Abstract
Purpose
This tutorial provides an introduction to cortical auditory spectral responses, focusing on event-related activity in the high-gamma frequencies (60–150 Hz), their recent emergence in neuroscience research, and potential clinical applications.
Method
Auditory high-gamma responses are described and compared with traditional cortical evoked responses, including the auditory evoked N1 response. Methods for acquiring and analyzing spectral responses, including time-frequency analyses, are discussed and contrasted with more familiar time-domain averaging approaches. Four cases are presented illustrating high-gamma response patterns associated with normal and impaired auditory processing.
Conclusions
Cortical auditory high-gamma responses may provide a useful clinical measure of auditory processing.
Keywords: high-gamma responses, auditory processing, time-frequency analysis, auditory cortex
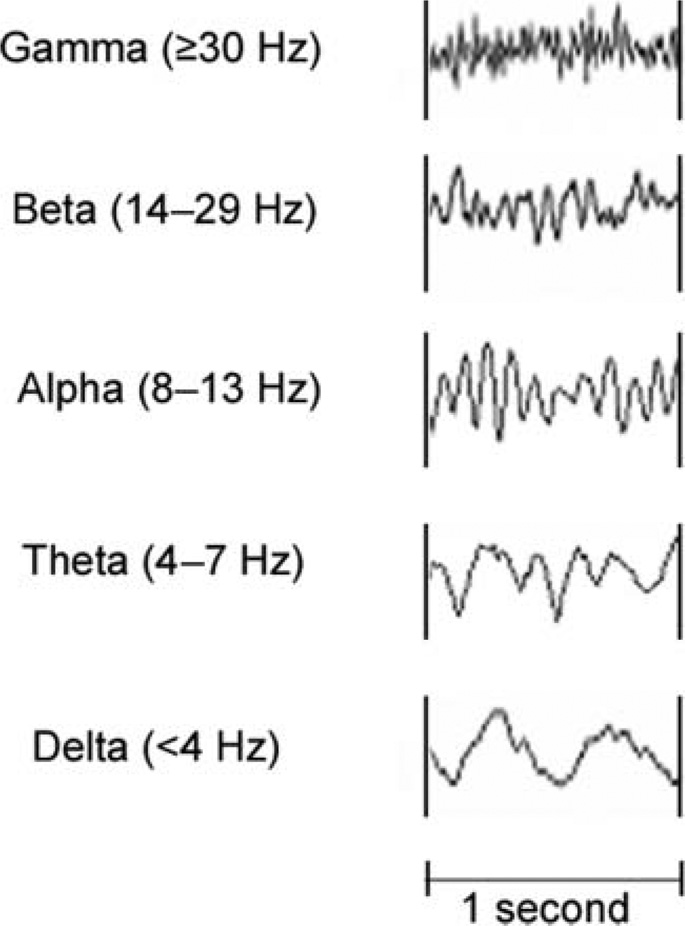
The human brain generates rhythmical electrical fluctuations, or oscillations, that are represented as waves of different frequencies in the electroencephalogram (EEG). EEG signals are recorded from the scalp or, in the case of intracranial recordings, directly from the surface of the cerebral cortex. By convention, the EEG is divided into five frequency (spectral) bands labeled with Greek symbols (see Figure 1): delta (δ, <4 Hz); theta (θ, 4–7 Hz); alpha (α, 8–13 Hz); beta (β, 14–29 Hz); and gamma (γ, ≥30 Hz). Specific EEG frequency bands can be modulated by level of alertness (e.g., awake, drowsy, or asleep), sensory input, and cognitive tasks. It is well established, for example, that alpha frequencies are modulated by attention and change in amplitude when eyes are open or closed (Adrian & Matthews, 1934). More recently, it has been shown that the gamma band, comprising the highest (e.g., fastest) of the EEG frequencies, is modulated by sensory input, including sounds, in humans and animals (Basar, Basar-Eroglu, Rahn, & Schurmann, 1991; Basar-Eroglu, Struber, Kruse, Basar, & Stadler, 1996; Tallon-Baudry & Bertrand, 1999). Event-related changes in amplitude are superimposed on the continuous background EEG and are measured in terms of power (amplitude squared). The distribution of EEG power changes over time (energy) form the basis of spectral responses.
Figure 1.
Single-channel normal electroencephalogram recordings showing the five major frequencies: delta, theta, alpha, beta, and gamma. Time (in seconds) is on the horizontal axis; amplitude (in decibels) is on the vertical axis.
The gamma band is subdivided into low gamma (30–60 Hz) and high gamma (60–150 Hz) based on functional and neurophysiological differences (Crone, Miglioretti, Gordon, & Lesser, 1998; Edwards, Soltani, Deouell, Berger, & Knight, 2005). Low gamma contains mostly phase-locked (evoked) components. An example of a low-gamma response is the evoked auditory steady-state response that occurs around 40 Hz in response to auditory stimuli that are amplitude- or frequency-modulated at the same rate. Phase-locked responses have a strong temporal relationship to the onset of the stimulus and are derived by trial averaging in the time domain.
In contrast, high gamma is composed primarily of non-phase-locked components. Although high-gamma responses are derived from the same electrophysiological signal as evoked responses, they are not evident in the averaged evoked waveform because jitter in their latencies results in cancellation during trial averaging in the time domain. Once considered “noise,” these non-phase-locked responses are now known to play an important role in activating and synchronizing local cortical networks involved in sensory processing and perception of simple and complex stimuli, including speech (Eckhorn et al., 1988; Fries, 2009; Gray & Singer, 1989; Palva et al., 2002; Sinai et al., 2009; Steinschneider et al., 2011; Tallon-Baudry & Bertrand, 1999). High-gamma responses have been used increasingly to measure cortical information processing in studies of auditory perception (Crone, Boatman, Gordon, & Hao, 2001; Edwards et al., 2005; Sinai et al., 2009), movement (Crone et al., 1998; Miller et al., 2007), and cognitive function (Herrmann & Knight, 2001; Herrmann et al., 2004; Ray, Niebur, Hsiao, Sinai, & Crone, 2008).
This tutorial provides an introduction to high-gamma responses for audiologists. Auditory high-gamma spectral responses are contrasted with traditional cortical evoked responses, focusing on the concurrent auditory evoked N1 response. Methods for recording and analyzing spectral responses, including time-frequency analysis, are described briefly and contrasted with traditional time-domain averaging methods. We begin with an overview of cortical oscillations and EEG signals. Four cases are presented to illustrate features and patterns of high-gamma responses associated with normal and impaired auditory processing. Current methodological challenges and potential clinical applications are discussed.
Auditory High-Gamma Responses
Auditory high-gamma responses are defined as event-related changes in spectral power in the 60–150-Hz frequency range. Changes in spectral power are determined statistically by comparison with baseline (i.e., prestimulus interval) and are represented using time-frequency plots (for an example, see Figure 2). The neural generators of auditory high-gamma responses are located in primary and nonprimary auditory cortex, including auditory association areas on the lateral superior temporal gyrus (Steinschneider et al., 2011; Trautner et al., 2006).
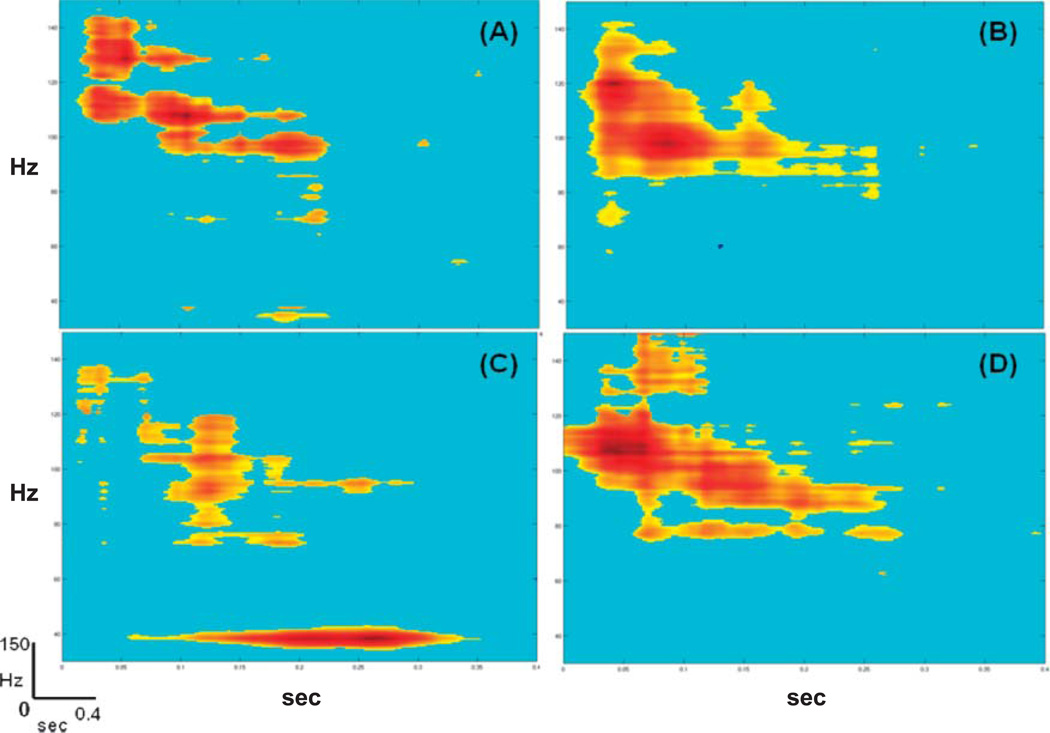
Figure 2.
Auditory high-gamma responses recorded with four different auditory stimuli from the same electrode site in a patient with normal auditory processing abilities (Case 1). Stimulus conditions are (A) tones (1200 Hz); (B) speech (/da/); (C) 40-Hz amplitude-modulated tone (carrier frequency = 1000 Hz); and (D) white noise. In (C), an auditory steady-state response is also visible at the tone amplitude modulation rate (40 Hz). Time is on the horizontal axis in seconds (0–0.4 s). Frequency is on the vertical axis in hertz (0–150 Hz). Statistically significant changes are color-coded, with red representing the largest increase in power (dB) from the prestimulus baseline, shown in blue.
High-gamma responses are associated with multiple aspects of auditory processing, including sound discrimination (Crone et al., 2001; Edwards et al., 2005; Fishman, Arezzo, & Steinschneider, 2004; Palva et al., 2002; Sinai et al., 2009), phonological processing (Chang et al., 2011; Steinschneider et al., 2011), auditory selective attention (Herrmann & Knight, 2001; Ray et al., 2008), auditory verbal memory (Herrmann et al., 2004; Kaiser, Ripper, Birbaumer, & Lutzenberger, 2003), and auditory comprehension (Towle et al., 2008). Because individuals with auditory processing disorders are often impaired in one or more of these auditory functions (American Speech-Language-Hearing Association, 1996, 2005; Moore, 2006), high-gamma responses are emerging as a potentially useful clinical tool for measuring auditory processing.
Auditory high-gamma-band responses occur approximately 100 ms (75–120 ms) after stimulus presentation (Brugge et al., 2009; Crone et al., 2001; Edwards et al., 2005; Howard et al., 2000). Although the cortical auditory evoked N1 response occurs in the same time period, peaking around 100 ms, it is distinguished from the high-gamma response by its low frequency (<10 Hz) and phase-locked activity, its neural generators that are located mainly in primary auditory cortex, and its functional role as an automatic detection response to sound onset or sound change.
Recording Methods
High-gamma responses are recorded with the same hardware and software used to record cortical evoked responses. Gamma responses can be recorded from the scalp using electrodes, or sensors in the case of magnetoencephalography, or from electrodes implanted directly on the cortex or in subcortical structures. Until recently, it has been difficult to identify high-gamma responses in scalp recordings due to the low-pass filtering effects of the skull (Nunez & Srinivasan, 2006). Therefore, much of what is known about high-gamma responses is based on direct cortical recording. Intracranial recordings, also known as electrocorticography (ECoG), are performed to localize seizures and map cortical functions (e.g., language or motor) in patients with epilepsy and cortical lesions who are neurosurgical candidates (Jerbi, Ossandon, & Hamame, 2009; Lachaux, Rudrauf, & Kahane, 2003).
Spectral Analysis
In contrast to traditional cortical evoked responses that are derived by signal averaging in the time domain, spectral responses are measured by averaging in the frequency domain (for a review, see Gilley & Sharma, 2010). Because the evoked waveform does not contain information about specific component frequencies, the recorded signal must be transformed from the time domain into the frequency domain. This transformation is usually performed by applying a Fourier analysis. Fourier analysis is a mathematical operation that decomposes the continuous signal into a set of sinusoidal component frequencies (sines and cosines) that are plotted as a power spectrum of the component frequencies.
Although frequency-domain analyses provide information about the magnitude of spectral responses, they do not provide information about when these changes occur in time. To preserve temporal information, time-frequency analyses are employed based on statistical comparisons of the pre-stimulus (baseline) and poststimulus EEG activity. The result is a distribution of statistically significant changes in frequency-specific power over time (energy) that are represented as time-frequency plots. There are a number of different time-frequency methods that have been used to analyze high-gamma responses, including the short-time (or short-term) Fourier transform (Zygierewicz, Durka, Klekowicz, Franaszczuk, & Crone, 2005), wavelet transforms (Gaona et al., 2011; Gurtubay et al., 2001), and matching pursuit algorithms (Franaszczuk, Bergey, Durka, & Eisenberg, 1998; Mallat & Zhang, 1993). Wavelets transform signals from the time domain to both the time and frequency domain and are commonly used because they are computationally efficient and provide good time and frequency resolution. The matching pursuit method is less computationally efficient but offers the advantage of being well suited for capturing brief, time-varying changes in neural signals characteristic of cortical sound processing (Boatman-Reich et al., 2010). A detailed discussion of these individual time-frequency methods is beyond the scope of this tutorial (for a review, see Wang, 2010).
Clinical Applications
Auditory gamma-band responses have been used to investigate abnormal cortical function in patients with neurological disorders such as Alzheimer’s disease and mild cognitive impairment (Missonnier et al., 2010), and psychiatric disorders such as schizophrenia (Uhlhaas et al., 2006; Uhlhaas & Singer, 2010); it has been shown that gamma-band responses correlate with the severity and duration of schizophrenia (Domjan, Csifcsak, Garab, Szendi, & Janka, 2009; Gallinat, Winterer, Herrmann, & Senkowski, 2004). Cortical mapping of auditory-related high-gamma responses has also been used to measure tinnitus severity (van der Loo et al., 2009), map cortical language functions for presurgical planning in patients with chronic epilepsy or tumors (Cervenka, Boatman-Reich, Ward, Franaszczuk, & Crone, 2011; Crone et al., 2001; Towle et al., 2008), and develop implantable brain–computer interfaces to enable paralyzed patients to communicate and to control prosthetic devices through neural signals (Guo, Gao, & Hong, 2010; Pfurtscheller et al., 2003). In the next section, we present a series of four cases to illustrate how high-gamma responses may also be useful for assessing auditory processing.
Case Studies
The following four cases are presented to illustrate patterns of auditory high-gamma responses associated with normal auditory processing (Patients 1 and 3) and impaired auditory processing (Patients 2 and 4). All four patients had medically refractory seizures and were admitted to our epilepsy monitoring unit for intracranial (ECoG) recordings to determine their candidacy for surgical treatment of their seizure disorders. All were left-hemisphere dominant for receptive and expressive language, as confirmed by intra-carotid amobarbital injection (Wada test). The auditory ECoG recordings were performed 3–4 days after electrode implantation surgery and while patients were awake and fully responsive, as in previous studies (Crone et al., 1998, 2001; Sinai et al., 2009). None of the patients experienced seizures during the recordings. For all patients, audiometric screening revealed normal hearing thresholds (≤25 dB HL) bilaterally at 500–4000 Hz and excellent word recognition scores in quiet (≥92%, Central Institute for the Deaf [CID] W-22 lists). Because auditory processing difficulties are common in patients with chronic epilepsy (Han et al., 2011; Korostenskaja et al., 2010), we routinely screen patients for auditory processing difficulties using the SCAN–A: A Test for Auditory Processing Disorders in Adolescents and Adults (Keith, 1994) administered through insert earphones at a 50-dB presentation level. The SCAN–A has four subtests to assess speech recognition under adverse listening conditions: filtered word recognition, word recognition in noise (8-dB signal-to-noise ratio; multispeaker babble), dichotic words, and dichotic sentences. All patients provided informed written consent for participation in the auditory recording studies.
Case 1
The first case involved a 16-year-old, right-handed girl who had developed right parietal lobe seizures at age 10. A magnetic resonance imaging (MRI) scan showed a small (approximately 1 cm) region of cortical dysplasia in the right postcentral gyrus. The patient was considered a candidate for resection surgery and had electrodes implanted over the right hemisphere for seizure localization and mapping of motor function. She had no history of hearing, speech, cognitive, or motor disorders. Her scores on all four subtests of the SCAN–A test were within normal limits (standard scores ≥ 9).
Auditory recording stimuli and paradigms
Auditory stimuli consisted of a 1200-Hz steady-state tone, two digitized consonant-vowel syllables (/da/ and /ba/; male speaker), an amplitude-modulated tone (1000-Hz carrier and 40-Hz modulation rate), and white noise. The tone and noise stimuli were presented sequentially, in pseudorandom order (no consecutive repetitions) at interstimulus intervals of 1–3 s, for a total of 180 trials, while the patient watched a silent animated video. The speech stimuli were presented using a traditional oddball paradigm: One syllable (/ba/) was designated as the standard and presented in 82% of the trials, while the other syllable (/da/) was the deviant and presented in 18% of the trials for a total of 300 trials. To explore potential effects on the auditory high-gamma response, both passive and active versions of the oddball task were administered. For the passive listening condition, the patient was asked to ignore the auditory stimuli and focus on the video; for the active listening task, she was asked to press a button, using her right hand, when she heard the target deviant stimulus (/da/). The ECoG recordings were preprocessed and then analyzed using a matching pursuit time-frequency analysis, as described above. For the oddball tasks, only responses to the deviant stimulus (/da/) were analyzed. Statistically significant (p < .05) increases in high-gamma power were represented as time-frequency plots.
Results
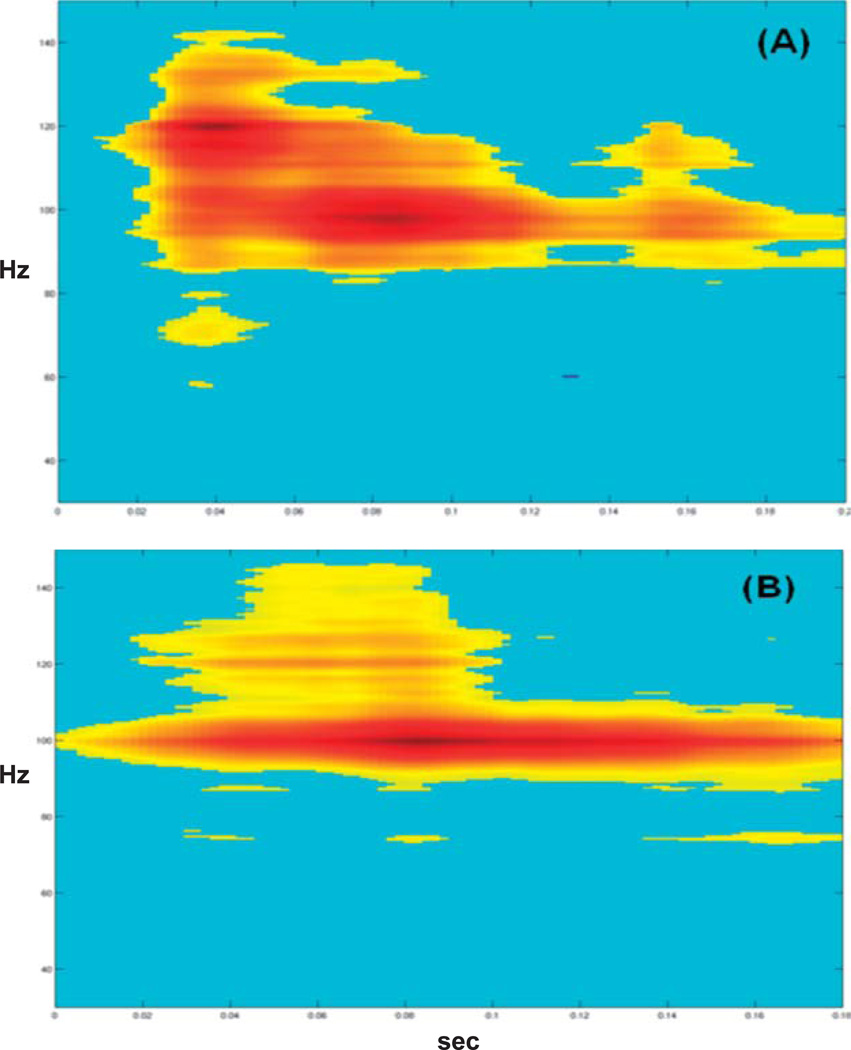
All auditory stimuli elicited high-gamma responses, as shown in the time-frequency plots in Figure 2. Responses were localized to a single electrode on the lateral right posterior superior temporal gyrus in auditory association cortex. High-gamma responses were evident 80–120 ms after stimulus presentation, peaking around 100 ms. For the amplitude-modulated tone, a later sustained steady-state response was also evident at 40 Hz (low gamma). Visual inspection of the time-frequency plots reveals differences in the size of high-gamma responses by stimulus type. Specifically, speech and white noise elicited broader responses than tones. Results for the passive and active speech oddball paradigm are shown for the same electrode in Figure 3. In the active listening condition, the high-gamma response bandwidth is narrower (e.g., sharper) than in the passive condition.
Figure 3.
Auditory high-gamma responses recorded with the syllable /da/ from the same electrode in a patient with normal auditory processing abilities (Case 1) using (A) a passive oddball paradigm and (B) an active oddball paradigm. Time is on the horizontal axis in seconds (0–0.2 s). Frequency is on the vertical axis in hertz (0–150 Hz). Statistically significant changes are color-coded, with red representing the largest increase in power (dB) from the prestimulus baseline, shown in blue.
Conclusions
Three features of auditory high-gamma responses were identified from a patient with normal auditory processing abilities: (a) High-gamma responses were elicited with a variety of auditory stimuli—simple, complex, periodic, and aperiodic; (b) the responses appeared to be modulated by stimulus and task conditions; and (c) the responses peaked around 100 ms after stimulus presentation, corresponding to the same time period as the cortical evoked N1 response (75–120 ms). These findings are consistent with previous reports of top-down attention effects on sensory processing in right auditory cortex (Alho et al., 1999; Bidet-Caulet et al., 2007; Gurtubay, Alegre, Labarga, Malanda, & Artieda, 2004), the time course of auditory high-gamma responses, and stimulus- and task-related effects (Gaona et al., 2011; Steinschneider et al., 2011). The presence of high-gamma responses under passive listening conditions underscores their potential clinical utility as objective measures of cortical processing.
Case 2
The second case involved a 20-year-old, right-handed woman who began having seizures originating in the right hemisphere at age 14. Her neurological examination and MRI scans were normal. There was no history of speech or hearing difficulties. The patient had a history of mild learning and attention problems. She was enrolled in a community college at the time of testing. On the SCAN–A test, she performed ≥2 SDs below the mean on all four subtests (standard scores ≤ 4). Auditory recordings were obtained using the same tones (1000 Hz and 1200 Hz), speech stimuli (/ba/ and /da/), and passive oddball paradigm described for Case 1.
Results
High-gamma responses to tones and speech were analyzed from a single electrode on the right lateral superior temporal gyrus. Both tones and speech elicited large prolonged broadband responses peaking 200 ms or later after stimulus onset (see Figure 4). Visual comparison revealed no clear differences in response morphology or size (bandwidth and magnitude) by stimulus type (tones vs. speech).
Figure 4.
Auditory high-gamma responses recorded from a patient with impaired auditory processing abilities (Case 2). Responses were elicited with (A) tones (1200 Hz) and (B) speech (/da/). Time is on the horizontal axis in seconds (0–0.3 s). Frequency is on the vertical axis in hertz (0–150 Hz). Statistically significant changes are color-coded, with red representing the largest increase in power (dB) from the prestimulus baseline, shown in blue.
Conclusions
High-gamma responses recorded from the right hemisphere of a patient with auditory processing impairments were relatively late and broadband, and showed no stimulus-modulation effects. This contrasts with results from the first patient (Case 1) who also had right hemisphere recordings but demonstrated normal auditory processing abilities. Large, broadband auditory gamma responses have also been observed in patients with neurological and psychiatric disorders who have auditory perceptual difficulties (Uhlhaas et al., 2006; Uhlhaas & Singer, 2010). Results from Case 2 suggest that poor behavioral performance on auditory testing cannot be attributed solely to impaired cognitive function (e.g., attention difficulties) and instead may reflect abnormalities in early stages of cortical sound processing.
No clear evoked N1 response was identified in this patient likely because the electrodes were implanted below the Sylvian fissure, on the lateral superior temporal gyrus, and may not have detected activity from primary auditory cortex. Therefore, it was not possible to determine whether the evoked N1 response was also abnormal in this patient. In the next two cases, the location of the electrodes allowed us to record and compare directly evoked N1 and high-gamma responses.
Cases 3 and 4
For Cases 3 and 4, two patients are presented: one with normal auditory processing abilities (Patient 3) and one with impaired auditory processing (Patient 4). Both had electrodes implanted over the left hemisphere including the temporal lobe, Sylvian fissure, and inferior parietal lobe—allowing us to record both auditory evoked N1 responses, which are generated in primary auditory cortex inside the Sylvian fissure, as well as auditory high-gamma responses. Both patients had three-dimensional volumetric MRI reconstructions with coregistered electrode locations and were relatively matched on a number of demographic variables (e.g., gender, age, and seizure side). The intracranial recording setup was the same for both patients. Auditory stimuli were tones (1000 Hz and 1200 Hz) presented in a passive oddball paradigm, as described in Case 1. High-gamma responses and evoked N1 responses were analyzed for both patients. Patient 3 also had recordings with the speech stimuli (/ba/ and /da/).
Patient 3 was a 38-year-old, left-handed man who had developed left-hemisphere seizures at age 29. He had no history of speech or hearing disorders. His MRI scan and neurological examination were normal. He performed within normal limits on the four subtests of the SCAN–A (standard scores ≥ 8).
Patient 4 was a 40-year-old, right-handed man who had begun having seizures at age 32. There was a family history of seizures (maternal). His MRI scan and neurological examination were normal. On the SCAN–A, he performed 2–3 SDs below the mean on all four subtests (standard scores ≤ 2).
Results
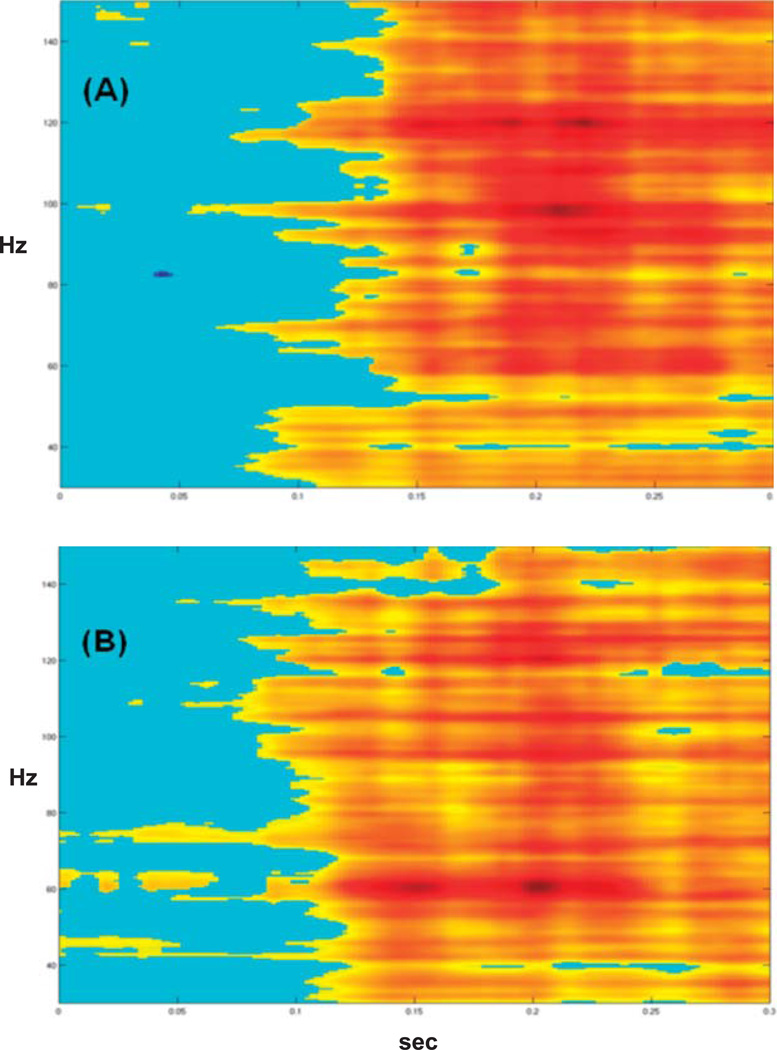
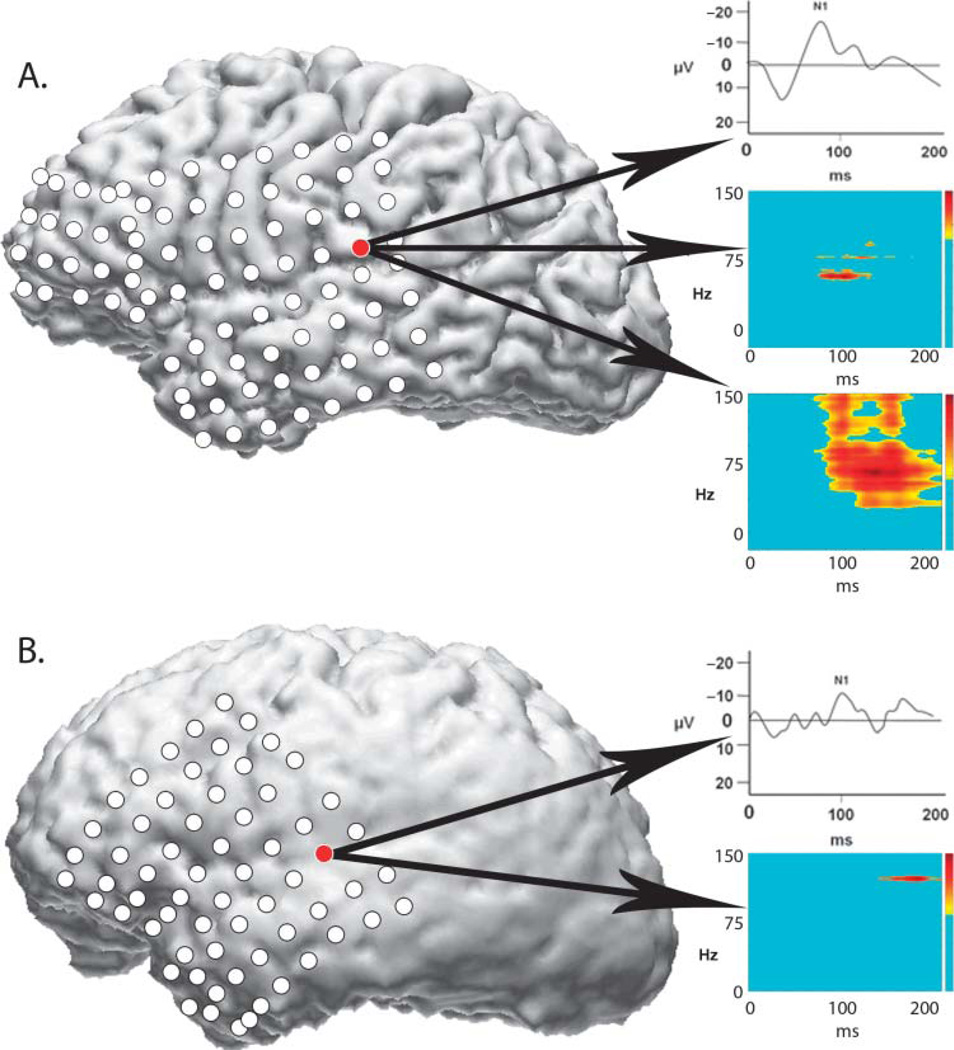
For both patients, evoked N1 responses and high-gamma responses to tones localized to electrode sites over the Sylvian fissure in the posterior half of the left temporal lobe (see Figure 5). For Patient 3 (see Figure 5A), there was partial temporal overlap between the high-gamma response and the evoked N1 response (top plot) at the same electrode site. The high-gamma response was visible at 80 ms (middle plot); the measured N1 peak latency was 81.93 ms. The high-gamma response to speech (bottom plot) at the same electrode location was broader than the response to tones and also overlapped the N1 tone response in time.
Figure 5.
Intracranial recording results from two patients with implanted left hemisphere electrodes. Electrode locations have been coregistered with each patient’s three-dimensional magnetic resonance imaging brain reconstructions; the lateral left view is shown. (A) Results from a patient with normal auditory processing abilities (Case 3). Top inset box shows evoked tone N1 response (waveform has been smoothed for display); middle inset box shows high-gamma response to same tone; bottom inset box shows high-gamma response to speech. (B) Results from a patient with auditory processing difficulties (Case 4). Evoked N1 response is shown in the top inset box (waveform has been smoothed for display); high-gamma response to tones is shown in the bottom inset box. Auditory speech recordings were not performed with the second patient.
For Patient 4 (see Figure 5B), the N1 response (top plot) had a peak latency of 102.17 ms (normal range = 75–120 ms). The high-gamma response (bottom plot) to the same tone stimulus occurred later, beginning around 130 ms and peaking at 180 ms. Recordings with speech stimuli were not performed due to clinical time constraints.
Conclusions
Both patients showed normal N1 responses to tones. For Patient 3, who had normal auditory function, high-gamma response overlapped the N1 response in time, consistent with findings from a recent study (Steinschneider et al., 2011). For Patient 4, who had impaired auditory processing abilities, the high-gamma response was delayed relative to the N1 response. This suggests that the temporal relationship between the high-gamma response and the evoked N1 response may be useful for differentiating normal and impaired auditory processing abilities. Recordings with speech were also performed with Patient 3 and elicited a broader high-gamma response than tones, consistent with results from the first case described.
Summary
High-gamma responses were elicited from both the right and left temporal lobe using a variety of auditory stimuli and task conditions, including passive listening. The two patients with normal auditory processing abilities showed stimulus- and task-modulation effects on the auditory high-gamma responses and temporal overlap with the time window of the evoked N1 response. High-gamma responses to speech and tones in one patient with impaired auditory abilities (Patient 2) showed broad, late responses to both stimuli. The lack of stimulus-modulation effects in the impaired listener is consistent with decreased functional specialization of auditory areas on the lateral temporal lobe for selective processing of complex sounds, including speech (Rauschecker, Tian, & Hauser, 1995), and has been associated previously with auditory processing impairments (Boatman & Miglioretti, 2005). Comparison of high-gamma and N1 responses in the patient with impaired auditory function (Patient 4) showed no temporal overlap (recall that that the location of electrodes in Patient 2 precluded identification of an N1 response). Larger studies are needed to test and verify these single-case observations. More comprehensive behavioral testing is also needed to better characterize the auditory processing abilities of epilepsy patients undergoing intracranial recordings.
Methodological Considerations
There are several limitations to using high-gamma responses in more standard clinical settings. One is that high-gamma responses have been difficult to record using noninvasive scalp electrodes due to the low-pass filtering effects of the skull (Nunez & Srinivasan, 2006). Recent advances in signal processing methods now make it possible to record high-gamma activity reliably from the scalp (Ball et al., 2008; Darvas et al., 2010). Another limitation is the lack of clinical norms and standardized protocols for obtaining and analyzing high-frequency spectral responses. As a result, it has been difficult to interpret individual results or to compare results across individuals or centers (Uhlhaas, Haenschel, Nikolic, & Singer, 2008). New analytic tools are needed to enable clinicians to quantify and compare spectral responses under different stimulus and task conditions.
Despite growing interest in auditory high-gamma responses, their role in auditory processing is not fully understood. It is not known, for example, whether these responses can selectively measure auditory processing abilities, such as sound localization, auditory discrimination, gap detection, and speech recognition in noise, or whether they can be used as reliable measures of treatment efficacy. It will be important to address these issues for high-gamma responses to be considered viable clinical measures of auditory processing (Hood, 1999; Jerger & Musiek, 2000). Similarly, it will be important to understand the subcortical contributions of auditory nerve and brainstem activity to these cortical responses (Hood, 2002; Kraus et al., 2000; Nagle & Musiek, 2009).
Conclusions
Cortical high-gamma responses are emerging as a potential clinical tool for evaluating auditory processing. Differences in high-gamma response patterns to simple and complex stimuli, including speech, may provide useful diagnostic information to complement traditional evoked response testing in the clinical setting.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1-DC05645 and K24-DC010028. Special thanks to Piotr Franaszczuk for assistance with the description of signal processing methods and to Sarah Colwell and Paras Bhatt for assistance with the figures.
References
- Adrian ED, Matthews BH. The Berger rhythm: Potential alterations form the occipital lobes in man. Brain. 1934;57:355–385. [Google Scholar]
- Alho K, Medvedev SV, Pakhomov SV, Roudas MS, Tervaniemi M, Reinikainen K, Naatanen R. Selective tuning of the left and right auditory cortices during spatially directed attention. Cognitive Brain Research. 1999;7:335–341. doi: 10.1016/s0926-6410(98)00036-6. [DOI] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association. (Central) auditory processing disorders [Technical report] 2005 Available from www.asha.org/policy. [Google Scholar]
- American Speech-Language-Hearing Association, Task Force on Central Auditory Processing Consensus Development. Central auditory processing: Current status of research and implications for clinical practice. American Journal of Audiology. 1996;5(2):41–54. [Google Scholar]
- Ball T, Demandt E, Mutschler I, Neitzel E, Mehring C, Vogt K, Schulze-Bonhage A. Movement related activity in the high gamma range of the human EEG. NeuroImage. 2008;41:302–310. doi: 10.1016/j.neuroimage.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Rahn E, Schurmann M. Sensory and cognitive components of brain resonance responses: An analysis of responsiveness in human and cat brain upon visual and auditory stimulation. Acta Oto-Laryngologica. 1991;111(Suppl 491):25–35. doi: 10.3109/00016489109136778. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Struber D, Kruse P, Basar E, Stadler M. Frontal gamma-band enhancement during multistable visual perception. International Journal of Psychophysiology. 1996;24:113–125. doi: 10.1016/s0167-8760(96)00055-4. [DOI] [PubMed] [Google Scholar]
- Bidet-Caulet A, Fischer C, Besle J, Aguera PE, Giard MG, Bertrand O. Effects of selective attention on the electrophysiological representation of concurrent sounds in the human auditory cortex. Journal of Neuroscience. 2007;27:9252–9261. doi: 10.1523/JNEUROSCI.1402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman DF, Miglioretti DL. Cortical sites critical for speech discrimination in normal and impaired listeners. Journal of Neuroscience. 2005;25:5475–5480. doi: 10.1523/JNEUROSCI.0936-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman-Reich D, Franaszczuk PJ, Korzeniewska A, Caffo B, Ritzl EK, Colwell S, Crone NE. Quantifying auditory event-related responses in multichannel human intracranial recordings. Frontiers in Computational Neuroscience. 2010;4(4) doi: 10.3389/fncom.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge JF, Nourski KV, Oya H, Reale RA, Kawasaki H, Steinschneider M, Howard MA. Coding of repetitive transients by auditory cortex on Heschl’s gyrus. Journal of Neurophysiology. 2009;102:2358–2374. doi: 10.1152/jn.91346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka M, Boatman-Reich D, Ward J, Franaszczuk P, Crone N. Language mapping in multilingual patients: Electrocorticography and cortical stimulation during naming. Frontiers in Human Neuroscience. 2011;5(13) doi: 10.3389/fnhum.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Edwards E, Nagarajan SS, Fogelson N, Dalal SS, Canolty RT, Knight R. Cortical spatio-temporal dynamics underlying phonological target detection in humans. Journal of Cognitive Neuroscience. 2011;23:1437–1446. doi: 10.1162/jocn.2010.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clinical Neurophysiology. 2001;112:565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Darvas F, Scherer R, Ojemann JG, Rao RP, Miller KJ, Sorensen LB. High gamma mapping using EEG. NeuroImage. 2010;49:930–938. doi: 10.1016/j.neuroimage.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan N, Csifcsak G, Garab EA, Szendi I, Janka Z. Gamma band response during auditory information processing in schizophrenia. European Neuropsychopharmacology. 2009;19(Suppl 1):S66. [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: A mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biological Cybernetics. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. Journal of Neurophysiology. 2005;94:4269–4280. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- Fishman YI, Arezzo JC, Steinschneider M. Auditory stream segregation in monkey auditory cortex: Effects of frequency separation, presentation rate, and tone duration. The Journal of the Acoustical Society of America. 2004;116:1656–1670. doi: 10.1121/1.1778903. [DOI] [PubMed] [Google Scholar]
- Franaszczuk PJ, Bergey GK, Durka PJ, Eisenberg HM. Time-frequency analysis using the matching pursuit algorithm applied to seizures originating from the mesial temporal lobe. Electroencephalography and Clinical Neurophysiology. 1998;106:513–521. doi: 10.1016/s0013-4694(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual Review of Neuroscience. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in un-medicated schizophrenic patients indicate impaired frontal network processing. Clinical Neurophysiology. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gaona C, Sharma M, Freudenburg Z, Breshears J, Bundy D, Roland J, Leuthardt E. Nonuniform high-gamma (60–500 Hz) power changes dissociate cognitive task and anatomy in human cortex. Journal of Neuroscience. 2011;31:2091–2100. doi: 10.1523/JNEUROSCI.4722-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley PM, Sharma A. Functional brain dynamics of evoked and event-related potentials from the central auditory system. Perspectives on Hearing and Hearing Disorders: Research and Diagnostics. 2010;14:12–20. [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proceedings of the National Academy of Sciences, USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Gao S, Hong B. An auditory brain-computer interface using active mental response. IEEE Transactions in Neural Systems Rehabilitation Engineering. 2010;18:230–235. doi: 10.1109/TNSRE.2010.2047604. [DOI] [PubMed] [Google Scholar]
- Gurtubay I, Alegre M, Labarga A, Malanda A, Artieda J. Gamma band responses to target and non-target auditory stimuli in humans. Neuroscience Letters. 2004;367:6–9. doi: 10.1016/j.neulet.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Gurtubay IG, Alegre M, Labarga A, Malanda A, Iriarte J, Artieda J. Gamma band activity in an auditory oddball paradigm studied with the wavelet transform. Clinical Neurophysiology. 2001;112:1219–1228. doi: 10.1016/s1388-2457(01)00557-0. [DOI] [PubMed] [Google Scholar]
- Han MW, Ahn JH, Kang JK, Lee EM, Lee JH, Bae JH, Chung JW. Central auditory processing impairments in patients with temporal lobe epilepsy. Epilepsy and Behavior. 2011;20:370–374. doi: 10.1016/j.yebeh.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Knight RT. Mechanisms of human attention: Event-related potentials and oscillations. Neuroscience & Biobehavioral Reviews. 2001;25:465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: Memory match and utilization. Trends in Cognitive Science. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hood LJ. A review of objective methods of evaluating auditory neural pathways. Laryngoscope. 1999;109:1745–1748. doi: 10.1097/00005537-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Hood LJ. Auditory neuropathy/auditory dys-synchrony: New insights. Hearing Journal. 2002;55(2):10–14. 17–18. [Google Scholar]
- Howard MA, Volkov IO, Mirsky R, Garell PD, Noh MD, Granner M, Brugge JF. Auditory cortex on the human posterior superior temporal gyrus. The Journal of Comparative Neurology. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Ossandon T, Hamame CM. Task-related gamma-band dynamics from an intracerebral perspective: Review and implications for surface EEG and MEG. Human Brain Mapping. 2009;30:1758–1771. doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger J, Musiek F. Report of the Consensus Conference on the Diagnosis of Auditory Processing Disorders in School-Aged Children. Journal of the American Academy of Audiology. 2000;11:467–474. [PubMed] [Google Scholar]
- Kaiser J, Ripper B, Birbaumer N, Lutzenberger W. Dynamics of gamma-band activity in human magnetoencephalogram during auditory pattern working memory. NeuroImage. 2003;20:816–827. doi: 10.1016/S1053-8119(03)00350-1. [DOI] [PubMed] [Google Scholar]
- Keith R. SCAN-A: A Test for Auditory Processing Disorders in Adolescents and Adults. San Antonio, TX: The Psychological Corporation; 1994. [Google Scholar]
- Korostenskaja M, Pardos M, Fujiwara H, Kujala T, Horn P, Rose D, Lee KH. Neuromagnetic evidence of impaired cortical auditory processing in pediatric intractable epilepsy. Epilepsy Research. 2010;92:63–73. doi: 10.1016/j.eplepsyres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham MA, Cunningham J, King CD, Koch DB, Wright BA. Consequences of neural asynchrony: A case of auditory neuropathy. Journal of the Association for Research in Otolaryngology. 2000;1:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rudrauf D, Kahane P. Intracranial EEG and human brain mapping. Journal of Physiology—Paris. 2003;97:613–628. doi: 10.1016/j.jphysparis.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Mallat S, Zhang Z. Matching pursuit with time-frequency dictionaries. IEEE Transactions on Signal Processing. 1993;41:3397–3415. [Google Scholar]
- Miller KJ, denNijs M, Shenoy P, Miller JW, Rao RP, Ojemann JG. Real-time functional brain mapping using electrocorticography. NeuroImage. 2007;37:504–507. doi: 10.1016/j.neuroimage.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Missonnier P, Herrmann FR, Michon A, Fazio-Costa L, Gold G, Giannakopoulos P. Early disturbances of gamma band dynamics in mild cognitive impairment. Journal of Neural Transmission. 2010;117:489–498. doi: 10.1007/s00702-010-0384-9. [DOI] [PubMed] [Google Scholar]
- Moore DR. Auditory processing disorder (APD): Definition, diagnosis, neural basis, and intervention. Audiological Medicine. 2006;4(1):4–11. [Google Scholar]
- Nagle S, Musiek FE. Morphological changes in the middle latency response using maximum length sequence stimuli. Journal of the American Academy of Audiology. 2009;20:492–502. doi: 10.3766/jaaa.20.8.4. [DOI] [PubMed] [Google Scholar]
- Nunez P, Srinivasan R. Electric fields of the brain: The neurophysics of EEG. 2nd ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Palva S, Palva JM, Shtyrov Y, Kujala T, Ilmoniemi RJ, Kaila K, Naatanen R. Distinct gamma-band evoked responses to speech and non-speech sounds in humans. Journal of Neuroscience. 2002;22:RC211. doi: 10.1523/JNEUROSCI.22-04-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Muller GR, Obermaier B, Krausz G, Schlogl A, Schrank C. Graz-BCI: State of the art and clinical applications. IEEE Transactions in Neural Systems Rehabilitation Engineering. 2003;11:177–180. doi: 10.1109/TNSRE.2003.814454. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995 Apr 7;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE. High-frequency gamma activity (80–150 Hz) is increased in human cortex during selective attention. Clinical Neurophysiology. 2008;119:116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai A, Crone NE, Wied HM, Franaszczuk PJ, Miglioretti D, Boatman-Reich D. Intracranial mapping of auditory perception: Event-related responses and electrocortical stimulation. Clinical Neurophysiology. 2009;120:140–149. doi: 10.1016/j.clinph.2008.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider M, Nourski K, Kawasaki H, Oya H, Brugge J, Howard M. Intracranial study of speech-elicited activity on the human posterolateral superior temporal gyrus. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr014. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, Kohrman MH. ECoG gamma activity during a language task: Differentiating expressive and receptive speech areas. Brain. 2008;131:2013–2027. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner P, Rosburg T, Dietl T, Fell J, Korzyukov OA, Kurthen M, Boutros NN. Sensory gating of auditory evoked and induced gamma band activity in intracranial recordings. NeuroImage. 2006;32:790–798. doi: 10.1016/j.neuroimage.2006.04.203. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophrenia Bulletin. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. Journal of Neuroscience. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews: Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van der Loo E, Gais S, Congedo M, Vanneste S, Plazier M, Menovsky T, De Ridder D. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One. 2009;4(10):e7396. doi: 10.1371/journal.pone.0007396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiology Reviews. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygierewicz J, Durka PJ, Klekowicz H, Franaszczuk PJ, Crone NE. Computationally efficient approaches to calculating significant ERD/ERS changes in the time-frequency plane. Journal of Neuroscience Methods. 2005;145:267–276. doi: 10.1016/j.jneumeth.2005.01.013. [DOI] [PubMed] [Google Scholar]