Abstract
This is a medical position statement developed by the Exocrine Pancreatic Insufficiency collaborative group which is a part of the Italian Association for the Study of the Pancreas (AISP). We covered the main diseases associated with exocrine pancreatic insufficiency (EPI) which are of common interest to internists/gastroenterologists, oncologists and surgeons, fully aware that EPI may also occur together with many other diseases, but less frequently. A preliminary manuscript based on an extended literature search (Medline/PubMed, Cochrane Library and Google Scholar) of published reports was prepared, and key recommendations were proposed. The evidence was discussed at a dedicated meeting in Bologna during the National Meeting of the Association in October 2012. Each of the proposed recommendations and algorithms was discussed and an initial consensus was reached. The final draft of the manuscript was then sent to the AISP Council for approval and/or modification. All concerned parties approved the final version of the manuscript in June 2013.
Keywords: Exocrine pancreatic insufficiency, Chronic pancreatitis, Gastric surgery, Pancreatic surgery, Pancreatic neoplasms, Risk factors, Clinical studies
Core tip: Pancreatic exocrine insufficiency represents a condition related to pancreatic and extrapancreatic disease. We have reviewed the evidence related to the pathophysiological aspects of exocrine pancreatic diseases and we have also reported the recommendations for treating this condition in the most common pancreatic and extrapancreatic diseases. Pancreatin minimicrospheres is a drug which is cost-effective according to a survey of Polish patients, but studies demonstrating its cost-efficacy in Italy are necessary.
INTRODUCTION
This is a medical position statement developed by the Exocrine Pancreatic Insufficiency collaborative group which is a part of the Italian Association for the Study of the Pancreas (AISP). We covered the main diseases associated with exocrine pancreatic insufficiency (EPI) of common interest to internists/gastroenterologists, oncologists and surgeons, fully aware that EPI may occur in many other diseases, but less frequently (Giardia and HIV infections, lymphoma, Whipple’s disease, amyloidosis).
LITERATURE SEARCH METHODS
A preliminary manuscript based on an extended literature search (Medline/PubMed, Cochrane Library and Google Scholar) of published reports was prepared, and key recommendations were proposed. A MESH term “EPI” was used for the search on Medline/PubMed and key words (exocrine pancreatic insufficiency) were used for both Cochrane Library and Google Scholar. A total of 1465 manuscript were retrieved on Medline/PubMed, 64 on Cochrane Library and 1234 on Google Scholar. After deduplication only 282 papers regarding the specific aims of the study were selected and 151 were utilized. The evidence and recommendations were discussed at a dedicated meeting in Bologna during the National Meeting of the Association in October 2012 in which was present 130 participants. Each of the proposed recommendations and algorithms was discussed and an initial consensus was reached. The final draft of the manuscript was then sent to the AISP Council for approval and/or modifications. All concerned parties approved the final version of the manuscript in June 2013.
PHYSIOLOGY OF PANCREATIC DIGESTION OF NUTRIENTS
The pancreatic secretion is a clear fluid liquid, 97% of which is water and electrolytes[1], and 3% proteins. In turn, these are made up of proteins (3%) mainly represented by proteases (80%), amylase (7%), lipase (4%) and nucleases (1%)[2]. The normal absorption of nutrients involves a complex mixture of digestive enzymes and bile salts, and an intact intestinal mucosa to enable the uptake of these hydrophobic complexes. Under normal condition, all major pancreatic enzymes act simultaneously with postprandial chyme decrease during duodenal-ileal transit[3]; the rate of intraluminal degradation differs widely among the major enzymes due to their different stability regarding inactivating mechanisms[4]. Pancreatic amylase is a very stable enzyme, probably because of its high resistance to enzymatic proteolysis[5]; the majority of that released into the duodenum reaches the terminal ileum in an active form[4,6,7] whereas approximately 60% of the protease activities released into the duodenum are delivered to the mid-jejunum, and only between 20% and 30% reach the terminal ileum[4]. As regards lipolytic enzymes, lipase is most susceptible to inactivation during small intestinal transit. In the absence of triglycerides, a large proportion of lipase activity is also lost between the duodenum and the jejunum, and only small quantities are delivered to the terminal ileum[4,5]. After ingestion, dietary lipids are initially emulsified in the stomach and then hydrolyzed by the action of gastric and pancreatic lipase and colipase; hydrolyzed lipids are then aggregated into micelles or liposomes with the addition of bile salts in the duodenum and jejunum, the micelles are absorbed across the intact intestinal villi by both active and passive processes and, finally, packaged into chylomicrons within intestinal epithelial cells and transported to the circulatory system via the lymphatic system[8].
MECHANISMS OF EXOCRINE PANCREATIC INSUFFICIENCY
Exocrine pancreatic insufficiency results from a progressive loss of acinar pancreatic cells which leads to the secretion of an insufficient amount of digestive enzymes into the duodenum. As indicated in Table 1, chronic pancreatitis is the most well-known cause of EPI[9] but also several other conditions, such as partial or total surgical resection of the gland, loss of function of pancreatic tissue or obstruction of the main pancreatic duct as well as diabetes, celiac disease, inflammatory bowel diseases, and gastrectomy should also be considered. Maldigestion results when exocrine (mainly lipase and trypsin) pancreatic function is reduced by more than 90%; other pancreatic and extra-pancreatic causes of maldigestion are reported in Table 2[10].
Table 1.
Causes of pancreatic insufficiency
| Chronic pancreatitis |
| Primary pancreatic insufficiency |
| Agenesis of the pancreas |
| Congenital pancreatic hypoplasia |
| Shwachman–Diamond syndrome |
| Johanson–Blizzard syndrome |
| Adult pancreatic lipomatosis or atrophy |
| Isolated lipase or colipase deficiency |
| Pancreatic resection |
| Pancreatic cancers |
| Secondary pancreatic insufficiency |
| Mucosal small bowel disease: Decreased cholecystokinin release |
| Somastatinoma or exogenous somatostatin analog intake: Decreased pancreatic secretion |
| Gastrinoma: Intraluminal destruction of enzymes |
| Surgery and Billroth II anastomosis: Poor mixing or decreased hormone release, disturbance of innervations |
| Periampullary tumors (pancreatic duct obstruction) |
Modified from reference 155.
Table 2.
Pathogenesis of maldigestion
| Mechanism | Explanation |
| Decreased pancreatic production | Lack of functional tissue or decreased endogenous neurohormonal stimulation |
| Decrease in delivery | Pancreatic duct obstruction |
| Decreased activation | Low duodenal pH |
| Premature enzymatic degradation | Decreased contact time due to increased motility, impaired interaction with chyme and biliary salts, and intestinal bacterial overgrowth |
CLINICAL MANIFESTATION AND ASSESSMENT OF EXOCRINE PANCREATIC INSUFFICIENCY
Patient complaints
Patients with steatorrhea typically report an increase in daily bowel movements, with fatty, bulky stools which are difficult to flush away. This occurs mainly after high fat-containing meals and is sometimes not a daily symptom. As steatorrhea occurs after meals, it typically happens 2 to 3 times a day in individuals with a normal lipid-content diet. Weight loss and anorexia may also develop over time due to malnutrition.
Physical examination
Chronic malabsorption results in weight loss, such as temporal scalloping, interosseous wasting, and lack of subcutaneous fat. Nail leukonychia due to hypoalbuminemia may be present in the late stages of chronic malabsorption. Signs of liposoluble vitamin lack may appear; ecchymoses due to clotting abnormalities in the case of vitamin K deficiency, ataxia and peripheral neuropathy resembling Friedreich ataxia due to vitamin E deficiency, abnormalities of night blindness and xerophthalmia (dry corneas) due to vitamin A deficiency; contraction or muscle spasms, osteomalacia and osteoporosis may also occur due to hypocalcemia. Examination of the stool is an important tool for recognizing steatorrhea.
Investigations
Exocrine pancreatic function is currently diagnosed using two groups of tests, usually referred to as direct and indirect (or tubeless) tests; the principal tests are reported in Table 3. The most sensitive test is a direct test based on aspiration of the pancreatic contents during secretin or secretin-cholecystokinin/cerulein administration[11]; this test is only available in a few centers, it is invasive and is not indicated in clinical practice. Other tests currently available in clinical practice are indirect tests. At present, fecal elastase-1 determination is the most diffuse test for screening pancreatic exocrine insufficiency[12], usually using a monoclonal test[13]. This test does not require the withdrawal of enzyme supplementation therapy and is based on analysis of a single stool sample. Concentrations of elastase-1 less than 200 μg/g in feces are compatible with exocrine pancreatic insufficiency and less than 100 μg/g are indicative of severe pancreatic insufficiency[14]. The 14C-triolein breath test and the cholesteryl-[1-13C] octanoate breath test have been used for assessing fat malabsorption[15]; the D-xylose test (normal serum D-xylose concentration greater than 1.33 mmol/L 1 h after an oral dose of D-xylose) for exploring the malabsorption of carbohydrates, and fecal chymotrypsin excretion (normal > 6 U/g) for evaluating the malabsorption of proteins[12]. Two other tests, not presently available commercially, are the N-benzoyl-L-tyrosyl-p-aminobenzoic acid (PABA) test and the pancreolauryl test which are based on the recovery of an ingested dose of PABA and fluorescein dilaurate from the urine[16]. In clinical trials, objective confirmation of excess fecal fat may be undertaken, and the following methods are usually used[9]: Sudan staining of random homogenized stool, steatocrit and quantitative fat analysis. Sudan staining evaluates the number and size of fat globules per high-power field (hpf), and the test results are scored as normal (≤ 20/hpf, 1 to 4 micrometers in size), moderately increased (> 20/hpf, 1 to 8 μm in size) and definitely increased (> 20/hpf, 6 to 75 μm in size)[17]. Compared to chemical fat analysis, Sudan staining has a sensitivity of 94% and a specificity of 95% for diagnosing abnormal fecal fat excretion[18]. Steatocrit is a quantitative measurement of fat and is expressed as a proportion of an entire centrifuged homogenized stool sample[19]. A spot acid steatocrit level (normal < 10%) has been reported as having a sensitivity of 100% and a specificity of 95% when compared to 72-h quantitative fat analysis[20]. The best reported method is the 72-h fat chemical analysis using the van de Kamer method. The patients need to keep a food diary to ensure that adequate dietary fat (100 g/d) is consumed during the test; the normal output is less than 7 g of fat per 24-h period[21]. Coefficient of fat absorption (CFA) should be used to better quantify the steatorrhea; it is calculated using the following equation: CFA (%) = 100 [(mean fat intake - mean stool fat)/mean fat intake[22]; in healthy subjects, the CFA is usually greater than 80%[23].
Table 3.
Indirect diagnostic tests for evaluating pancreatic exocrine insufficiency
| Test | In favour | Against |
| CFA | Gold standard | 72 h stool collection; 100 g standard diet; no simultaneous PERT; not pancreas specific |
| Acid steatocrit | Linear correlation with CFA also in a single sample; Good as screening | High fat diet needed; 24-72 h stool collection is ideal |
| Fecal elastase 1 | Single stool sample; PERT can be continued | Poor sensitivity in mild EPI, watery stools and small bowel disease |
| 13C-mixed triglyceride breath test | Simple; Also for mild forms of EPI and therapy assessment | Requires further validation |
| Fecal chymotrypsin | Good for compliance control; Single small stool sample | Sensitivity low for clinical practice (chymotrypsin is variably inactivated during intestinal transit); not for mild EPI; watery stools decrease enzyme activity; PERT must be discontinued |
| Secretin-enhanced magnetic resonance cholangiopancreatograpgy | Morphological and semi-quantitative functional changes | Requires further validation |
| Nutritional status (magnesium < 2.05 mg/dL, ↓prealbumin, ↓albumin, ↓retinol binding protein, ↓ferritin, ↓hemoglobin) | Simple | Requires further validation |
CFA: Coefficient of fat absorption; PERT: Pancreatic enzyme supplementation therapy; EPI: Pancreatic exocrine insufficiency.
A new assessment for pancreatic malabsorption which takes into consideration some serum parameters reflecting nutritional status (magnesium < 2.05 mg/dL, reduced serum levels of prealbumin, albumin, retinol binding protein, ferritin, and hemoglobin) has recently been reported[24], but it requires further validation[25].
Finally, bioelectrical impedance has been proposed for assessing nutritional status in patients with pancreatic cancer[26]. This method is based on the different conductive and resistive properties of the various body tissues; it is not invasive, it is inexpensive and it can be performed at the bedside. In brief, fixed low-voltage and high-frequency alternating current introduced into the body is conducted through the fluid compartment of the fat-free mass and it is able to measure both body resistance and capacitance. Capacitance causes the current to lag behind the voltage, creating a phase shift; this shift is quantified geometrically as the angular transformation of the capacitance: resistance ratio, also called phase angle.
Pancreatic enzyme replacement therapy
In order to avoid maldigestion and ameliorate the nutritional status of patients with EPI, the cornerstone of treatment is pancreatic enzyme replacement therapy (PERT). Available formulations contain pancreatic enzymes encapsulated in microgranules or minimicrospheres with a pH sensitive coating in order to either prevent the release and the subsequent inactivation of enzymes by gastric acidity or to release the enzymes into the intestinal lumen where the pH is higher and optimal for the digestion and absorption of food. Currently, the Italian guidelines also suggest minimicrospheres to be the ideal pancreatin formulation[9].
The initial recommended dose of pancreatic extract which should be given is 40000-50000 units of lipase per meal and 25000 U per snack, and this dose should be progressively increased until the steatorrhea is totally or sufficiently reduced[27,28]; this dosage should be maintained over time.
Dietary and drug recommendation
Food intake should be distributed between three main meals per day, and two or three snacks. The pancreatic extracts should be ingested during the meals.
Even if a diet which is low in fat reduces steatorrhea and improves maldigestion, it restricts caloric intake and is not a good option.
Medium-chain triglycerides (MCTs) have not been shown to be effective in patients suffering from chronic pancreatitis with EPI. Moreover, their poor palatability and high cost reduce patient compliance. Evidence exists that MCTs also require enzyme supplements for proper digestion and absorption[29]. They should be used only in patients with persistence of symptoms or weight loss despite adequate enzyme supplementation[30]. Medium-chain triglycerides have been proposed in PERT non-responders as an “ultima ratio”. The quantity of energy administered by MCTs is limited (ca 8.3 kcal/g) and the dose must be increased slowly in order to achieve intestinal adaptation, even when using enteral nutrition[31]. However, trials have shown no advantage between a normal balanced diet and MCT-enriched preparations[29,32,33].
A diet rich in fiber content is contraindicated because the fibrous material will interfere with proteolytic and amylolytic enzyme activity; lipolytic activity is most affected[30,34], whereas enzymes contained in gastroprotected minimicrospheres can be assumed also with food having a pH less than 5.5. Acid-suppressing agents should be utilized only in patients who continue to experience symptoms of maldigestion despite the adequate administration of PERT[35].
Goal of the treatment
Steatorrhea in severe pancreatic insufficiency is very difficult to resolve completely, and only a 60%-70% reduction is usually achieved using PERT[36]. This may be due the fact that there are numerous interactions between pancreatic maldigestion, intestinal ecology and intestinal inflammation; consequently, to the methods of achieving optimal management of pancreatic maldigestion need to be fully re-evaluated, considering not only the correction of pancreatic insufficiency using PERT and, the best duodenal pH to allow for the optimal efficacy of these extracts, but also the decontamination of the intestinal lumen, the supplementation of bile acids and, probably, the use of probiotics to attenuate intestinal inflammation in chronic pancreatitis patients[37]. Fat soluble vitamins and micronutrients, such as zinc and selenium, should be routinely assessed and administered whenever necessary[38].
Warnings regarding PERT
An appropriate clinical response to PERT does not allow predicting a normal nutritional status in patients with chronic pancreatitis. Up to 2/3 of patients with an apparently good clinical response have some residual nutritional deficiency[39]. Crushing, chewing or holding the pancreatic extract capsules in the mouth may cause local irritation. The fine powder of the pancreatic enzymes may also be irritating to the nasal mucosa and the respiratory tract and can precipitate an asthma attack. Extremely high doses of pancreatic extracts have been associated with hyperuricemia and hyperuricosuria[40]. Submucosal strictures in the proximal colons of children with cystic fibrosis have been reported (“fibrosing colonopathy”), and it is now recommended that not more than 10000 units of lipase per kg of body weight per day be given to children[41]; to our knowledge, this complication has been never reported in adults[42].
RECOMMENDATION FOR SPECIFIC DISEASES
Acute pancreatitis
In Italy, there are approximately 20000 admissions per year for acute pancreatitis (AP)[43]. Acute pancreatitis is an inflammatory disease most commonly caused by gallstones or alcohol abuse, and is associated with significant morbidity and mortality[44]. Pathological values of fecal elastase-1 have been found in 12.0% of patients with AP (9.3% with mild and 2.7% with severe pancreatitis). Pathological fecal elastase-1 was not significantly related to sex, age or day of refeeding. Finally, only 4.0% of patients may have severe EPI (i.e., fecal elastase-1 concentrations less than 100 μg/g). Thus, in selected cases (approximately 800 Italian AP patients per year), there is the need for enzyme supplementation during refeeding if the elastase-1 fecal determination is clearly abnormal[45]. The suggestion is that these patients be monitored for EPI for at least 6-18 mo and treated with oral pancreatic enzymes at a dosage of 40000-50000 U per meal and 25000 U per snack unless otherwise indicated[27] (Figure 1).
Figure 1.
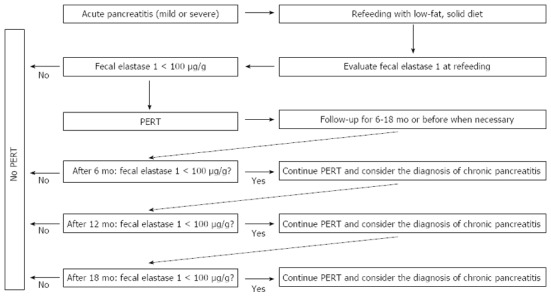
Algorithm for monitoring and treating exocrine pancreatic insufficiency in patients hospitalized for acute pancreatitis. PERT: Pancreatic enzyme replacement therapy.
Chronic pancreatitis
The greatest benefit of PERT is in the patients who excrete more than 15 g of fecal fat per day or have weight loss[46,47]. However, German and Spanish guidelines treat patients with a daily fecal fat output < 15 g in the presence of symptoms of malabsorption (weight loss, osteopenia, loss of muscular mass)[9,31,48,49]. Alcohol should also be avoided to prevent additional impairment of the pancreatic exocrine function[50].
The initial dose of pancreatic enzymes should be 40000 units as a starting dose for a meal and 20000 units for a snack[9,31,48,51].
Increasing doses of PERT are recommended in non-responder patients[9,48,51]. Furthermore, acid suppression is also suggested to ensure optimal enzymatic delivery into the duodenum, despite the lack of clinical trials[52]. Moreover, as reported by Domínguez-Muñoz et al[53], gastric acid inhibition avoids bile acid precipitation and allows lipase release in the proximal gut. It has been shown that patients with EPI respond properly to PERT if bicarbonate secretion is preserved and/or gastric secretion reduced. Calcium and magnesium-containing antacids should be avoided as they produce soaps, precipitate with glycine conjugated bile salts in the intestine and worsen steatorrhea[54].
Lack of patient compliance may be a cause of treatment failure and can be discovered by measuring fecal chymotrypsin[55]. If chymotrypsin activity in the stool is low, the patient should be educated to take supplements during or just after meals[9,56]. Intestinal bacterial overgrowth, found in up to 40% of the patients with chronic pancreatitis[57,58], intestinal giardiasis or other intestinal malabsorption disorders, should be ruled out in non-responder patients.
Parameters to be used for the assessment of therapy include clinical improvement/normalization of nutritional parameters and clinical symptoms[9,24]. In non-responder patients, laboratory methods for assessing fat absorption (CFA, C-13 mixed triglyceride breath test) may be used. Fat soluble vitamin deficiency should be corrected parenterally[9].
Before starting PERT, evaluation of the fasting glucose levels and quantification of the malabsorption is suggested, if possible. Moreover, determining glucose fasting levels during the first 1-2 wk of treatment is also suggested[51]. An algorithm for PERT in chronic pancreatitis patients is summarized in Figure 2.
Figure 2.
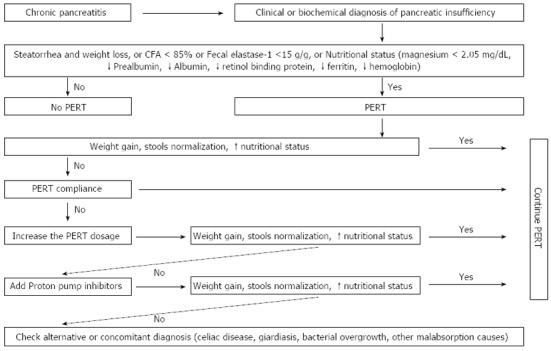
Algorithm for monitoring and treating exocrine pancreatic insufficiency. Algorithm for monitoring and treating exocrine pancreatic insufficiency summarized from Italian[9], German[47] and Spanish[31] guidelines, and a synopsis of the guidelines[51]. CFA: Coefficient of fat absorption; FE1: Fecal elastase-1; PERT: Pancreatic enzymes replacement therapy; PPI: Proton pump inhibitor.
Unresectable pancreatic ductal adenocarcinoma
The prevalence of EPI is high but of moderate degree in the majority of cases; it has been reported that 65% of pancreatic cancer patients have fat malabsorption, and 50% protein malabsorption[59,60]. The causes of the EPI are mainly related to the obstruction of and/or the loss of the pancreatic parenchyma[61]. Thus, the most important predictors of the onset of EPI malabsorption in pancreatic cancer patients are the site of the tumor in the pancreatic head, the tumor replacing at least 90% of the normal pancreatic tissue and main duct obstruction[62-64].
Even if the most widely accepted prognostic factors in unresectable pancreatic carcinoma are the presence of metastases and the value of CA 19-9 at presentation[65,66], the prognostic factor “weight loss” has received particular attention from the “Eastern Cooperative Oncology Group” study[66] and in this study the weight loss ranged from 30% in patients with non-Hodgkin’s lymphoma to 87% in patients with gastric cancer; patients with pancreatic cancer showed weight loss in 65% of cases and it correlates with worsening of the performance status even if this factor was not a negative prognostic factor for survival[66]. In contrast, a more recent retrospective study found a direct relationship between the percentage of weight loss and the risk of death, with a value greater than 7 times the expected value when the decrease exceeded 10%[67] and these data were confirmed by a retrospective study regarding 58 patients with unresectable pancreatic carcinomas showing that a phase angle of less than 5 degrees was a negative prognostic factor[26] and by a prospective non-randomized study enrolling 194 patients with unresectable advanced pancreatic cancer showing that a value of fecal elastase-1 less than 20 μg/g was a negative prognostic factor for survival. Of interest, a value of fecal elastase-1 of less than 20 μg/g and extremely severe pancreatic insufficiency were found more frequently in the group of patients with tumors in the head of the pancreas[68].
The main question is whether replacement therapy with pancreatic enzymes and nutritional therapy have a positive impact on the quality of life and survival in patients with advanced pancreatic cancer. Pancreatic enzyme replacement therapy can partially prevent weight loss in patients with unresectable tumors of the pancreatic head, at least in the period before biliary endoprosthesis placement[69]. Two different phase II studies have shown that, in patients with advanced pancreatic cancer, having a weight loss of more than 5% in the previous 4 wk and a body mass index of less than 19, parenteral nutrition improved all nutritional parameters, as evaluated by the bioelectrical impedance without, however, reaching normality[70,71].
The algorithm for monitoring EPI and malnutrition in unresectable pancreatic ductal adenocarcinoma patients is reported in Figure 3. Of course, appropriate amounts of pancreatic extracts should be administered during each meal (40000-50000 U of lipase) and per snack (25000 U).
Figure 3.
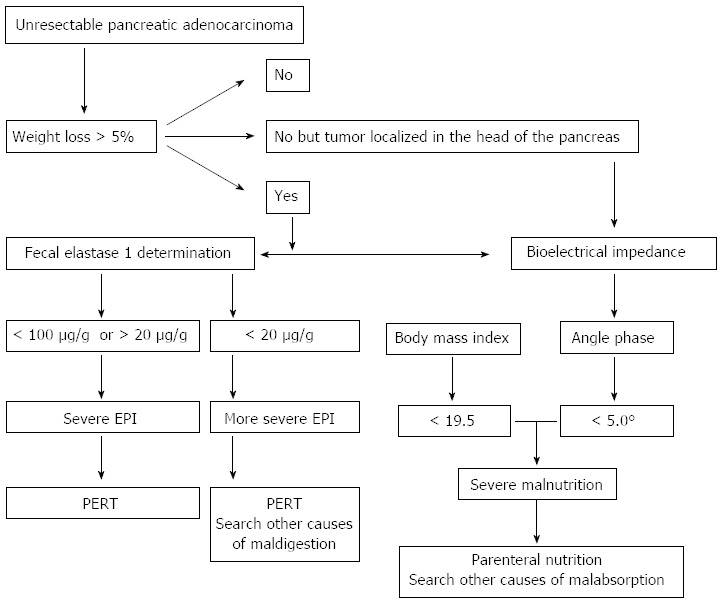
Algorithm for monitoring and treating exocrine pancreatic insufficiency and malnutrition in unresectable pancreatic ductal adenocarcinoma patients. EPI: Exocrine pancreatic insufficiency; PERT: Pancreatic enzymes replacement therapy.
Diabetes mellitus
Exocrine pancreatic insufficiency was demonstrated in approximately 50% of patients with insulin-dependent diabetes, and in 30%-50% of those with non insulin-dependent diabetes[72-78]. Nine prospective reports evaluated EPI by means of fecal elastase-1 estimation in patients with either type-1 or type-2 diabetes[79-87] (Table 4) and included 2770 diabetic patients, 837 of them (30%) with type-1, and the remaining 1933 with type-2 diabetes mellitus (DM). Overall, fecal elastase-1 concentrations were abnormal (i.e., < 200 μg/g) in 904 patients (32.6%) and the impairment was mild (i.e., fecal elastase-1 > 100 but < 200 μg/g) in 439 patients (15.8%, overall); severe EPI (< 100 μg/g) was documented in 465 (16.8%). Of the 904 diabetics with abnormal fecal elastase-1, exocrine impairment was mild in 48.9%, and severe in 51.4%. The prevalence of EPI differed slightly between type 1 and type 2 DM. Abnormal (< 200 μ/g) fecal elastase-1 concentrations were found in 346 (41.3%) of 837 type 1 diabetic patients, and in 558 (28.9%) of 1933 type 2 diabetic patients, a 12.4% difference in prevalence rates. More patients with type 1 DM (188 of 837, 22.5%) had signs of severe EPI, as compared to the 14.3% rate (277 of 1933) in type 2 DM. Exocrine pancreatic insufficiency is usually only of a mild to a moderate degree, and will not lead to clinically overt steatorrhea in the majority of diabetics. Thus, the clinical relevance of EPI and the role of functional tests in these patients are questionable. However, patients with DM frequently suffer from a wide range of abdominal complaints which contribute to impairment of the quality of life[88]. Although data are controversial, at least some of these symptoms may be attributable in part to EPI (mild to moderate) and might respond to enzyme treatment[81,89-94]. Thus, pancreatic tests should be part of the diagnostic work-up in patients with symptoms and do not respond to simple therapeutic measures. As reported in Figure 4, patients with fecal elastase-1 < 100 μg/g should be given pancreatic enzymes in adequate daily doses (40000-50000 U of lipase) administered during meals. Treatment improves symptoms significantly, the supply of soluble fat vitamins is normalized, and the risk of osteoporosis is reduced. Enzyme replacement therapy might have an impact on glucose metabolism since it can reduce the insulin requirement and contribute to improved control of the glucose metabolism, but the evidence is contradictory[93,94] as improvement of glucose metabolism was not seen in all studies[95,96].
Table 4.
Fecal elastase 1 concentrations in type 1 and type 2 diabetes mellitus n (%)
| Ref. |
Type 1 DM |
Type 2 DM |
||||
| Overall | FE-1 (100-200 μg/g) | FE-1 (< 100 μg/g) | Overall | FE-1 (100-200 μg/g) | FE-1 (< 100 μg/g) | |
| Hardt et al[80] | 322 | 73 (23) | 92 (28) | 697 | 108 (15) | 138 (20) |
| Vesterhus et al[81] | 140 | 10 (7) | 16 (11) | 63 | 2 (3) | 6 (9) |
| Larger et al[82] | 195 | 28 (14) | 38 (19) | 472 | 35 (7) | 50 (10) |
| Icks et al[83] | 112 | 22 (20) | 29 (26) | |||
| Cavalot et al[84] | 37 | 17 (46) | 4 (11) | |||
| Rathmann et al[85] | 544 | 100 (18) | 65 (12) | |||
| Nunes et al[86] | 42 | 6 (14) | 9 (21) | |||
| Yilmaztepe et al[87] | 32 | 9 (28) | 1 (3) | |||
| Overall | 837 | 158 (18.9) | 188 (22.5) | 1933 | 275 (14.2) | 283 (14.6) |
DM: Diabetes mellitus; FE-1: Fecal elastase-1.
Figure 4.
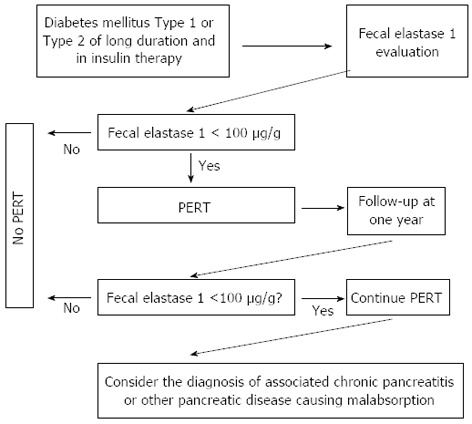
Algorithm for monitoring and treating exocrine pancreatic insufficiency in patients with diabetes mellitus. PERT: Pancreatic enzymes replacement therapy.
Celiac disease
The prevalence of adult celiac disease (CD) in the general population is reported to be 1%-2%[96-99]; diarrhea remains a common presenting symptom[100] and it is usually attributed to continued gluten ingestion; however, other causes of chronic diarrhea in patients who are compliant with their gluten-free diet exist, and one of them is exocrine pancreatic insufficiency. Using a secretin test, it has been found a mild reduction in the pancreatic secretion of bicarbonates and pancreatic enzymes (especially lipase) in untreated celiac patients these alterations revert to normal after going on a gluten-free diet; mild pancreatic insufficiency is present in about 40% of untreated CD patients and severe pancreatic insufficiency in 10%[101,102]. More recently, other authors using tubeless test, such as fecal chymotrypsin or elastase 1 determination and the C mixed-triglyceride breath test, confirmed that pancreatic insufficiency in untreated CD patients in percentages ranging from 11.4% to 56.2%[103-107].
It has also been suggested exocrine pancreatic function impairment may be related to the degree of mucosal villous atrophy and that the level of fecal elastase may improve once the mucosa has recovered after an appropriate gluten-free diet[104,108]. In addition, it seems that pancreatic insufficiency does not depend on nutritional status[105]. Regarding the use of PERT in these patients, the data come from a double blind randomized study carried out on children showing that pancreatic enzyme therapy is certainly useful in the first 30 d after the diagnosis of CD[106]. In fact, after 30 d of a gluten-free diet associated with pancreatic extracts, body weight significantly increases with respect to patients treated with only a gluten-free diet. Similar results were obtained in a longitudinal study[109]. The conclusion is that pancreatic enzyme therapy is certainly useful in the first 30 d after the diagnosis of CD and that enzyme supplementation may possibly be discontinued as symptoms improve and fecal elastase-1 concentrations normalize. The dosage of pancreatic extracts should be 40000-50000 U per meal and 25000 U per snack. In CD patients who continue to experience clinical steatorrhea despite being on a gluten-free diet, a search for possible exocrine pancreatic insufficiency must be carried out[110]. In addition, we should bear in mind that, in adult CD patients have a risk developing chronic pancreatitis more than 3 times as compared to general population and there is also and increased need for PERT[111]. An algorithm for PERT in CD is reported in Figure 5.
Figure 5.
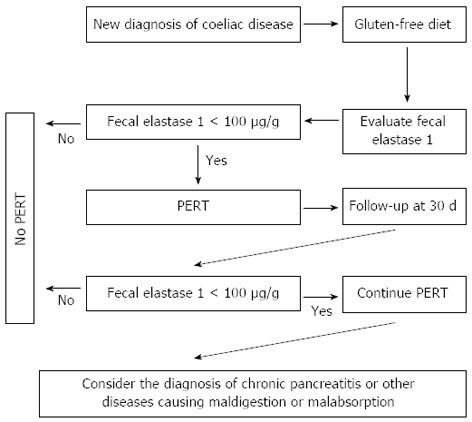
Algorithm for monitoring and treating exocrine pancreatic insufficiency in patients with celiac disease. PERT: Pancreatic enzymes replacement therapy.
Inflammatory bowel diseases
Crohn’s disease: About 35% of the patients with Crohn’s disease have an impaired exocrine pancreatic function[112] and no relationship are present between exocrine pancreatic insufficiency and age or nutritional status. Interestingly, patients having steatorrhea had a defect of lipase output ranging from 10% to 67% and, especially in this latter group of patients, the use of PERT was hypothesized. In patients with Crohn’s disease, enzyme activities were not correlated to the duration of disease or to the extent or localization of a previous bowel resection[113]. The lowest enzyme values were found in patients with the most extensive bowel involvement, and they were significantly lower than in patients with disease confined to the terminal ileum. Thus, the factors related to the impaired pancreatic function in Crohn’s disease seem to be disease activity, and the localization and extent of the disease. Finally, patients with Crohn’s disease may have an autoimmune involvement of the pancreatic gland and those having positive serum pancreatic autoantibodies may also have impaired exocrine pancreatic function more frequently[114]. However, we have no evidence that PERT can be utilized in patients with Crohn’s disease and exocrine pancreatic insufficiency to improve the maldigestion present in these patients.
Ulcerative colitis: Pancreatic exocrine insufficiency, assessed using a secretin-cerulein test, may be present in about 40%-50% of patients with ulcerative colitis[112,115,116] especially during a active phase of the disease and the majority of patients with pancreatic insufficiency had active disease with loose stools; thus, the reduced fecal elastase-1 concentration could have been due to dilution of the enzyme and not to pancreatic involvement. In addition, in those patients who were also studied during the remission phase of the disease and had a solid stool, the fecal elastase-1 concentration became normal[112]. More recently, the possibility of autoimmune pancreatitis associated with ulcerative colitis has been reported. Thus, it is possible that only a small number of ulcerative colitis patients having severe pancreatitis insufficiency due to autoimmune pancreatitis may benefit from PERT.
Gastric surgery
Exocrine pancreatic insufficiency is a common clinical problem after gastric surgery[117]. The side effects of gastric resections, in particular total gastrectomy, include diarrhea, anorexia, weight loss and EPI that are responsible for a global status of malnutrition, malabsorption and maldigestion[118]. Malnutrition is considered one of the major complications after gastric surgery for gastric cancer[119] and EPI contributes to the pathogenesis of global malnutrition. After gastric surgery, EPI can result from various causes, such as a deficient trituration of nutrients, altered gastric emptying, alteration of pancreatic denervation and post-cibal asynchrony[120,121].
Any surgical procedure, such as total or subtotal gastrectomy, total or subtotal pancreatectomy, with or without duodenal resection (e.g., in the context of a Whipple procedure), causing distortion in the anatomo-physiology of digestion can be responsible for EPI[122-127]. Several events can be considered as being responsible for EPI after gastrectomy. Alterations of gastric relaxation due to the absence of nervous gastric reflexes; the absence of nervous gastric stimulation responsible for pancreatic secretion caused by the lack of fundus relaxation and the reduction of exocrine pancreatic secretion due to the absence of cholecystokinin after intestinal resection. Rapid gastric emptying and asynchrony between gastric emptying and biliopancreatic secretion due to new tracts of various reconstructions, bacterial overgrowth after gastrectomy, extensive denervation of the pancreas due to lymph node dissection and truncal vagotomy are the most frequent alterations involved in the pathogenesis of EPI[123,128,129]. The latter has been shown to cause mild to moderate EPI by itself[130,131]. In 1996, Friess et al[123] demonstrated that 100% of patients develop severe primary EPI three mo after a total gastrectomy. Chymotrypsin and trypsin were the most severely deficient enzymes after gastric surgery, with a decreased production of up to 91% three mo after surgery. Low levels of gastrin and postprandial pancreatic polypeptides, and high levels of cholecystokinin were also reported[123]. Exocrine pancreatic insufficiency is reported in both total and partial gastrectomy; Büchler et al[127] demonstrated that the pancreolauryl test was pathological in 47%-64% of patients after Billroth-I surgery and in 64%-70% after Billroth-II surgery. On the contrary, Heptner et al[124] reported EPI after gastric resection in only 30% of patients, even if the pancreolauryl test was abnormal in 90% of these patients. Armbrecht et al[132] conducted a double-blind, crossover study of 15 patients who underwent surgery for gastric cancer (total gastrectomy) and compared PERT with a placebo. The authors concluded that PERT reduced massive steatorrhea and improved stool consistency after total gastrectomy. Nevertheless, Bragelmann and coworkers reported an overall improvement in abdominal symptoms, fecal frequency and fecal consistency when following 52 institutionalized patients with a fecal fat output greater than or equal to 14 g/d after gastric resection for cancer, but no differences were found regarding body mass index, bowel habits or fat malabsorption[133]. Interestingly, Huddy and coworkers found that EPI contributes to postoperative morbidity after an esophagectomy, and that these patients can benefit from PERT[134].
The main goal of the therapy in patients suffering from EPI is to reverse all the secondary events caused by enzyme deficiency (Figure 6). Therapeutic efficacy is closely connected with two important aspects: time and dosage of the pancreatic enzymes administered, and dietary changes[135]. Nutritional changes should include a high carbohydrate diet, with normal fat and medium-chain triglycerides[136]. It is also recommended that a personalized diet be created after major gastrointestinal surgery in order to prevent weight loss, anorexia, inflammation and changes in homeostasis[53]. Following a total gastrectomy and in patients receiving therapy with proton pump inhibitors (PPIs) for some reason, unprotected pancreatin powder is preferred[120]. In addition to dietary changes it is mandatory to resort to PERT, orally ingested, during meals. The dose must be adapted to the meal and should not be less than 40000-50000 U of lipase per meal[135]. For partial gastric resection, patients receiving uncoated pancreatic enzyme formulations should also require simultaneously administered proton pump inhibitors[53] since lipase is irreversibly deactivated by gastric acid. However, the administration of PPIs can improve fat digestion even in patients who do not benefit from PERT[53]. Several authors agree with the need for liposoluble vitamin supplementation, especially for patients with severe EPI[135].
Figure 6.
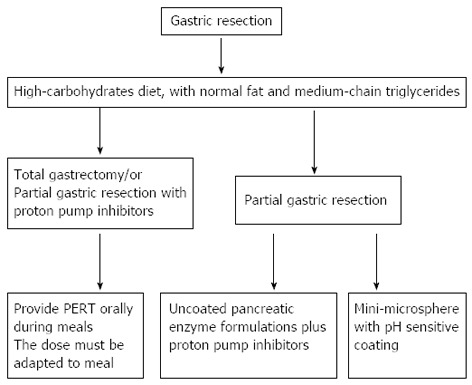
Algorithm for treating exocrine pancreatic insufficiency in patients who undergo gastric resection. PERT: Pancreatic enzymes replacement therapy.
Pancreatic resections
Suggestions on this topic are based on a consensus reached by the experts and are not fully based on data coming from literature. Partial or total pancreatectomy (TP) is frequently associated with EPI. In this setting, PERT is essential for maintaining adequate digestion. In a TP, the removal of the entire pancreatic parenchyma produces inevitable exocrine failure while, in a partial pancreatectomy, the severity of EPI depends on both the underlying disease, the preoperative pancreatic function, and the extent and type of the resection. Most importantly, any pancreatic neoplasm can be associated with chronic obstructive pancreatitis (focal or extended) which might affect pancreatic function/secretion, leading to EPI before any type of resection.
Extent and type of the resection
Pancreaticoduodenectomy: Anatomical changes secondary to reconstruction after a pancreaticoduodenectomy (PD) lead to important physiological alterations which frequently correlate with the severity of postoperative EPI. A PD (either Whipple or pylorus-preserving) is associated with several and complex patho-physiological events such as: (1) disturbance of gastric fundus relaxation caused by the disappearance of antro-fundic and duodeno-fundic reflexes; (2) the absence of neurally stimulated pancreatic excretion caused by the lack of fundus relaxation; (3) the reduction of cholecystokinin-mediated stimulation of pancreatic secretion secondary to duodenal resection; (4) large and hard to digest nutrient particles reaching the jejunal lumen due to resection of the distal stomach (Whipple procedure); (5) reduction in exocrine pancreatic secretion due to pancreatic head resection; and (6) asynchrony between the gastric emptying of nutrients and bilio-pancreatic secretion as a result of anatomical reconstruction[122,128,137,138].
For these reasons, every patient who is candidate for a PD should be considered at increased risk for EPI regardless of the underlying disease 128138. Therefore, it has been suggested that, after PD, PERT be given to all patients with pancreatic cancer, especially those with impending adjuvant therapy. Furthermore, it should be considered that pancreatic cancer is often associated with obstructive chronic pancreatitis, a preoperative risk factor for the development of EPI by itself[128,139]. The development of pancreatic insufficiency after PD could also be related to the different techniques used for the pancreatic anastomosis[117,140,141].
Distal pancreatectomy: Distal pancreatectomy (DP) is the procedure of choice for treating lesions affecting the body-tail of the gland. A DP may affect pancreatic exocrine function depending on the amount of normal tissue removed[142,143]. Based on these data, permanent postoperative EPI, as a result of parenchymal loss from pancreatic resection, was not observed and these conclusions have been subsequently confirmed[139].
Atypical resections: Middle pancreatectomy and enucleation: Atypical resections are usually performed for benign or borderline pancreatic tumors, such as small (< 2 cm) pancreatic neuroendocrine tumors, cystic papillary tumors, low grade intraductal papillary mucinous neoplasms and serous cystic adenomas. Enucleation is usually performed for tumors smaller than 2 cm located in any part of the pancreas but sufficiently far from the main pancreatic duct. A MP is indicated for neoplasm in the neck of the pancreas which could not be safely enucleated[139,144-146]. Using the 13C-mixed triglyceride breath test (normal test > 5%), it has been found an EPI rate of 5% after a median follow up of 71 mo[147]. In addition, Crippa et al[148], after a median follow-up of 54 mo, observed a rate of clinical EPI of 5% in a cohort of 100 patients who had undergone MP for benign or borderline tumors. The authors compared this result with an EPI rate of 15.6% in patients who underwent an extended left pancreatectomy (at the right side of the superior mesenteric vein), the alternative surgical procedure to MP for lesions located in the pancreatic neck. These data are consistent with those of others[149,150].
Treatment of EPI in pancreatic resection
It has been reported that in patients undergoing a pylorus-preserving pancreaticoduodenectomy for pancreatic neoplasia, gastro-protected microspheres were less effective than those in patients who had undergone a classic Whipple technique[151] probably because microspheres are retained in the stomach. One of the few randomized studies explaining the efficacy of pancreatic extracts for the control of malabsorption was carried out on a small group of patients with chronic pancreatitis who had undergone a pancreatic resection with longitudinal pancreaticojejunostomy[152] and showed that treatment with pancreatic extracts ameliorated not only the nitrogen balance but also the fat and protein absorption. Another randomized controlled double-blinded crossover study explored the comparative efficacy of two pancreatin preparations of gastroprotected microspheres with different doses in pancreatectomized patients having chronic pancreatitis[153]. All patients were stabilized before enrollment in the study with a standard dose of pancreatic extracts. After this stabilization period, 56% of the patients still had a fecal fat excretion greater than 7 g/d, and 38% greater than 15 g/d. The results demonstrated that there was a significant relationship between fecal fat excretion, fecal volume and evacuation frequency but there was no relationship between fecal fat excretion, and abdominal pain or malabsorption. Both the pancreatin standard dose and the elevated dose demonstrated equal efficacy; in pancreatectomized patients, high dose pancreatic extracts significantly reduced the number of capsules needed per day with a better compliance to substitutive therapy. From the clinical point of view, pancreatic enzyme replacement therapy needs to be routinely considered and based on pragmatic clinical evaluation of the patient[22,38,63]. The suggested algorithms for PERT in patients undergoing surgery, according to the type of pancreatic resection, are reported in Figures 7-9, taking into consideration that the dosage should be no less than 40000-50000 U of lipase per meal and 25000 per snack.
Figure 7.
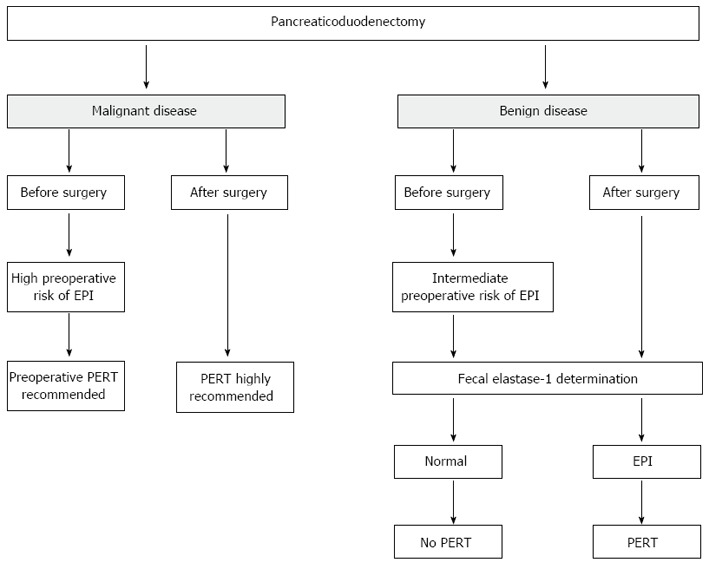
Algorithm for monitoring and treating exocrine pancreatic insufficiency in patients who receive pancreaticoduodenectomy. EPI: Exocrine pancreatic insufficiency; PERT: Pancreatic enzyme replacement therapy.
Figure 9.
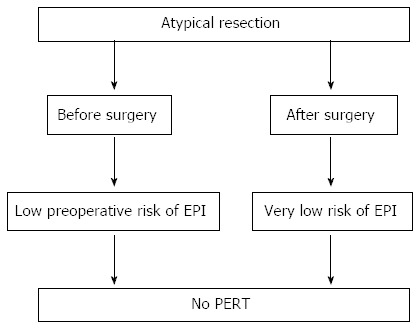
Algorithm for monitoring and treating exocrine pancreatic insufficiency in patients who undergo atypical resection of the pancreas. EPI: Exocrine pancreatic insufficiency; PERT: Pancreatic enzymes replacement therapy.
Figure 8.
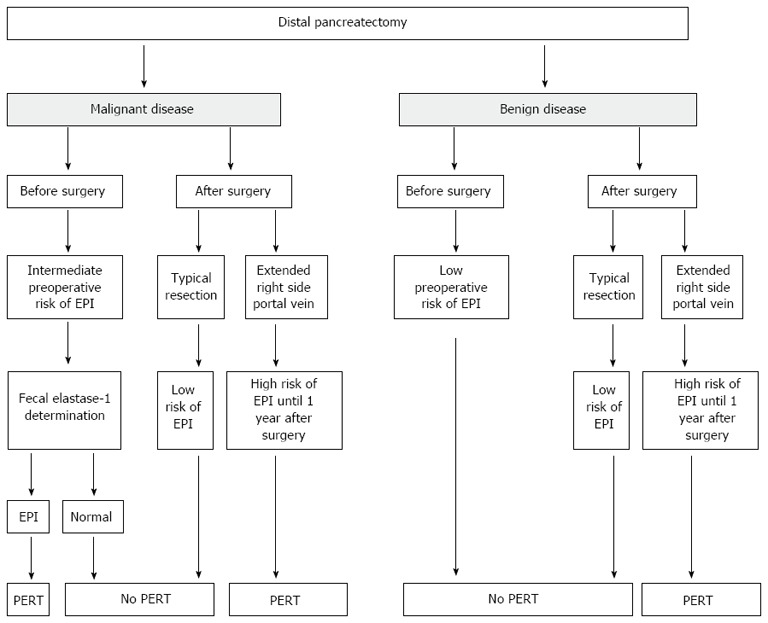
Algorithm for monitoring and treating exocrine pancreatic insufficiency in patients who undergo distal pancreatectomy. EPI: Exocrine pancreatic insufficiency; PERT: Pancreatic enzymes replacement therapy.
CONCLUSION
We should point out that there is a paucity of information regarding some areas of managing EPI there is a lack of good quality of literature. Finally, studies on the economic aspects of this treatment with the different formulations commercially available are also necessary; it has been calculated that the treatment of chronic pancreatitis-related EPI with pancreatin minimicrospheres is cost-effective according to a survey of Polish patients but it is necessary that these results also be evaluated for the Italian National Health System[154].
Footnotes
Supported by An unrestricted grant from Abbott Italia s.r.l.
P- Reviewers: Chiaro MD, Maluf F, Regimbeau JM, Sperti C S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Gullo L, Pezzilli R, Priori P, Baldoni F, Paparo F, Mattioli G. Pure pancreatic juice collection over 24 consecutive hours. Pancreas. 1987;2:620–623. doi: 10.1097/00006676-198709000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Scheele G, Bartelt D, Bieger W. Characterization of human exocrine pancreatic proteins by two-dimensional isoelectric focusing/sodium dodecyl sulfate gel electrophoresis. Gastroenterology. 1981;80:461–473. [PubMed] [Google Scholar]
- 3.Borgstrom B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957;36:1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Layer P, Go VL, DiMagno EP. Fate of pancreatic enzymes during small intestinal aboral transit in humans. Am J Physiol. 1986;251:G475–G480. doi: 10.1152/ajpgi.1986.251.4.G475. [DOI] [PubMed] [Google Scholar]
- 5.Granger M, Abadie B, Marchis-Mouren G. Limited action of trypsin on porcine pancreatic amylase: characterization of the fragments. FEBS Lett. 1975;56:189–193. doi: 10.1016/0014-5793(75)81088-x. [DOI] [PubMed] [Google Scholar]
- 6.Layer P, Jansen JB, Cherian L, Lamers CB, Goebell H. Feedback regulation of human pancreatic secretion. Effects of protease inhibition on duodenal delivery and small intestinal transit of pancreatic enzymes. Gastroenterology. 1990;98:1311–1319. [PubMed] [Google Scholar]
- 7.Holtmann G, Kelly DG, Sternby B, DiMagno EP. Survival of human pancreatic enzymes during small bowel transit: effect of nutrients, bile acids, and enzymes. Am J Physiol. 1997;273:G553–G558. doi: 10.1152/ajpgi.1997.273.2.G553. [DOI] [PubMed] [Google Scholar]
- 8.Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G519–G524. doi: 10.1152/ajpgi.00189.2007. [DOI] [PubMed] [Google Scholar]
- 9.Frulloni L, Falconi M, Gabbrielli A, Gaia E, Graziani R, Pezzilli R, Uomo G, Andriulli A, Balzano G, Benini L, et al. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis. 2010;42 Suppl 6:S381–S406. doi: 10.1016/S1590-8658(10)60682-2. [DOI] [PubMed] [Google Scholar]
- 10.DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme ouputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–815. doi: 10.1056/NEJM197304192881603. [DOI] [PubMed] [Google Scholar]
- 11.Gullo L, Costa PL, Fontana G, Labò G. Investigation of exocrine pancreatic function by continuous infusion of caerulein and secretin in normal subjects and in chronic pancreatitis. Digestion. 1976;14:97–107. doi: 10.1159/000197914. [DOI] [PubMed] [Google Scholar]
- 12.Gullo L, Ventrucci M, Tomassetti P, Migliori M, Pezzilli R. Fecal elastase 1 determination in chronic pancreatitis. Dig Dis Sci. 1999;44:210–213. doi: 10.1023/a:1026691209094. [DOI] [PubMed] [Google Scholar]
- 13.Pezzilli R, Morselli-Labate AM, Palladoro F, Campana D, Piscitelli L, Tomassetti P, Corinaldesi R. The ELISA fecal elastase-1 polyclonal assay reacts with different antigens than those of the monoclonal assay. Pancreas. 2005;31:200–201. doi: 10.1097/01.mpa.0000167002.96641.70. [DOI] [PubMed] [Google Scholar]
- 14.Leodolter A, Kahl S, Domínguez-Muñoz JE, Gerards C, Glasbrenner B, Malfertheiner P. Comparison of two tubeless function tests in the assessment of mild-to-moderate exocrine pancreatic insufficiency. Eur J Gastroenterol Hepatol. 2000;12:1335–1338. doi: 10.1097/00042737-200012120-00012. [DOI] [PubMed] [Google Scholar]
- 15.Ventrucci M, Cipolla A, Ubalducci GM, Roda A, Roda E. 13C labelled cholesteryl octanoate breath test for assessing pancreatic exocrine insufficiency. Gut. 1998;42:81–87. doi: 10.1136/gut.42.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventrucci M, Gullo L, Daniele C, Priori P, Labò G. Pancreolauryl test for pancreatic exocrine insufficiency. Am J Gastroenterol. 1983;78:806–809. [PubMed] [Google Scholar]
- 17.Drummey GD, Benson JA, Jones CM. Microscopical examination of the stool for steatorrhea. N Engl J Med. 1961;264:85–87. doi: 10.1056/NEJM196101122640207. [DOI] [PubMed] [Google Scholar]
- 18.Fine KD, Ogunji F. A new method of quantitative fecal fat microscopy and its correlation with chemically measured fecal fat output. Am J Clin Pathol. 2000;113:528–534. doi: 10.1309/0T2W-NN7F-7T8Q-5N8C. [DOI] [PubMed] [Google Scholar]
- 19.Sugai E, Srur G, Vazquez H, Benito F, Mauriño E, Boerr LA, Bai JC. Steatocrit: a reliable semiquantitative method for detection of steatorrhea. J Clin Gastroenterol. 1994;19:206–209. doi: 10.1097/00004836-199410000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Amann ST, Josephson SA, Toskes PP. Acid steatocrit: a simple, rapid gravimetric method to determine steatorrhea. Am J Gastroenterol. 1997;92:2280–2284. [PubMed] [Google Scholar]
- 21.Gullo L, Pezzilli R, Cassano A, Ligabue A, Ventrucci M, Barbara L. Clinical effectiveness of a new enteric-coated pancreatic enzyme extract in the treatment of pancreatic steatorrhoea. Curr Ther Res. 1988;44:105–109. [Google Scholar]
- 22.Seiler CM, Izbicki J, Varga-Szabó L, Czakó L, Fiók J, Sperti C, Lerch MM, Pezzilli R, Vasileva G, Pap A, et al. Randomised clinical trial: a 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment Pharmacol Ther. 2013;37:691–702. doi: 10.1111/apt.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowitz D, Konstan MW, O’Rourke A, Cohen M, Hendeles L, Murray FT. Coefficients of fat and nitrogen absorption in healthy subjects and individuals with cystic fibrosis. J Pediatr Pharmacol Ther. 2007;12:47–52. doi: 10.5863/1551-6776-12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindkvist B, Domínguez-Muñoz JE, Luaces-Regueira M, Castiñeiras-Alvariño M, Nieto-Garcia L, Iglesias-Garcia J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology. 2012;12:305–310. doi: 10.1016/j.pan.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Talukdar R, Reddy DN. Rational use of pancreatic enzymes in patients with chronic pancreatitis. Pancreatology. 2012;12:480–481; author reply 480-481. doi: 10.1016/j.pan.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, Lammersfeld CA. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr. 2004;92:957–962. doi: 10.1079/bjn20041292. [DOI] [PubMed] [Google Scholar]
- 27.Layer P, Keller J, Lankisch PG. Pancreatic enzyme replacement therapy. Curr Gastroenterol Rep. 2001;3:101–108. doi: 10.1007/s11894-001-0005-8. [DOI] [PubMed] [Google Scholar]
- 28.Domínguez-Muñoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9:116–122. doi: 10.1007/s11894-007-0005-4. [DOI] [PubMed] [Google Scholar]
- 29.Caliari S, Benini L, Sembenini C, Gregori B, Carnielli V, Vantini I. Medium-chain triglyceride absorption in patients with pancreatic insufficiency. Scand J Gastroenterol. 1996;31:90–94. doi: 10.3109/00365529609031633. [DOI] [PubMed] [Google Scholar]
- 30.Isaksson G, Lilja P, Lundquist I, Ihse I. Influence of dietary fiber on exocrine pancreatic function in the rat. Digestion. 1983;27:57–62. doi: 10.1159/000198930. [DOI] [PubMed] [Google Scholar]
- 31.de-Madaria E, Abad-González A, Aparicio JR, Aparisi L, Boadas J, Boix E, de-Las-Heras G, Domínguez-Muñoz E, Farré A, Fernández-Cruz L, et al. The Spanish Pancreatic Club’s recommendations for the diagnosis and treatment of chronic pancreatitis: part 2 (treatment) Pancreatology. 2013;13:18–28. doi: 10.1016/j.pan.2012.11.310. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Midha S, Singh N, Joshi YK, Garg PK. Dietary counseling versus dietary supplements for malnutrition in chronic pancreatitis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2008;6:353–359. doi: 10.1016/j.cgh.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 33.Caliari S, Benini L, Bonfante F, Brentegani MT, Fioretta A, Vantini I. Pancreatic extracts are necessary for the absorption of elemental and polymeric enteral diets in severe pancreatic insufficiency. Scand J Gastroenterol. 1993;28:749–752. doi: 10.3109/00365529309098285. [DOI] [PubMed] [Google Scholar]
- 34.Dutta SK, Hlasko J. Dietary fiber in pancreatic disease: effect of high fiber diet on fat malabsorption in pancreatic insufficiency and in vitro study of the interaction of dietary fiber with pancreatic enzymes. Am J Clin Nutr. 1985;41:517–525. doi: 10.1093/ajcn/41.3.517. [DOI] [PubMed] [Google Scholar]
- 35.Guarner L, Rodríguez R, Guarner F, Malagelada JR. Fate of oral enzymes in pancreatic insufficiency. Gut. 1993;34:708–712. doi: 10.1136/gut.34.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarner M. Treatment of pancreatic exocrine deficiency. World J Surg. 2003;27:1192–1195. doi: 10.1007/s00268-003-7237-8. [DOI] [PubMed] [Google Scholar]
- 37.Pezzilli R. Chronic pancreatitis: maldigestion, intestinal ecology and intestinal inflammation. World J Gastroenterol. 2009;15:1673–1676. doi: 10.3748/wjg.15.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toouli J, Biankin AV, Oliver MR, Pearce CB, Wilson JS, Wray NH. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. Med J Aust. 2010;193:461–467. doi: 10.5694/j.1326-5377.2010.tb04000.x. [DOI] [PubMed] [Google Scholar]
- 39.Domínguez-Muñoz JE, Iglesias-García J. Oral pancreatic enzyme substitution therapy in chronic pancreatitis: is clinical response an appropriate marker for evaluation of therapeutic efficacy? JOP. 2010;11:158–162. [PubMed] [Google Scholar]
- 40.Sack J, Blau H, Goldfarb D, Ben-Zaray S, Katznelson D. Hyperuricosuria in cystic fibrosis patients treated with pancreatic enzyme supplements. A study of 16 patients in Israel. Isr J Med Sci. 1980;16:417–419. [PubMed] [Google Scholar]
- 41.FitzSimmons SC, Burkhart GA, Borowitz D, Grand RJ, Hammerstrom T, Durie PR, Lloyd-Still JD, Lowenfels AB. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med. 1997;336:1283–1289. doi: 10.1056/NEJM199705013361803. [DOI] [PubMed] [Google Scholar]
- 42.Gullo L, Pezzilli R, Gaiani S. Tolerability and safety of the long-term administration of pancreatic extracts. Pancreas. 1997;14:210–212. doi: 10.1097/00006676-199703000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Pezzilli R, Uomo G, Zerbi A, Gabbrielli A, Frulloni L, De Rai P, Delle Fave G, Di Carlo V. Diagnosis and treatment of acute pancreatitis: the position statement of the Italian Association for the study of the pancreas. Dig Liver Dis. 2008;40:803–808. doi: 10.1016/j.dld.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Uomo G, Pezzilli R, Gabbrielli A, Castoldi L, Zerbi A, Frulloni L, De Rai P, Cavallini G, Di Carlo V. Diagnostic assessment and outcome of acute pancreatitis in Italy: results of a prospective multicentre study. ProInf-AISP: Progetto informatizzato pancreatite acuta, Associazione Italiana Studio Pancreas, phase II. Dig Liver Dis. 2007;39:829–837. doi: 10.1016/j.dld.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Pezzilli R, Simoni P, Casadei R, Morselli-Labate AM. Exocrine pancreatic function during the early recovery phase of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:316–319. [PubMed] [Google Scholar]
- 46.Bruno MJ, Haverkort EB, Tytgat GN, van Leeuwen DJ. Maldigestion associated with exocrine pancreatic insufficiency: implications of gastrointestinal physiology and properties of enzyme preparations for a cause-related and patient-tailored treatment. Am J Gastroenterol. 1995;90:1383–1393. [PubMed] [Google Scholar]
- 47.Domínguez-Muñoz JE, Iglesias-García J, Vilariño-Insua M, Iglesias-Rey M. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2007;5:484–488. doi: 10.1016/j.cgh.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmeister A, Mayerle J, Beglinger C, Büchler MW, Bufler P, Dathe K, Fölsch UR, Friess H, Izbicki J, Kahl S, et al. [S3-Consensus guidelines on definition, etiology, diagnosis and medical, endoscopic and surgical management of chronic pancreatitis German Society of Digestive and Metabolic Diseases (DGVS)] Z Gastroenterol. 2012;50:1176–1224. doi: 10.1055/s-0032-1325479. [DOI] [PubMed] [Google Scholar]
- 49.Meier R, Ockenga J, Pertkiewicz M, Pap A, Milinic N, Macfie J, Löser C, Keim V. ESPEN Guidelines on Enteral Nutrition: Pancreas. Clin Nutr. 2006;25:275–284. doi: 10.1016/j.clnu.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 50.Gullo L, Barbara L, Labò G. Effect of cessation of alcohol use on the course of pancreatic dysfunction in alcoholic pancreatitis. Gastroenterology. 1988;95:1063–1068. doi: 10.1016/0016-5085(88)90184-9. [DOI] [PubMed] [Google Scholar]
- 51.Lohr JM, Oliver MR, Frulloni L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. United Eur Gastroenterol J. 2013:In press. doi: 10.1177/2050640613476500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiMagno EP. Gastric acid suppression and treatment of severe exocrine pancreatic insufficiency. Best Pract Res Clin Gastroenterol. 2001;15:477–486. doi: 10.1053/bega.2001.0195. [DOI] [PubMed] [Google Scholar]
- 53.Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, Vilariño-Insua M. Optimising the therapy of exocrine pancreatic insufficiency by the association of a proton pump inhibitor to enteric coated pancreatic extracts. Gut. 2006;55:1056–1057. doi: 10.1136/gut.2006.094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham DY, Sackman JW. Mechanism of increase in steatorrhea with calcium and magnesium in exocrine pancreatic insufficiency: an animal model. Gastroenterology. 1982;83:638–644. [PubMed] [Google Scholar]
- 55.Lankisch PG. What to do when a patient with exocrine pancreatic insufficiency does not respond to pancreatic enzyme substitution, a practical guide. Digestion. 1999;60 Suppl 1:97–103. doi: 10.1159/000051463. [DOI] [PubMed] [Google Scholar]
- 56.Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, Figueiras A, Vilariño-Insua M. Effect of the administration schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005;21:993–1000. doi: 10.1111/j.1365-2036.2005.02390.x. [DOI] [PubMed] [Google Scholar]
- 57.Casellas F, Guarner L, Vaquero E, Antolín M, de Gracia X, Malagelada JR. Hydrogen breath test with glucose in exocrine pancreatic insufficiency. Pancreas. 1998;16:481–486. doi: 10.1097/00006676-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Pieramico O, Dominguez-Muñoz JE, Nelson DK, Böck W, Büchler M, Malfertheiner P. Interdigestive cycling in chronic pancreatitis: altered coordination among pancreatic secretion, motility, and hormones. Gastroenterology. 1995;109:224–230. doi: 10.1016/0016-5085(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 59.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.el-Kamar FG, Grossbard ML, Kozuch PS. Metastatic pancreatic cancer: emerging strategies in chemotherapy and palliative care. Oncologist. 2003;8:18–34. doi: 10.1634/theoncologist.8-1-18. [DOI] [PubMed] [Google Scholar]
- 61.Perez MM, Newcomer AD, Moertel CG, Go VL, Dimagno EP. Assessment of weight loss, food intake, fat metabolism, malabsorption, and treatment of pancreatic insufficiency in pancreatic cancer. Cancer. 1983;52:346–352. doi: 10.1002/1097-0142(19830715)52:2<346::aid-cncr2820520228>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 62.Yuasa Y, Murakami Y, Nakamura H, Uemura K, Ohge H, Sudo T, Hashimoto Y, Nakashima A, Hiyama E, Sueda T. Histological loss of pancreatic exocrine cells correlates with pancreatic exocrine function after pancreatic surgery. Pancreas. 2012;41:928–933. doi: 10.1097/MPA.0b013e31823d837d. [DOI] [PubMed] [Google Scholar]
- 63.Halloran CM, Cox TF, Chauhan S, Raraty MG, Sutton R, Neoptolemos JP, Ghaneh P. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: a prospective study. Pancreatology. 2011;11:535–545. doi: 10.1159/000333308. [DOI] [PubMed] [Google Scholar]
- 64.DiMagno EP, Malagelada JR, Go VL. The relationships between pancreatic ductal obstruction and pancreatic secretion in man. Mayo Clin Proc. 1979;54:157–162. [PubMed] [Google Scholar]
- 65.Weber A, Kehl V, Mittermeyer T, Herberich E, Röthling N, Schmid RM, Prinz C. Prognostic factors for survival in patients with unresectable pancreatic cancer. Pancreas. 2010;39:1247–1253. doi: 10.1097/MPA.0b013e3181e21b1b. [DOI] [PubMed] [Google Scholar]
- 66.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO, Engstrom PF, Ezdinli EZ, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 67.Papadoniou N, Kosmas C, Gennatas K, Polyzos A, Mouratidou D, Skopelitis E, Tzivras M, Sougioultzis S, Papastratis G, Karatzas G, et al. Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. Anticancer Res. 2008;28:543–549. [PubMed] [Google Scholar]
- 68.Partelli S, Frulloni L, Minniti C, Bassi C, Barugola G, D’Onofrio M, Crippa S, Falconi M. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig Liver Dis. 2012;44:945–951. doi: 10.1016/j.dld.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 69.Bruno MJ, Haverkort EB, Tijssen GP, Tytgat GN, van Leeuwen DJ. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut. 1998;42:92–96. doi: 10.1136/gut.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pelzer U, Arnold D, Gövercin M, Stieler J, Doerken B, Riess H, Oettle H. Parenteral nutrition support for patients with pancreatic cancer. Results of a phase II study. BMC Cancer. 2010;10:86. doi: 10.1186/1471-2407-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richter E, Denecke A, Klapdor S, Klapdor R. Parenteral nutrition support for patients with pancreatic cancer--improvement of the nutritional status and the therapeutic outcome. Anticancer Res. 2012;32:2111–2118. [PubMed] [Google Scholar]
- 72.Chey WY, Shay H, Shuman CR. External pancreatic secretion in diabetes mellitus. Ann Intern Med. 1963;59:812–821. doi: 10.7326/0003-4819-59-6-812. [DOI] [PubMed] [Google Scholar]
- 73.el Newihi H, Dooley CP, Saad C, Staples J, Zeidler A, Valenzuela JE. Impaired exocrine pancreatic function in diabetics with diarrhea and peripheral neuropathy. Dig Dis Sci. 1988;33:705–710. doi: 10.1007/BF01540434. [DOI] [PubMed] [Google Scholar]
- 74.Frier BM, Saunders JH, Wormsley KG, Bouchier IA. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut. 1976;17:685–691. doi: 10.1136/gut.17.9.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gröger G, Layer P. Exocrine pancreatic function in diabetes mellitus. Eur J Gastroenterol Hepatol. 1995;7:740–746. [PubMed] [Google Scholar]
- 76.Harano Y, Kim CI, Kang M, Shichiri M, Shimizu Y, Li H, Yoshida M, Shigeta Y, Abe H. External pancreatic dysfunction associated with diabetes mellitus. J Lab Clin Med. 1978;91:780–790. [PubMed] [Google Scholar]
- 77.Lankisch PG, Manthey G, Otto J, Koop H, Talaulicar M, Willms B, Creutzfeldt W. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion. 1982;25:211–216. doi: 10.1159/000198833. [DOI] [PubMed] [Google Scholar]
- 78.Vacca JB, Henke WJ, Knight WA. The exocrine pancreas in diabetes mellitus. Ann Intern Med. 1964;61:242–247. doi: 10.7326/0003-4819-61-2-242. [DOI] [PubMed] [Google Scholar]
- 79.Hardt PD, Krauss A, Bretz L, Porsch-Ozcürümez M, Schnell-Kretschmer H, Mäser E, Bretzel RG, Zekhorn T, Klör HU. Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol. 2000;37:105–110. doi: 10.1007/s005920070011. [DOI] [PubMed] [Google Scholar]
- 80.Hardt PD, Hauenschild A, Nalop J, Marzeion AM, Jaeger C, Teichmann J, Bretzel RG, Hollenhorst M, Kloer HU. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology. 2003;3:395–402. doi: 10.1159/000073655. [DOI] [PubMed] [Google Scholar]
- 81.Vesterhus M, Raeder H, Aurlien H, Gjesdal CG, Bredrup C, Holm PI, Molven A, Bindoff L, Berstad A, Njølstad PR. Neurological features and enzyme therapy in patients with endocrine and exocrine pancreas dysfunction due to CEL mutations. Diabetes Care. 2008;31:1738–1740. doi: 10.2337/dc07-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larger E, Philippe MF, Barbot-Trystram L, Radu A, Rotariu M, Nobécourt E, Boitard C. Pancreatic exocrine function in patients with diabetes. Diabet Med. 2012;29:1047–1054. doi: 10.1111/j.1464-5491.2012.03597.x. [DOI] [PubMed] [Google Scholar]
- 83.Icks A, Haastert B, Giani G, Rathmann W. Low fecal elastase-1 in type I diabetes mellitus. Z Gastroenterol. 2001;39:823–830. doi: 10.1055/s-2001-17867. [DOI] [PubMed] [Google Scholar]
- 84.Cavalot F, Bonomo K, Perna P, Bacillo E, Salacone P, Gallo M, Mattiello L, Trovati M, Gaia E. Pancreatic elastase-1 in stools, a marker of exocrine pancreas function, correlates with both residual beta-cell secretion and metabolic control in type 1 diabetic subjects. Diabetes Care. 2004;27:2052–2054. doi: 10.2337/diacare.27.8.2052. [DOI] [PubMed] [Google Scholar]
- 85.Rathmann W, Haastert B, Icks A, Giani G, Hennings S, Mitchell J, Curran S, Wareham NJ. Low faecal elastase 1 concentrations in type 2 diabetes mellitus. Scand J Gastroenterol. 2001;36:1056–1061. doi: 10.1080/003655201750422657. [DOI] [PubMed] [Google Scholar]
- 86.Nunes AC, Pontes JM, Rosa A, Gomes L, Carvalheiro M, Freitas D. Screening for pancreatic exocrine insufficiency in patients with diabetes mellitus. Am J Gastroenterol. 2003;98:2672–2675. doi: 10.1111/j.1572-0241.2003.08730.x. [DOI] [PubMed] [Google Scholar]
- 87.Yilmaztepe A, Ulukaya E, Ersoy C, Yilmaz M, Tokullugil HA. Investigation of fecal pancreatic elastase-1 levels in type 2 diabetic patients. Turk J Gastroenterol. 2005;16:75–80. [PubMed] [Google Scholar]
- 88.Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, Horowitz M. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71–76. doi: 10.1111/j.1572-0241.2001.03350.x. [DOI] [PubMed] [Google Scholar]
- 89.Hardt PD, Hauenschild A, Jaeger C, Teichmann J, Bretzel RG, Kloer HU. High prevalence of steatorrhea in 101 diabetic patients likely to suffer from exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations: a prospective multicenter study. Dig Dis Sci. 2003;48:1688–1692. doi: 10.1023/a:1025422423435. [DOI] [PubMed] [Google Scholar]
- 90.Cavalot F, Bonomo K, Fiora E, Bacillo E, Salacone P, Chirio M, Gaia E, Trovati M. Does pancreatic elastase-1 in stools predict steatorrhea in type 1 diabetes? Diabetes Care. 2006;29:719–721. doi: 10.2337/diacare.29.03.06.dc05-1389. [DOI] [PubMed] [Google Scholar]
- 91.Hardt PD. Comment on “Lw fecal elastase 1 levels do not indicate exocrine pancreatic insufficiency in type-1 diabetes mellitus (pancreas. 2008; 36: 274-278)”. Pancreas. 2009;38:471–42; author reply 471-42;. doi: 10.1097/MPA.0b013e31818b0060. [DOI] [PubMed] [Google Scholar]
- 92.Ewald N, Bretzel RG, Fantus IG, Hollenhorst M, Kloer HU, Hardt PD. Pancreatin therapy in patients with insulin-treated diabetes mellitus and exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations. Results of a prospective multi-centre trial. Diabetes Metab Res Rev. 2007;23:386–391. doi: 10.1002/dmrr.708. [DOI] [PubMed] [Google Scholar]
- 93.Teichmann J, Mann ST, Stracke H, Lange U, Hardt PD, Bretzel RG, Klör HU. Parathormone levels and Vitamin D metabolism in female patients with various grades of fecal elastase 1 deficiency. Eur J Med Res. 2008;13:563–567. [PubMed] [Google Scholar]
- 94.Glasbrenner B, Malfertheiner P, Kerner W, Scherbaum WA, Ditschuneit H. [Effect of pancreatin on diabetes mellitus in chronic pancreatitis] Z Gastroenterol. 1990;28:275–279. [PubMed] [Google Scholar]
- 95.Mohan V, Poongothai S, Pitchumoni CS. Oral pancreatic enzyme therapy in the control of diabetes mellitus in tropical calculous pancreatitis. Int J Pancreatol. 1998;24:19–22. doi: 10.1007/BF02787526. [DOI] [PubMed] [Google Scholar]
- 96.Weitgasser R, Abrahamian H, Clodi M, Fortunat W, Hammer H. [Position paper: Exocrine pancreatic insufficiency and diabetes mellitus] Wien Klin Wochenschr. 2012;124 Suppl 2:100–103. doi: 10.1007/s00508-012-0290-2. [DOI] [PubMed] [Google Scholar]
- 97.West J, Logan RF, Hill PG, Lloyd A, Lewis S, Hubbard R, Reader R, Holmes GK, Khaw KT. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut. 2003;52:960–965. doi: 10.1136/gut.52.7.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 99.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–391. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 100.Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74–S78. doi: 10.1053/j.gastro.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 101.Dreiling DA. The pancreatic secretion in the malabsorption syndrome and related malnutrition states. J Mt Sinai Hosp N Y. 1957;24:243–250. [PubMed] [Google Scholar]
- 102.Regan PT, DiMagno EP. Exocrine pancreatic insufficiency in celiac sprue: a cause of treatment failure. Gastroenterology. 1980;78:484–487. [PubMed] [Google Scholar]
- 103.Perri F, Pastore M, Festa V, Clemente R, Quitadamo M, D’Altilia MR, Niro G, Paolucci P, Andriulli A. Intraduodenal lipase activity in celiac disease assessed by means of 13C mixed-triglyceride breath test. J Pediatr Gastroenterol Nutr. 1998;27:407–410. doi: 10.1097/00005176-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 104.Walkowiak J, Herzig KH. Fecal elastase-1 is decreased in villous atrophy regardless of the underlying disease. Eur J Clin Invest. 2001;31:425–430. doi: 10.1046/j.1365-2362.2001.00822.x. [DOI] [PubMed] [Google Scholar]
- 105.Carroccio A, Iacono G, Montalto G, Cavataio F, Lorello D, Soresi M, Di Martino D, Notarbartolo A. Pancreatic insufficiency in celiac disease is not dependent on nutritional status. Dig Dis Sci. 1994;39:2235–2242. doi: 10.1007/BF02090377. [DOI] [PubMed] [Google Scholar]
- 106.Carroccio A, Iacono G, Montalto G, Cavataio F, Lorello D, Greco L, Soresi M, Notarbartolo A. Pancreatic enzyme therapy in childhood celiac disease. A double-blind prospective randomized study. Dig Dis Sci. 1995;40:2555–2560. doi: 10.1007/BF02220441. [DOI] [PubMed] [Google Scholar]
- 107.Carroccio A, Iacono G, Montalto G, Cavataio F, Di Marco C, Balsamo V, Notarbartolo A. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut. 1991;32:796–799. doi: 10.1136/gut.32.7.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gomez JC, Morán CE, Mauriño EC, Bai JC. Exocrine pancreatic insufficiency in celiac disease. Gastroenterology. 1998;114:621–623. doi: 10.1016/s0016-5085(98)70562-1. [DOI] [PubMed] [Google Scholar]
- 109.Evans KE, Leeds JS, Morley S, Sanders DS. Pancreatic insufficiency in adult celiac disease: do patients require long-term enzyme supplementation? Dig Dis Sci. 2010;55:2999–3004. doi: 10.1007/s10620-010-1261-y. [DOI] [PubMed] [Google Scholar]
- 110.Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. 1997;112:1830–1838. doi: 10.1053/gast.1997.v112.pm9178673. [DOI] [PubMed] [Google Scholar]
- 111.Sadr-Azodi O, Sanders DS, Murray JA, Ludvigsson JF. Patients with celiac disease have an increased risk for pancreatitis. Clin Gastroenterol Hepatol. 2012;10:1136–1142.e3. doi: 10.1016/j.cgh.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Angelini G, Cavallini G, Bovo P, Brocco G, Castagnini A, Lavarini E, Merigo F, Tallon N, Scuro LA. Pancreatic function in chronic inflammatory bowel disease. Int J Pancreatol. 1988;3:185–193. doi: 10.1007/BF02798930. [DOI] [PubMed] [Google Scholar]
- 113.Hegnhøj J, Hansen CP, Rannem T, Søbirk H, Andersen LB, Andersen JR. Pancreatic function in Crohn’s disease. Gut. 1990;31:1076–1079. doi: 10.1136/gut.31.9.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn’s disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22:202–206. doi: 10.1097/00004836-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 115.Maconi G, Dominici R, Molteni M, Ardizzone S, Bosani M, Ferrara E, Gallus S, Panteghini M, Bianchi Porro G. Prevalence of pancreatic insufficiency in inflammatory bowel diseases. Assessment by fecal elastase-1. Dig Dis Sci. 2008;53:262–270. doi: 10.1007/s10620-007-9852-y. [DOI] [PubMed] [Google Scholar]
- 116.Beharry S, Ellis L, Corey M, Marcon M, Durie P. How useful is fecal pancreatic elastase 1 as a marker of exocrine pancreatic disease? J Pediatr. 2002;141:84–90. doi: 10.1067/mpd.2002.124829. [DOI] [PubMed] [Google Scholar]
- 117.Nakamura H, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Sueda T. Predictive factors for exocrine pancreatic insufficiency after pancreatoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg. 2009;13:1321–1327. doi: 10.1007/s11605-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 118.Ryan AM, Healy LA, Power DG, Rowley SP, Reynolds JV. Short-term nutritional implications of total gastrectomy for malignancy, and the impact of parenteral nutritional support. Clin Nutr. 2007;26:718–727. doi: 10.1016/j.clnu.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 119.Saito A, Noguchi Y, Yoshikawa T, Doi C, Fukuzawa K, Matsumoto A, Ito T, Tsuburaya A, Nagahara N. Gastrectomized patients are in a state of chronic protein malnutrition analyses of 23 amino acids. Hepatogastroenterology. 2001;48:585–589. [PubMed] [Google Scholar]
- 120.Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54 Suppl 6:vi1–v28. doi: 10.1136/gut.2005.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keller J, Aghdassi AA, Lerch MM, Mayerle JV, Layer P. Tests of pancreatic exocrine function - clinical significance in pancreatic and non-pancreatic disorders. Best Pract Res Clin Gastroenterol. 2009;23:425–439. doi: 10.1016/j.bpg.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 122.Domínguez-Muñoz JE. Pancreatic enzyme replacement therapy: exocrine pancreatic insufficiency after gastrointestinal surgery. HPB (Oxford) 2009;11 Suppl 3:3–6. doi: 10.1111/j.1477-2574.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Friess H, Böhm J, Müller MW, Glasbrenner B, Riepl RL, Malfertheiner P, Büchler MW. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol. 1996;91:341–347. [PubMed] [Google Scholar]
- 124.Heptner G, Domschke S, Domschke W. Exocrine pancreatic function after gastrectomy. Specificity of indirect tests. Gastroenterology. 1989;97:147–153. doi: 10.1016/0016-5085(89)91428-5. [DOI] [PubMed] [Google Scholar]
- 125.Gullo L, Costa PL, Ventrucci M, Mattioli S, Viti G, Labò G. Exocrine pancreatic function after total gastrectomy. Scand J Gastroenterol. 1979;14:401–407. [PubMed] [Google Scholar]
- 126.Ihse I, Arnesjö B, Kugelberg C, Lilja P. Intestinal activities of trypsin, lipase, and phospholipase after a test meal. An evaluation of 474 examinations. Scand J Gastroenterol. 1977;12:663–668. doi: 10.3109/00365527709181700. [DOI] [PubMed] [Google Scholar]
- 127.Büchler M, Malfertheiner P, Glasbrenner B, Beger HG. [Secondary pancreatic insufficiency following distal stomach resection] Langenbecks Arch Chir. 1985;367:41–50. doi: 10.1007/BF01241944. [DOI] [PubMed] [Google Scholar]
- 128.Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:947–955. doi: 10.1016/j.bpg.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 129.Domínguez Muñoz JE. [Physiopathology, diagnosis, and treatment of exocrine pancreatic insufficiency in patients following gastrointestinal or pancreatic surgery] Gastroenterol Hepatol. 2005;28 Suppl 1:39–42. doi: 10.1157/13071386. [DOI] [PubMed] [Google Scholar]
- 130.Malagelada JR, Go VL, Summerskill WH. Altered pancreatic and biliary function after vagotomy and pyloroplasty. Gastroenterology. 1974;66:22–27. [PubMed] [Google Scholar]
- 131.Wormsley KG. The effect of vagotomy on the human pancreatic response to direct and indirect stimulation. Scand J Gastroenterol. 1972;7:85–91. doi: 10.3109/00365527209180742. [DOI] [PubMed] [Google Scholar]
- 132.Armbrecht U, Lundell L, Stockbrügger RW. The benefit of pancreatic enzyme substitution after total gastrectomy. Aliment Pharmacol Ther. 1988;2:493–500. doi: 10.1111/j.1365-2036.1988.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 133.Brägelmann R, Armbrecht U, Rosemeyer D, Schneider B, Zilly W, Stockbrügger RW. The effect of pancreatic enzyme supplementation in patients with steatorrhoea after total gastrectomy. Eur J Gastroenterol Hepatol. 1999;11:231–237. doi: 10.1097/00042737-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 134.Huddy JR, Macharg FM, Lawn AM, Preston SR. Exocrine pancreatic insufficiency following esophagectomy. Dis Esophagus. 2013;26:594–597. doi: 10.1111/dote.12004. [DOI] [PubMed] [Google Scholar]
- 135.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 136.Ockenga J. Importance of nutritional management in diseases with exocrine pancreatic insufficiency. HPB (Oxford) 2009;11 Suppl 3:11–15. doi: 10.1111/j.1477-2574.2009.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ghaneh P, Neoptolemos JP. Pancreatic exocrine insufficiency following pancreatic resection. Digestion. 1999;60 Suppl 1:104–110. doi: 10.1159/000051464. [DOI] [PubMed] [Google Scholar]
- 138.Matsumoto J, Traverso LW. Exocrine function following the whipple operation as assessed by stool elastase. J Gastrointest Surg. 2006;10:1225–1229. doi: 10.1016/j.gassur.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 139.Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 140.Lemaire E, O’Toole D, Sauvanet A, Hammel P, Belghiti J, Ruszniewski P. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br J Surg. 2000;87:434–438. doi: 10.1046/j.1365-2168.2000.01388.x. [DOI] [PubMed] [Google Scholar]
- 141.Jang JY, Kim SW, Park SJ, Park YH. Comparison of the functional outcome after pylorus-preserving pancreatoduodenectomy: pancreatogastrostomy and pancreatojejunostomy. World J Surg. 2002;26:366–371. doi: 10.1007/s00268-001-0234-x. [DOI] [PubMed] [Google Scholar]
- 142.Morrow CE, Cohen JI, Sutherland DE, Najarian JS. Chronic pancreatitis: long-term surgical results of pancreatic duct drainage, pancreatic resection, and near-total pancreatectomy and islet autotransplantation. Surgery. 1984;96:608–616. [PubMed] [Google Scholar]
- 143.Speicher JE, Traverso LW. Pancreatic exocrine function is preserved after distal pancreatectomy. J Gastrointest Surg. 2010;14:1006–1011. doi: 10.1007/s11605-010-1184-0. [DOI] [PubMed] [Google Scholar]
- 144.Balzano G, Zerbi A, Veronesi P, Cristallo M, Di Carlo V. Surgical treatment of benign and borderline neoplasms of the pancreatic body. Dig Surg. 2003;20:506–510. doi: 10.1159/000073646. [DOI] [PubMed] [Google Scholar]
- 145.Iacono C, Bortolasi L, Serio G. Is there a place for central pancreatectomy in pancreatic surgery? J Gastrointest Surg. 1998;2:509–516; discussion 516-517. doi: 10.1016/s1091-255x(98)80050-4. [DOI] [PubMed] [Google Scholar]
- 146.Warshaw AL, Rattner DW, Fernández-del Castillo C, Z’graggen K. Middle segment pancreatectomy: a novel technique for conserving pancreatic tissue. Arch Surg. 1998;133:327–331. doi: 10.1001/archsurg.133.3.327. [DOI] [PubMed] [Google Scholar]
- 147.Sudo T, Murakami Y, Uemura K, Hayashidani Y, Hashimoto Y, Ohge H, Sueda T. Middle pancreatectomy with pancreaticogastrostomy: a technique, operative outcomes, and long-term pancreatic function. J Surg Oncol. 2010;101:61–65. doi: 10.1002/jso.21430. [DOI] [PubMed] [Google Scholar]
- 148.Crippa S, Bassi C, Warshaw AL, Falconi M, Partelli S, Thayer SP, Pederzoli P, Fernández-del Castillo C. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg. 2007;246:69–76. doi: 10.1097/01.sla.0000262790.51512.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roggin KK, Rudloff U, Blumgart LH, Brennan MF. Central pancreatectomy revisited. J Gastrointest Surg. 2006;10:804–812. doi: 10.1016/j.gassur.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 150.Falconi M, Zerbi A, Crippa S, Balzano G, Boninsegna L, Capitanio V, Bassi C, Di Carlo V, Pederzoli P. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17:1621–1627. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 151.Bruno MJ, Borm JJ, Hoek FJ, Delzenne B, Hofmann AF, de Goeij JJ, van Royen EA, van Gulik TM, de Wit LT, Gouma DJ, et al. Comparative effects of enteric-coated pancreatin microsphere therapy after conventional and pylorus-preserving pancreatoduodenectomy. Br J Surg. 1997;84:952–956. doi: 10.1002/bjs.1800840712. [DOI] [PubMed] [Google Scholar]
- 152.Van Hoozen CM, Peeke PG, Taubeneck M, Frey CF, Halsted CH. Efficacy of enzyme supplementation after surgery for chronic pancreatitis. Pancreas. 1997;14:174–180. doi: 10.1097/00006676-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 153.Neoptolemos JP, Ghaneh P, Andrén-Sandberg A, Bramhall S, Patankar R, Kleibeuker JH, Johnson CD. Treatment of pancreatic exocrine insufficiency after pancreatic resection. Results of a randomized, double-blind, placebo-controlled, crossover study of high vs standard dose pancreatin. Int J Pancreatol. 1999;25:171–180. [PubMed] [Google Scholar]
- 154.Morawski JH, Prüfert A, van Engen A, Foerster D, Sander-Struckmeier S, Małecka-Panas E, Pezzilli R. Cost-effectiveness analysis of pancreatin minimicrospheres in patients with pancreatic exocrine insufficiency due to chronic pancreatitis. J Med Econ. 2012;15 Suppl 1:15–25. doi: 10.3111/13696998.2012.737882. [DOI] [PubMed] [Google Scholar]
- 155.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]


