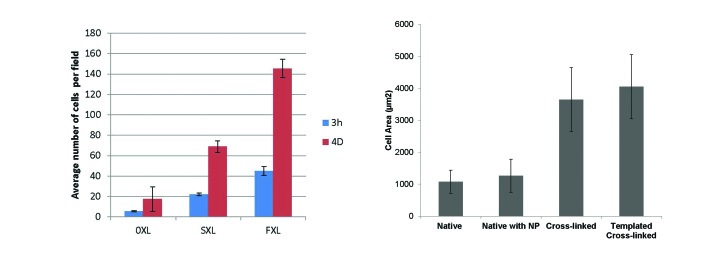
To what extent do surface cross-linked and nanoparticle-templated films enhance the initial cell response? We answer this question by considering the interaction with MC3T3-E1 pre-osteoblastic cells. In Figure 8, we show the number of adherent cells on surface cross-linked films to be intermediate between that on non-cross-linked (negative control) and fully cross-linked (positive control) films. We also show the degree of cell spreading on a nanoparticle templated (i.e., porous) film to be comparable to that on a fully cross-linked (i.e., non-porous positive control) film and much greater than that on native (i.e., non-cross-linked control) films.
Figure 8.
Left) Number of adherent MC3T3-E1 pre-osteoblastic cells on native (0XL), surface cross-linked (SXL), and fully cross-linked (FXL) nanofilm biomaterials. Right) Average MC3T3-E1 pre-osteoblastic cell area on native, native with nanoparticle (i.e., without dissolution), fully cross-linked, and templated cross-linked (i.e., porous) nanofilm biomaterials. Reproduced with permission from John Wiley and Sons [25].
Discussion
The world of biology is filled with amazing structures whose detailed functions are only now becoming understood. With hierarchical structure and advanced function, the world of modern materials is a marvel in its own right and an important cog in the continual advancement of the human condition. Marrying these two worlds represents a key scientific challenge, and nanofilm biomaterials ― acting as the interface between material and biology — offer great promise toward a number of new and emerging biomedical applications.
Carbon nanotube-based nanofilm biomaterials are capable of destroying harmful pathogens without the use of antibiotics or other potentially harmful (bio)chemical agents. This capability offers great promise to many areas of health care ― potentially affecting the quality of human life. In addition to biomedical device applications, one could imagine other surfaces — within health care facilities or even households ― being rendered anti-infective using this approach. A remaining challenge is to better understand mechanistically the microbe-nanotube interaction. Current evidence suggests both physical disruption (i.e., piercing the cell wall) and oxidative stress to contribute, but details remain unclear [9,10]. A more complete mechanistic picture is needed to improve the CNT-based film approach and to tailor it toward specific pathogens. Another challenge involves the question of carbon nanotube toxicity to human cells. Some examples from the literature show toxicity [48-50], while others indicate compatibility [51-53]. The discrepancy may be due to differences in CNT properties, e.g., diameter, length, and purity (i.e., metal content); and more studies appear to be needed here. In any event, approval of CNT-based materials for clinical use will likely require extensive evidence as to their safety.
Nanofilm biomaterials of independently controllable bioactivity and mechanical rigidity are expected to have an important impact in many applications involving cell-material contact, e.g., as biomedical device coatings that promote a confluent endothelial cell layer, cell-based bioreactor supports that induce production of a pharmaceutical product, and tissue engineering scaffolds signaling fetal or even stem cells to form mature cellular structures. “Standard” nanofilm biomaterials already show great promise but suffer from strong coupling between bioactivity and mechanical rigidity. Our strategies significantly enhance the potential utility of these thin film systems. A remaining challenge is to better understand the biological response to film-embedded bioactive agents, e.g., cell sensitivity to intra-film concentration, surface cross-linked polymer layers, and strength of bioactive agent-nanofilm interaction. The importance of high film loadings may differ among biomedical applications — a broader understanding of what systems can benefit most from high and controllable local bioactive agent exposures would help guide the development of these promising materials.
Conclusions
By matching the key material and biomolecular length scales, nanotechnology is poised to make significant contributions to biomedicine. We summarize here recent work in our lab in the area of nanofilm biomaterials: thin polymer-based films of thickness 10 to 100 nm. In one avenue, films containing carbon nanotubes act as permanent antimicrobial surfaces, offering the possibility of infection-resistant biomedical and other health care-related devices. In another avenue, films of independently controllable bioactivity and mechanical rigidity are achieved by either surface cross-linking or nanoparticle templating approaches and offer great promise to interface the material and biological worlds.
Acknowledgments
We graciously acknowledge collaborations with Dr. Maria Sammalkorpi of Aalto University, Finland, and Prof. Emmanuel Pauthe of the Université de Cergy-Pontoise, France, and financial support from the National Science Foundation via CBET-0756323 and CBET-1066994.
Abbreviations
- CNT
carbon nanotube
- LbL
layer-by-layer
- PLGA
poly(lactic-co-glycolic acid)
- PLL
poly(L-lysine)
- PGA
poly(L-glutamic acid)
References
- Iijima S. Helical Microtubules of Graphitic Carbon. Nature. 1991;354:56–58. [Google Scholar]
- Sinnott SB, Andrews R. Carbon nanotubes: Synthesis, properties, and applications. Critical Reviews in Solid State and Materials Sciences. 2001;26(3):145–249. [Google Scholar]
- Lu X, Chen ZF. Curved Pi-conjugation, aromaticity, and the related chemistry of small fullerenes (< C-60) and single-walled carbon nanotubes. Chemical Reviews. 2005;105:3643–3696. doi: 10.1021/cr030093d. [DOI] [PubMed] [Google Scholar]
- Yang WR, Thordarson P, Gooding JJ, Ringer SP, Braet F. Carbon nanotubes for biological and biomedical applications. Nanotechnology. 2007;18(41):412001. [Google Scholar]
- Aoki N, Yokoyama A, Nodasaka Y. et al. Cell Culture on a Carbon Nanotube Scaffold. J Biomed Nantechnol. 2005;1:402–405. [Google Scholar]
- Pantarotto D, Singh R, McCarthy D. et al. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed Engl. 2004;43(39):5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- Singh R, Pantarotto D, McCarthy D. et al. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: Toward the construction of nanotube-based gene delivery vectors. J Am Chem Soc. 2005;127:4388–4396. doi: 10.1021/ja0441561. [DOI] [PubMed] [Google Scholar]
- Guo C, Al-Jamal K, Bianco A, Prato M, Kostarelos K. Carbon nanotube-dendron series for siRNA delivery: mechanisms of cellular internalisation. Drug Discov Today. 2010;15:1100. [Google Scholar]
- Kang S, Pinault M, Pfefferle LD, Elimelech M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007;23:8670–8673. doi: 10.1021/la701067r. [DOI] [PubMed] [Google Scholar]
- Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir. 2008;24(13):6409–6413. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- Nepal D, Balasubramanian S, Simonian AL, Davis VA. Strong antimicrobial coatings: Single-walled carbon nanotubes armored with biopolymers. Nano Lett. 2008;8:1896–1901. doi: 10.1021/nl080522t. [DOI] [PubMed] [Google Scholar]
- Liu SB, Wei L, Hao L. et al. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. Acs Nano. 2009;3:3891–3902. doi: 10.1021/nn901252r. [DOI] [PubMed] [Google Scholar]
- Ahmed F, Santos SM, Vergara R, Tria MCR, Advincula R, Rodrigues DF. Antimicrobial Applications of Electroactive PVK-SWNT Nanocomposites. Environ Sci Technol. 2012;46(3):1804–1810. doi: 10.1021/es202374e. [DOI] [PubMed] [Google Scholar]
- Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- Hammond PT. Form and function in multilayer assembly: New applications at the nanoscale. Advanced Materials. 2004;16:1271–1293. [Google Scholar]
- Hammond PT. Engineering Materials Layer-by-Layer: Challenges and Opportunities in Multilayer Assembly. AIChE Journal. 2011;57:2928–2940. [Google Scholar]
- Tang ZY, Wang Y, Podsiadlo P, Kotov NA. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Advanced Materials. 2006;18:3203–3224. [Google Scholar]
- Ren KF, Crouzier T, Roy C, Picart C. Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cell differentiation. Advanced Functional Materials. 2008;18:1378–1389. doi: 10.1002/adfm.200701297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudou T, Crouzier T, Ren KF, Blin G, Picart C. Multiple Functionalities of Polyelectrolyte Multilayer Films: New Biomedical Applications. Advanced Materials. 2010;22:441–467. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- Gribova V, Auzely-Velty R, Picart C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering. Chemistry of Materials. 2012;24:854–869. doi: 10.1021/cm2032459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan S, Loebick CZ, Kang S, Elimelech M, Pfefferle LD, Van Tassel PR. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly(lactic-co-glycolic acid) Nanoscale. 2010;2:1789–1794. doi: 10.1039/c0nr00329h. [DOI] [PubMed] [Google Scholar]
- Aslan S, Deneufchatel M, Hashmi S. et al. Carbon nanotube-based antimicrobial biomaterials formed via layer-by-layer assembly with polypeptides. J Colloid Interface Sci. 2012;388:268–273. doi: 10.1016/j.jcis.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Aslan S, Maatta J, Haznedaroglu BZ. et al. Carbon nanotube bundling: influence on layer-by-layer assembly and antimicrobial activity. Soft Matter. 2013;9:2136–2144. [Google Scholar]
- Phelps JA, Morisse S, Hindie M, Degat MC, Pauthe E, Van Tassel PR. Nanofilm Biomaterials: Localized Cross-Linking To Optimize Mechanical Rigidity and Bioactivity. Langmuir. 2011;27:1123–1130. doi: 10.1021/la104156c. [DOI] [PubMed] [Google Scholar]
- Wu C, Aslan S, Gand A, Wolenski JS, Pauthe E, Van Tassel PR. Porous Nanofilm Biomaterials Via Templated Layer-by-Layer Assembly. Advanced Functional Materials. 2013;23:66–74. [Google Scholar]
- Jarvis WR. The United States approach to strategies in the battle against healthcare-associated infections, 2006: transitioning from benchmarking to zero tolerance and clinician accountability. J Hosp Infect. 2007;65:3–9. doi: 10.1016/S0195-6701(07)60005-X. [DOI] [PubMed] [Google Scholar]
- Banerjee I, Pangule RC, Kane RS. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Advanced Materials. 2011;23:690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- Glinel K, Thebault P, Humblot V, Pradier CM, Jouenne T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomaterialia. 2012;8:1670–1684. doi: 10.1016/j.actbio.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Munoz-Bonilla A, Fernandez-Garcia M. Polymeric materials with antimicrobial activity. Progress in Polymer Science. 2012;37:281–339. [Google Scholar]
- Siedenbiedel F, Tiller JC. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers. 2012;4:46–71. [Google Scholar]
- Busscher HJ, van der Mei HC, Subbiahdoss G. et al. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Science Translational Medicine. 2012;4 doi: 10.1126/scitranslmed.3004528. [DOI] [PubMed] [Google Scholar]
- Rizzello L, Cingolani R, Pompa PP. et al. Nanotechnology tools for antibacterial materials. Nanomedicine. 2013;8:807–821. doi: 10.2217/nnm.13.63. [DOI] [PubMed] [Google Scholar]
- Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. International Journal of Nanomedicine. 2012;7:2767–2781. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DF. Definitions of biomaterials. Consensus conference of the Europen Society for Biomaterials. Chester, UK: Elsevier; 1986. [Google Scholar]
- Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomaterialia. 2010;6:3824–3846. doi: 10.1016/j.actbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Hubbell JA. Bioactive biomaterials. Curr Opin Biotechnol. 1999;10:123–129. doi: 10.1016/s0958-1669(99)80021-4. [DOI] [PubMed] [Google Scholar]
- Sakiyama-Elbert SE, Hubbell JA. Functional biomaterials: Design of novel biomaterials. Annual Review of Materials Research. 2001;31:183–201. [Google Scholar]
- Mendelsohn JD, Yang SY, Hiller J, Hochbaum AI, Rubner MF. Rational design of cytophilic and cytophobic polyelectrolyte multilayer thin films. Biomacromolecules. 2003;4:96–106. doi: 10.1021/bm0256101. [DOI] [PubMed] [Google Scholar]
- Richert L, Boulmedais F, Lavalle P. et al. Improvement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linking. Biomacromolecules. 2004;5:284–294. doi: 10.1021/bm0342281. [DOI] [PubMed] [Google Scholar]
- Richert L, Engler AJ, Discher DE, Picart C. Elasticity of native and cross-linked polyelectrolyte multilayer films. Biomacromolecules. 2004;5:1908–1916. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- Picart C, Schneider A, Etienne O. et al. Controlled degradability of polysaccharide multilayer films in vitro and in vivo. Advanced Functional Materials. 2005;15:1771–1780. [Google Scholar]
- Jessel N, Atalar F, Lavalle P. et al. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Advanced Materials. 2003;15:692–695. [Google Scholar]
- Benkirane-Jessel N, Lavalle P, Hubsch E. et al. Short-time timing of the biological activity of functionalized polyelectrolyte multilayers. Advanced Functional Materials. 2005;15:648–654. [Google Scholar]
- Nadiri A, Kuchler-Bopp S, Mjahed H. et al. Cell apoptosis control using BMP4 and noggin embedded in a potyetectrotyte multilayer film. Small. 2007;3:1577–1583. doi: 10.1002/smll.200700115. [DOI] [PubMed] [Google Scholar]
- Crouzier T, Ren K, Nicolas C, Roy C, Picart C. Layer-By-Layer Films as a Biomimetic Reservoir for rhBMP-2 Delivery: Controlled Differentiation of Myoblasts to Osteoblasts. Small. 2009;5:598–608. doi: 10.1002/smll.200800804. [DOI] [PubMed] [Google Scholar]
- Crouzier T, Fourel L, Boudou T, Albiges-Rizo C, Picart C. Presentation of BMP-2 from a Soft Biopolymeric Film Unveils its Activity on Cell Adhesion and Migration. Advanced Materials. 2011;23:H111–H118. doi: 10.1002/adma.201004637. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Castranova V, Kisin ER. et al. Exposure to carbon nanotube material: Assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66(20):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- Manna SK, Sarkar S, Barr J. et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappa B in human keratinocytes. Nano Lett. 2005;5(9):1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36(3):189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- Chen X, Tam UC, Czlapinski JL. et al. Interfacing carbon nanotubes with living cells. J Am Chem Soc. 2006;128(19):6292–6293. doi: 10.1021/ja060276s. [DOI] [PubMed] [Google Scholar]
- Fadel TR, Steenblock ER, Stern E. et al. Enhanced cellular activation with single walled carbon nanotube bundles presenting antibody stimuli. Nano Lett. 2008;8:2070–2076. doi: 10.1021/nl080332i. [DOI] [PubMed] [Google Scholar]
- Lobo AO, Corat MAF, Antunes EF. et al. An evaluation of cell proliferation and adhesion on vertically-aligned multi-walled carbon nanotube films. Carbon. 2010;48:245–254. [Google Scholar]
- Wang FH, Itkis ME, Bekyarova E, AHaddonntunes RC. Charge-compensated, semiconducting single-walled carbon nanotube thin film as an electrically configurable optical medium. Nature Photonics. 2013;7:460–466. [Google Scholar]