Abstract
A latitudinal cline in flowering time in accessions of Arabidopsis thaliana has been widely predicted because the environmental cues that promote flowering vary systematically with latitude, but evidence for such clines has been lacking. Here, we report evidence of a significant latitudinal cline in flowering time among 70 Northern European and Mediterranean ecotypes when grown under ecologically realistic conditions in a common garden environment. The detected cline, however, is found only in ecotypes with alleles of the flowering time gene FRIGIDA (FRI) that lack major deletions that would disrupt protein function, whereas there is no relationship between flowering time and latitude of origin among accessions with FRI alleles containing such deletions. Analysis of climatological data suggests that late flowering in accessions with putatively functional FRI was associated with reduced January precipitation at the site of origin, consistent with previous reports of a positive genetic correlation between water use efficiency and flowering time in Arabidopsis, and the pleiotropic effects of FRI of increasing water use efficiency. In accessions collected from Southern latitudes, we detected that putatively functional FRI alleles were associated with accelerated flowering relative to accessions with nonfunctional FRI under the winter conditions of our experiment. These results suggest that the ecological function of the vernalization requirement conferred by FRI differs across latitudes. More generally, our results indicate that by combining ecological and molecular genetic data, it is possible to understand the forces acting on life history transitions at the level of specific loci.
A major goal in evolutionary biology is to understand the genetic basis and evolutionary dynamics of life history traits (1). In plants, the shift from vegetative to reproductive growth often has dramatic consequences for fitness and represents a fundamental transition in development (2-4), and for this reason the timing of reproduction has been the subject of intensive ecological, molecular, and genetic investigation.
The model plant Arabidopsis thaliana presents a promising study system to understand the genetic basis and evolutionary dynamics of life history traits such as flowering time for two reasons: first, rapid progress has been made characterizing the developmental and genetic pathways underlying the transition from vegetative to reproductive growth; and second, accessions of Arabidopsis have been collected over a wide geographic area that includes a diversity of climatological and photoperiod regimes. By combining knowledge of the genetic pathways that lead to flowering with data on the site of collection of accessions, it is possible to evaluate the effects of macroscale ecological forces such as climate and photoperiod on life history transitions at the level of specific genetic loci (see ref. 5 for an example related to seedling emergence traits).
In A. thaliana, the transition to reproduction is accelerated by long days (LDs), as mediated by the photoperiod pathway (6-8), and by early exposure to cold temperatures, through the vernalization pathway (4, 9, 10). The environmental cues for these genetic and developmental pathways (photoperiod amplitude and temperature) vary systematically with latitude (10, 11), and for this reason a latitudinal cline in flowering time has been predicted (e.g., refs. 12-19). Although Arabidopsis ecotypes collected over a wide latitudinal range do show significant variation in life history traits (e.g., ref. 12), clear empirical evidence in support of latitudinal clines in flowering time has been lacking despite numerous investigations (e.g., refs. 12-19). It is ironic that in a plant species where knowledge of the interactions between genetic pathways and environmental cues is the most detailed there is so little evidence of simple geographic patterns that would be predicted from that knowledge. These results have led some authors to suggest that ecotypic variation in flowering time is the result of genetic drift (17).
The molecular genetics of flowering time also fail to provide a clear explanation as to why latitudinal clines in flowering time have not been observed. The flowering time gene FRIGIDA (FRI) is considered to be the major determinant of flowering time variation in natural accessions of Arabidopsis (e.g., refs. 4 and 20-24). Functional FRI alleles lead to an accumulation of Flowering Locus C (FLC) mRNA, which in turn inhibits flowering (10), unless down-regulated by vernalization; in essence, functional FRI prevents plants from flowering until winter has passed. The loss of functional FRI alleles is thus thought to promote early flowering in the absence of vernalization (4, 9). Among ecotypes examined in detail, loss-of-function mutations in FRI are associated with earlier flowering time under controlled conditions in 13 of 18 ecotypes screened to date (4, 10). Early-flowering ecotypes appear to have evolved from late-flowering ancestors at least twice, independently, based on analysis of the allelic variation at FRI (10). Moreover, an excess of nonsynonymous polymorphisms have been described in the first exon of FRI, which has been interpreted as evidence of natural selection for early flowering time (25).
Given these results, one would expect a latitudinal divergence in FRI functionality, with early-flowering ecotypes containing nonfunctional FRI restricted to either Southern latitudes or climates with mild winters as a result of natural selection. However, to date there is no evidence of a relationship between winter temperatures at the site of origin of accessions and flowering time as measured under controlled conditions (10), or of a predominance of ecotypes with nonfunctional FRI alleles in Southern latitudes [P = 0.43, our analysis of Johanson's (10) table 1 with a Wilcoxon two-sample test].
Table 1. Mean monthly temperatures and day lengths for the duration of the common garden experiment.
| Month | Mean observed temperature, °C | Day length, h |
|---|---|---|
| October | 12.3 | 10.9 |
| November | 8.6 | 9.7 |
| December | 4.1 | 9.1 |
| January | 1.8 | 9.5 |
| February | 2 | 10.5 |
| March | 4.6 | 11.9 |
Temperature data was gathered from the National Climatic Data Center (National Oceanic and Atmospheric Adminitration) for T. F. Green Airport in Providence.
Here, we report a latitudinal cline in days until bolting in Arabidopsis ecotypes allowed to overwinter under natural conditions. These results demonstrate a latitudinal cline in a life history trait closely related to flowering time in A. thaliana. However, this cline is detected only in ecotypes with FRI alleles lacking deletions that would disrupt protein function. Surprisingly, in ecotypes from Southern latitudes, such putatively functional FRI alleles are associated with accelerated flowering relative to ecotypes with nonfunctional FRI under the winter conditions of our experiment. These results suggest that the ecological function of the vernalization requirement conferred by FRI may differ across latitudes.
Materials and Methods
Common Garden Experiment. On October 2, 2001, we planted seeds of 70 Northern European and Mediterranean ecotypes in a common garden experiment. These plants germinated, overwintered, and bolted under the natural seasonal environmental conditions typically experienced by plants after a winter annual life history. Three to five seeds from each of the 70 ecotypes were deposited into randomized and blocked peat pots that had been sunk into the soil in three raised beds outside of the Brown University greenhouses. Pots were 2 in × 2 in (5.08 cm × 5.08 cm), with 1 in (2.54 cm) between adjacent pots, and they were filled with Metromix 360 soil (Scotts-Sierra Horticultural Products, Marysville, OH). Within each bed, pots were arranged in four spatial blocks, with the position of any ecotype assigned randomly. The raised beds were covered with metal window screening to prevent rain drops from disturbing seeds in the pots while still allowing precipitation and sunlight to reach the seeds.
Seeds were allowed to germinate naturally, and germination was monitored on a regular basis. After germination, the window screening was removed, and the germinant closest to the center of the pot was designated the focal plant; all other plants were then thinned. Germination was relatively synchronous, and thinning began after ≈14 days. Plants experienced Rhode Island winter conditions, including chilling, short-day (SD) photoperiods, and snow (e.g., Fig. 4, which is published as supporting information on the PNAS web site). Mean monthly temperatures and day lengths for the common garden experiment are given in Table 1. Plants were exposed, on average, to vernalizing or near-vernalizing temperatures for 5 of the 6 months of the experiment and to SDs or nearly equal day lengths. For each focal plant we recorded the number of days until bolting (differentiation of the inflorescence from the apical meristem) from the time of planting. Preliminary analysis indicated that days until bolting was highly correlated with rosette leaf number (r = 0.72, P < 0.0001), a commonly used index of flowering time in A. thaliana (e.g., refs. 26 and 27). As a part of a larger study (M.U. and K.M.O., unpublished data), we also measured days until flowering on the same sample of ecotypes in growth chambers at the Phytotron facility of North Carolina State University under SD and LD photoperiods.
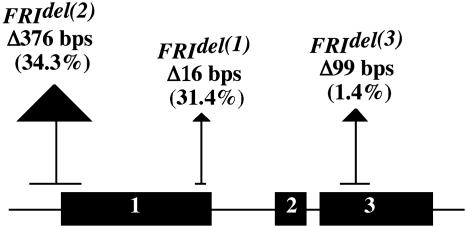
FRI Genotyping. Seed for A. thaliana ecotypes were obtained from single-seed propagated material provided by the Arabidopsis Biological Resource Center, Ohio State University, Columbus, OH. Genomic DNA was extracted from these plants by using DNeasy plant mini kits (Qiagen, Chatsworth, CA). PCR was performed with Taq polymerase by using standard protocols and previously published primers (25). PCR products were gel-purified and sequenced directly by using BigDye terminator reactions. Internal primers for sequencing were designed by using the Primer3 web site (www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi) based on the published GenBank sequence (accession no. AF228499). Sequences were run on a Perkin-Elmer 3700 capillary automated sequencer. Sequences were aligned to the published sequence, and deletions predicted to disrupt FRI protein expression were identified (refs. 10 and 25; Fig. 1) and genotyped in all 70 ecotypes. We refer to FRI alleles lacking deletions that would disrupt protein function as putatively functional FRI and those with deletions that would disrupt protein function as nonfunctional FRI. Although definitive evidence of FRI functionality requires genetic transformation or controlled crosses to known FRI nulls, we note that past studies have found a high correspondence between functionality and nonfunctionality identified by genotyping and functionality and nonfunctionality identified by genetic transformation or controlled crosses (e.g., refs. 10 and 26).
Fig. 1.
Structure of the FRI gene showing the location, size, and frequency of the three assayed deletions.
Latitudinal, Longitudinal, and Climatological Data. We gathered information on the site of collection of the 70 ecotypes from The Arabidopsis Information Resource web site (www.arabidopsis.org), and then determined the latitude and longitude for these locations. For cases where latitudinal or longitudinal ranges were given, we used mean values. We downloaded data describing the mean 1961-1990 climatology at locations of origin for the ecotypes from the high-resolution (10′) surface climate data set developed by New et al. (28). Data on January temperature and precipitation and July temperature and precipitation were chosen to represent winter and summer climates. Specific climatology data at the site of collection for ecotypes were obtained with the “get grid value” command of GIS software ARCVIEW 3.3. Our complete data set of ecotype stock numbers, geographic coordinates, putative FRI functionality, and bolting time is given in Table 3, which is published as supporting information on the PNAS web site.
Statistical Analysis. To evaluate the relationship between ecotype bolting dates and longitude, latitude, and climate, we used the mean bolting date from the replicates of each ecotype. We calculated ecotype least-square means for bolting date from a statistical model that included the main effects of bed, spatial blocks nested within beds, ecotype, and ecotype × bed interactions. Sample sizes for determining ecotype least-square means ranged between 9 and 12 and replicate plants per ecotypes. Analysis of amplified fragment length polymorphism loci described by Sharbel et al. (29) by K.M.O. and M.D.P. (unpublished manuscript) indicated that the ecotypes used in our study behave as a single genetic population (30, 31).
Results and Discussion
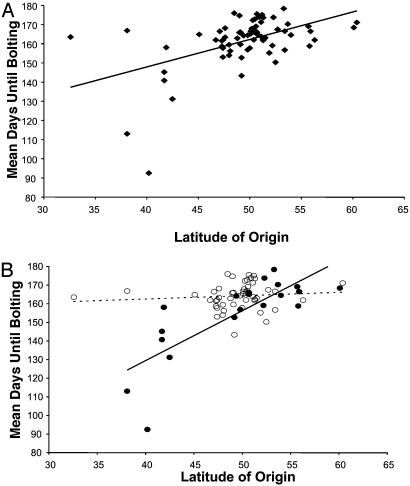
Latitudinal Cline. Under our experimental conditions, there was a significant, positive relationship between latitude of origin and average days until bolting, as determined by linear regression (latitude regression coefficient ± SE = 1.43 ± 0.30, P < 0.0001; Fig. 2A), indicating that ecotypes from Northern latitudes bolted later than ecotypes from Southern latitudes. These data indicate clear genetic differentiation of Arabidopsis ecotypes along a latitudinal gradient. Moreover, the observation of significant differences between ecotypes in a common garden indicates that these differences are genetically based, presumably as a result of selection caused by factors that covary with latitude. (The large number of generations of self-fertilization of the ecotypes under uniform conditions in the Arabidopsis stock center suggests that maternal effects are unlikely.) Our inference that the latitudinal cline is caused by genetic differentiation in response to selection pressures covarying with latitude is strengthened because the ecotypes used were drawn from a large geographic area and ecotypic means calculated from a common garden will decrease the contribution of random environmental variation (32).
Fig. 2.
The relationship between latitude of origin and mean days until bolting for 70 ecotypes grown in a common garden in Rhode Island. (A) Data portrayed for all ecotypes regardless of FRI. (B) Data for all ecotypes, broken down by FRI class. Open circles indicate nonfunctional FRI, and solid circles indicate putatively functional FRI. The regression for the putatively functional FRI class, shown with the solid line, is significant, whereas the regression for the nonfunctional FRI class is not significant. The slopes of the two lines are significantly different, based on analysis of covariance and permutation testing.
FRI Effects. Based on the molecular genetics of flowering time in A. thaliana, we considered two nonexclusive hypotheses that could explain these data. On the one hand, the observed cline could be caused by latitudinal divergence in the functionality of FRI, with early-flowering ecotypes possessing loss-of-function mutations predominantly found in Southern latitudes. On the other hand, the observed cline could be modulated by FRI functionality, in that functional FRI genes may have allowed ecotypes to respond and adapt to environmental cues that covary with latitude, such as vernalization. We failed to detect any support for the first hypothesis: there was no difference in the mean latitude of origin between putatively functional and null FRI allele classes, (t test for unequal variances, t = 0.02, df = 27.3, P = 0.99), as would be required if latitudinal divergence in FRI function were the cause of the observed relationship between latitude and days until bolting.
In contrast, we detected striking evidence that the putative functionality of FRI modulates the relationship between latitude and days until bolting (Fig. 2B): the effect of latitude on days until bolting differs significantly depending on FRI functionality as determined by analysis of covariance (FRI functionality × latitude F1,66 = 27.89, P < 0.0001). Separate regressions for the two FRI allele classes revealed that ecotypes with putatively functional FRI exhibit a significant latitudinal cline in bolting time (latitude regression coefficient ± SE = 2.68 ± 0.48, P < 0.0001; Fig. 2B), whereas those with nonfunctional FRI show no relationship between latitude and bolting time (latitude regression coefficient ± SE = 0.18 ± 0.25, P = 0.49; Fig. 2B).
We used two approaches to verify the robustness of this statistical result. First, to determine whether this result could be caused by simply dividing the data into two categories according to a single genetic locus (in this case, FRI), we performed the same analyses with 59 of the amplified fragment length polymorphism (AFLP) loci from Sharbel et al.'s data set (29) that had sufficient variation for analysis. In only three cases (P = 0.051) did we detect AFLP locus × latitude interactions with F statistics greater than the observed FRI functionality × latitude F statistic, suggesting that our FRI results are unlikely to occur by random chance alone. However, because the AFLP loci do not represent all possible combinations of potential outcomes, we designed a permutation test to obtain a more rigorous estimate of the P value for the interaction between FRI functionality and latitude. In each iteration of the permutation test, ecotypes retained their observed days until bolting and latitude of origin, but were randomly assigned a functional or nonfunctional “pseudomarker;” the frequency of functional and nonfunctional pseudomarkers was constrained to equal the frequency of putatively functional and nonfunctional FRI in our sample. For each of 10,000 permutations, we evaluated an analysis of covariance with days until bolting as the response variable and latitude, pseudomarker, and latitude × pseudomarker interactions as the predictor variables. The frequency of permutations with latitude × pseudomarker F statistics greater than the observed FRI functionality × latitude F statistic was quite low (P = 0.011), indicating that the FRI × latitude interaction we observed is unlikely to occur by chance alone. These results and Fig. 2B suggest two conclusions: (i) the association between FRI and flowering time differs depending on the latitude of origin of the ecotype, and (ii) ecological factors that covary with latitude have likely imposed natural selection on flowering time variation, but only in ecotypes with putatively functional FRI alleles.
Effects of FRI on Flowering Time. The data presented in Fig. 2B suggest that in Southern latitudes, putatively functional FRI alleles are associated with accelerated flowering under natural winter conditions. To address this possibility quantitatively, we divided the data from the common garden and growth chamber experiments into subsamples consisting of Northern and Southern ecotypes, as determined by the median latitude of origin in our sample, 50.3° north. The frequency of FRI functional and nonfunctional ecotypes above and below this median latitude is approximately equal to the frequency for the entire sample of ecotypes, suggesting that the Northern and Southern ecotypes represent unbiased subsamples with respect to FRI.
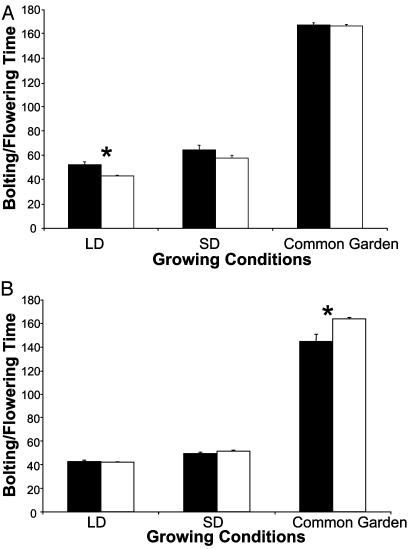
For Northern ecotypes (Fig. 3A), the patterns observed in our common garden experiment, and the growth chamber experiments with the same collection of ecotypes under SD and LD photoperiods, mirror previous results on effects of FRI deficiency (e.g., refs. 10 and 23). In the growth chamber experiments, we find that loss-of-function mutations in FRI lead to significantly earlier flowering under LD, as has been reported (t test for unequal variances, t = 3.64, df = 11.8, P = 0.0035), and a nonsignificant trend for earlier flowering under SD (t = 1.58, df = 31, P = 0.12). In the common garden, we find no difference in flowering time (t = 0.39, df = 31, P = 0.70), as would be expected given that the experimental plants experienced at least 30-40 days of vernalization (cf. ref. 23).
Fig. 3.
The effect of putatively functional and nonfunctional FRI on mean days until bolting (common garden) or mean days until flowering (growth chamber), portrayed separately for ecotypes from Northern latitudes (>50.3 N) (A) and Southern latitudes (<50.3 N) (B). Filled bars portray data for ecotypes with putatively functional FRI alleles, and open bars portray data ecotypes with nonfunctional alleles. Means and standard errors are indicated (standard errors are sometimes very small and hard to distinguish) for three growing conditions (LD = LDs in growth chamber, SD = SDs in growth chamber, and Common Garden in Rhode Island). * indicates significant difference between FRI classes based on t test.
In Southern ecotypes of Arabidopsis (Fig. 3B), the effect of loss-of-function mutations in FRI is not associated with flowering time under LD (t test for unequal variances, t = 0.51, df = 12.9, P = 0.62). Under SD, there is a nonsignificant trend for later flowering of ecotypes that have FRI nonfunctional alleles (t = 1.45, df = 38, P = 0.16). In the common garden, however, we found that putatively functional FRI is significantly associated with earlier flowering (t test for unequal variances, t = 3.0, df = 11.9, P = 0.01, Fig. 3B). The difference between late-flowering FRI nulls and early-flowering putatively functional FRI in Southern ecotypes is sufficiently large that if we were to naively analyze the entire data set without controlling for latitude, it would appear that ecotypes that have FRI null alleles, in general, flower later than ecotypes with putatively functional FRI alleles under the winter conditions of our experiment. However, controlling for latitude indicates that these results are not found in Northern latitudes, where the majority of early work on late-flowering FRI-dominant (functional) ecotypes was performed [e.g., Napp-Zinn's work on Swiss, German, and French ecotypes (33)]. Nonetheless, the results for Southern ecotypes (Figs. 2B and 3B) suggest that functional FRI is necessary for early flowering under winter conditions, at least in some ecotypes. The comparison between SD growth chamber conditions and the common garden suggests that these effects cannot be entirely explained by effects of SD photoperiod per se, suggesting that other environmental or ecological factors, such as exposure to vernalizing temperatures in the common garden, contribute to these results.
Ecological Correlates of Clinal Variation. To address the possibility that the observed latitudinal cline in ecotypes with putatively functional FRI was caused by local adaptation to climate at the site of collection (e.g., ref. 34), we evaluated a multiple regression containing latitude, longitude, mean January temperature and precipitation, and mean July temperature and precipitation as independent variables (28) and days until bolting as the response variable. This approach controls for the correlations between the independent variables; significant effects of latitude would indicate that the observed genetic differentiation and cline are caused by environmental factors, such as seasonal amplitude of photoperiod variation, other than those included in the regression.
After controlling for climatological factors, the average number of days until bolting was still significantly influenced by latitude (Table 2; latitude effect, P = 0.029), but also January precipitation (P = 0.015). In particular, later bolting in the common garden was significantly associated with lower January precipitation at the site of origin (Table 2). Because flowering time and water use efficiency have been shown to be genetically correlated in Arabidopsis (35), these data suggest an ecological model for the evolution of late-flowering phenotypes in Arabidopsis. If in sites with less January precipitation natural selection acts to increase water use efficiency, later flowering may evolve as a correlated response, as has been noted in some crop species [see McKay et al. (35) for details]. Interestingly, McKay et al. (35) also found strong pleiotropic effects of both FRI and FLC, with near isogenic lines containing functional FRI and FLC increasing both flowering time and water use efficiency in both the Col and Ler genomic backgrounds. These data are consistent with our findings that less January precipitation at the site of origin is associated with later flowering, but only in ecotypes with putatively functional FRI. [We note again that the observed genetic differentiation of bolting time in response to latitude and climate reported here is unlikely to be caused by the population structure described by Sharbel et al. (29), as the collection of ecotypes analyzed here behave as a single genetic population (K.M.O., unpublished data)].
Table 2. Multiple regression analysis to test the effects of geographic origin and climatic variables on days until bolting for ecotypes with putatively functional FRI.
| Source | Parameter estimate | Standard error | t statistic | P |
|---|---|---|---|---|
| Longitude | −0.28 | 0.75 | 0.37 | 0.714 |
| Latitude | 2.64 | 1.09 | 2.42 | 0.029 |
| January temperature | 2.24 | 1.78 | 1.26 | 0.230 |
| January precipitation | −0.37 | 0.13 | 2.78 | 0.015 |
| July temperature | 0.44 | 3.24 | 0.14 | 0.894 |
| July precipitation | 0.46 | 0.27 | 1.68 | 0.115 |
Significant effects are shown in bold; total sample size (n = 21).
Genetic Mechanisms of Early Flowering for Putatively Functional FRI Alleles. The predominant view on the genetic basis of late-flowering in A. thaliana is that this phenotype arises through the interaction of two dominant genes, FRI and FLC (see above and e.g. refs. 4, 10, 26, 27, and 36). Accordingly, our finding that Southern ecotypes with putatively functional FRI alleles flower, on average, 19 days (≈6.5 rosette leaves) earlier under natural conditions than ecotypes with nonfunctional FRI alleles requires explanation. It is worth noting that Southern ecotypes with nonfunctional FRI alleles flower at approximately the same time as Northern ecotypes with either type of FRI allele (e.g., the nonsignificant regression line of Fig. 2B for ecotypes with nonfunctional FRI, and comparisons of Fig. 3 A and B). These data suggest that Southern ecotypes with putatively functional FRI exhibit accelerated flowering, rather than Southern ecotypes with nonfunctional FRI exhibiting a delay in flowering.
One possible explanation for these data are that these early-flowering Southern ecotypes with putatively functional FRI alleles have nonfunctional or weak FLC alleles, as has been described or implicated for other early flowering ecotypes [e.g., Da(1)-12, Shakhdara (27), Kondara, and KZ9 (26)]. Although this possibility is consistent with the current genetic model of the early-flowering habit, it would require a higher frequency of weak or null FLC genes than has been reported previously [for example, Michaels et al. (27) report a frequency of weak or null FLC genes of 11% (3 of 27 randomly chosen accessions)] and that these weak or null FLC genes be found in predominantly in Southern latitudes and in ecotypes with putatively functional FRI. In like fashion, it is possible that Southern ecotypes with putatively functional FRI carry dominant suppressors of FRI function, as has been suggested for the ecotype Wil-2 (26); however, this explanation would also require dominant suppressors of FRI to be found in primarily Southern latitudes.
A third possibility is that the observed cline reflects differential photoperiod sensitivity among the ecotypes that would be observed only among vernalized plants such as those in our experiment, as vernalization down-regulates FLC levels (e.g., ref. 36). However, this possibility is also unlikely, as we do not observe clinal variation among the ecotypes with nonfunctional FRI, which would also have low levels of FLC expression. The restrictive assumptions necessary for these three scenarios to lead to an acceleration of flowering in Southern ecotypes with putatively functional FRI suggest to us that they are unlikely.
A more plausible, ecologically reasonable hypothesis is that Southern ecotypes with putatively functional FRI are more sensitive to vernalization cues (e.g., refs. 9, 36, and 37). Although exposure to cold temperatures eliminates the late-flowering effects of functional FRI and FLC (23, 36), ecotypes from some Southern latitudes will be exposed to typical vernalization temperatures [1-7°C (37)] less often. One potential consequence of reduced exposure to vernalization is that Southern ecotypes may have increased sensitivity to vernalization cues, as has been observed for other European species with similar geographic distributions (e.g., ref. 38). If, in fact, Southern ecotypes, do exhibit increased sensitivity to vernalization cues (for example, because of past selection to ensure flowering before the onset of summer heat) exposure to vernalization cues in Rhode Island that are stronger and longer in duration (e.g., Table 1) may have had a disproportionate effect on flowering time. In this scenario, the selective forces leading to a vernalization requirement might differ between Northern and Southern latitudes. In Northern latitudes, a vernalization requirement ensures that plants do not flower until winter has passed, presumably because of the risks of winter mortality and the benefits of waiting for favorable spring conditions (4). In contrast, in Southern latitudes, where winters are, on average, milder, and summer conditions harsher, increased sensitivity to vernalization may actually cue flowering under SD conditions to ensure that plants do not wait too long to flower and risk mortality from summer heat. Ecological genetic studies that combine crosses to null mutants, differential vernalization exposure, and ecologically realistic experiments that expose plants to the natural variation and distribution of environmental conditions will be necessary to fully evaluate these ecological and genetic hypotheses.
Supplementary Material
Acknowledgments
We thank M. Israel, A. Aguilera, M. Jackson, N. Kraft, A. Fisher, Z. German, K. Heebink, C. Wessinger, H. Urabe, A. Pahuja, N. Reese, B. Singh, F. Jackson, J. Kelley, and M. Vadeboncoeur for technical assistance and T. Korves, L. Dorn, R. Amasino, and three anonymous reviewers for discussions and suggestions on the manuscript. Our work was supported by National Science Foundation Grants DEB-0129018 (to J.S.) and DEB-9976997 (to J.S., T. F. C. Mackay, and M.D.P.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FRI, FRIGIDA; FLC, Flowering Locus C; SD, short day; LD, long day.
References
- 1.Roff, D. A. (1992) The Evolution of Life History: Theory and Analysis (Chapman & Hall, New York).
- 2.Geber, M. A. (1990) Evolution 44, 799-819. [DOI] [PubMed] [Google Scholar]
- 3.Stratton, D. A. (1998) Heredity 81, 144-155. [DOI] [PubMed] [Google Scholar]
- 4.Simpson, G. G. & Dean, C. (2002) Science 296, 285-289. [DOI] [PubMed] [Google Scholar]
- 5.Maloof, J. N., Borevitz, J. O., Dabi, T., Lutes, J., Nehring, R. B., Redfern, J. L., Trainer, G. T., Wilson, J. M., Asami, T., Berry, C. C., et al. (2001) Nat. Genet. 29, 441-446. [DOI] [PubMed] [Google Scholar]
- 6.Koornneef, M., Alonso-Blanco, C., Peeters, A. J. M. & Soppe, W. (1998) Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 345-370. [DOI] [PubMed] [Google Scholar]
- 7.Levy, Y. Y. & Dean, C. (1998) Plant Cell 10, 1973-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazquez, M. A. & Weigel, D. (1999) Plant Physiol. 120, 1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheldon, C. C., Rouse, D. T., Finnegan, E. J., Peacock, W. J. & Dennis, E. S. (2000) Proc. Natl. Acad. Sci. USA 97, 3753-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johanson, U., West, J., Lister, C., Michaels, S., Amasino, R. & Dean, C. (2000) Science 290, 344-347. [DOI] [PubMed] [Google Scholar]
- 11.Thomas, B. & Vince-Prue, D. (1997) Photoperiodism in Plants (Academic, New York).
- 12.Nordborg, M. & Bergelson, J. (1999) Am. J. Bot. 86, 470-475. [PubMed] [Google Scholar]
- 13.Kuittinen, H., Mattila, A. & Savolainen, O. (1997) Heredity 79, 144-152. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson, B. H., Sills, G. R. & Nienhuis, J. (1993) Am. J. Bot. 80, 646-648. [Google Scholar]
- 15.Zhang, J. & Lechowicz, M. J. (1994) Am. J. Bot. 81, 1336-1342. [Google Scholar]
- 16.Botto, J. F. & Smith, H. (2002) Plant Cell Environ. 25, 53-63. [Google Scholar]
- 17.Stenoien, H. K., Fenster, C. B., Kuittinen, H. & Savolainen, O. (2002) Am. J. Bot. 89, 1604-1608. [DOI] [PubMed] [Google Scholar]
- 18.Pollard, H., Cruzan, M. & Pigliucci, M. (2001) Evol. Ecol. Res. 3, 129-155. [Google Scholar]
- 19.Pigliucci, M. & Marlow, E. T. (2001) Oecologia 127, 501-508. [DOI] [PubMed] [Google Scholar]
- 20.Lee, I., Bleecker, A. & Amasino, R. (1993) Mol. Gen. Genet. 237, 171-176. [DOI] [PubMed] [Google Scholar]
- 21.Burn, J. E., Smyth, D. R., Peacock, W. J. & Dennis, E. S. (1993) Genetica 90, 147-155. [Google Scholar]
- 22.Clarke, J. H. & Dean, C. (1994) Mol. Gen. Genet. 242, 81-89. [DOI] [PubMed] [Google Scholar]
- 23.Lee, I. & Amasino, R. M. (1995) Plant Physiol. 108, 157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanda, S., John, M. & Amasino, R. (1997) J. Hered. 88, 69-72. [DOI] [PubMed] [Google Scholar]
- 25.Le Corre, V., Roux, F. & Reboud, X. (2002) Mol. Biol. Evol. 19, 1261-1271. [DOI] [PubMed] [Google Scholar]
- 26.Gazzani, S., Gendall, A. R., Lister, C. & Dean, C. (2003) Plant Physiol. 132, 1107-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaels, S. D., He, Y., Scortecci, K. C. & Amasino, R. M. (2003) Proc. Natl. Acad. Sci. USA 100, 10102-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.New, M., Lister, D., Hulme, M. & Makin, I. (2002) Climate Res. 21, 1-25. [Google Scholar]
- 29.Sharbel, T. F., Haubold, B. & Mitchell-Olds, T. (2000) Mol. Ecol. 9, 2109-2118. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard, J. K., Stephens, M. & Donnelly, P. (2000) Genetics 155, 945-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pritchard, J. K., Stephens, M., Rosenberg, N. A. & Donnelly, P. (2000) Am. J. Hum. Genet. 67, 170-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endler, J. A. (1986) Natural Selection in the Wild (Princeton Univ. Press, Princeton).
- 33.Napp-Zinn, K. (1979) in La Physiologie de la Floraison, eds. Champagnat, P. & Jacques, R. (Collogues Internationaux, Centre National de la Recherche Scientifique, Paris), pp. 217-220.
- 34.Hoffman, M. H. (2002) J. Biogeogr. 29, 125-134. [Google Scholar]
- 35.McKay, J. K., Richards, J. H. & Mitchell-Olds, T. (2003) Mol. Ecol. 12, 1137-1151. [DOI] [PubMed] [Google Scholar]
- 36.Michaels, S. D. & Amasino, R. M. (2001) Plant Cell 13, 935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaels, S. D. & Amasino, R. M. (2000) Plant Cell Environ. 23, 1145-1153. [Google Scholar]
- 38.Boudry, P., McCombie, H. & Van Dijk, H. (2002) J. Ecol. 90, 693-703. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.