Abstract
AIM: To conduct a meta-analysis comparing laparoscopic total gastrectomy (LTG) with open total gastrectomy (OTG) for the treatment of gastric cancer.
METHODS: Major databases such as Medline (PubMed), Embase, Academic Search Premier (EBSCO), Science Citation Index Expanded and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library were searched for studies comparing LTG and OTG from January 1994 to May 2013. Evaluated endpoints were operative, postoperative and oncological outcomes. Operative outcomes included operative time and intraoperative blood loss. Postoperative recovery included time to first flatus, time to first oral intake, hospital stay and analgesics use. Postoperative complications comprised morbidity, anastomotic leakage, anastomotic stenosis, ileus, bleeding, abdominal abscess, wound problems and mortality. Oncological outcomes included positive resection margins, number of retrieved lymph nodes, and proximal and distal resection margins. The pooled effect was calculated using either a fixed effects or a random effects model.
RESULTS: Fifteen non-randomized comparative studies with 2022 patients were included (LTG - 811, OTG - 1211). Both groups had similar short-term oncological outcomes, analgesic use (WMD -0.09; 95%CI: -2.39-2.20; P = 0.94) and mortality (OR = 0.74; 95%CI: 0.24-2.31; P = 0.61). However, LTG was associated with a lower intraoperative blood loss (WMD -201.19 mL; 95%CI: -296.50--105.87 mL; P < 0.0001) and overall complication rate (OR = 0.73; 95%CI: 0.57-0.92; P = 0.009); fewer wound-related complications (OR = 0.39; 95%CI: 0.21-0.72; P = 0.002); a quicker recovery of gastrointestinal motility with shorter time to first flatus (WMD -0.82; 95%CI: -1.18--0.45; P < 0.0001) and oral intake (WMD -1.30; 95%CI: -1.84--0.75; P < 0.00001); and a shorter hospital stay (WMD -3.55; 95%CI: -5.13--1.96; P < 0.0001), albeit with a longer operation time (WMD 48.25 min; 95%CI: 31.15-65.35; P < 0.00001), as compared with OTG.
CONCLUSION: LTG is safe and effective, and may offer some advantages over OTG in the treatment of gastric cancer.
Keywords: Gastric cancer, Laparoscopic total gastrectomy, Laparoscopic assisted total gastrectomy, Open total gastrectomy, Meta-analysis
Core tip: Currently, surgical resection is the mainstay treatment for gastric cancer. With technical advances and improved instrumentation, laparoscopic total gastrectomy (LTG) is being used increasingly to treat this malignant disease. However, compared with conventional open total gastrectomy (OTG), the safety and technical feasibility of LTG have not been adequately evaluated. This study clarified that, compared with OTG, LTG has similar short-term oncological outcomes, analgesic use and mortality. Furthermore, LTG was associated with lower intraoperative blood loss and overall complication rate, fewer wound-related complications, quicker recovery of gastrointestinal motility and a shorter hospital stay, albeit with a longer operation time.
INTRODUCTION
Gastric cancer is one of the most common cancers worldwide and is a leading cause of cancer death[1]. Despite improvements in diagnosis and systemic therapy, surgery, in the form of gastrectomy with lymph node dissection, still forms the mainstay of treatment[2]. Since it was first described in 1994[3], laparoscopic surgery, and more specifically laparoscopic distal gastrectomy, has been used widely in the far East to treat early gastric cancers and is associated with many advantages over open surgery[4-7]. On the other hand, laparoscopic total gastrectomy (LTG) with lymph node dissection, which was reported in 1999[8], is practiced less widely and is more challenging to perform[9]. The procedure is associated with a high risk of bleeding and a technically demanding anastomosis, all within a narrow operating field[9,10]. However, with technical advances and improved instrumentation, LTG is now being used increasingly to treat gastric cancer[11-14].
A number of studies comparing the short-term or long-term outcomes, of LTG vs conventional open total gastrectomy (OTG) for early and advanced gastric carcinoma have shown it to be feasible, oncologically effective and safe in experienced hands[14-17]. LTG offers the potential advantage of being less invasive, causing less surgical trauma with less postoperative pain and a quicker recovery[18,19]. However, most studies were too small to adequately evaluate the surgical outcomes of LTG. The aim of the current study was to inform future surgical practice by comparing the technical feasibility, effectiveness, and safety of LTG and OTG in the treatment of early and advanced gastric cancer, through a systematic review and meta-analysis of published comparative studies.
MATERIALS AND METHODS
Literature search
A comprehensive literature search in Medline (PubMed), Embase, Academic Search Premier (EBSCO), Science Citation Index Expanded and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library was carried out for relevant studies, between January 1994 and May 2013, comparing OTG and LTG in the treatment of gastric cancer. The following search terms were used: “gastric cancer; laparoscopic total gastrectomy; laparoscopic assisted total gastrectomy; minimally invasive surgery; open total gastrectomy” along with their synonyms or abbreviations. Reference lists of selected articles were also examined to identify relevant studies that were not identified in the database searches. Investigators and experts in the field of laparoscopic surgery were contacted to ensure that all relevant studies were identified. Only comparative clinical trials with full-text descriptions were included. Final inclusion of articles was determined by consensus; when this failed, a third author adjudicated.
Inclusion criteria
Studies included: (1) English language articles published in peer-reviewed journals; (2) human studies; (3) studies with at least one of the outcomes mentioned; (4) clear documentation of the operative techniques as “open” or “laparoscopic” or “laparoscopic-assisted”; and (5) where multiple studies came from the same institute and/or authors, either the higher quality study or the more recent publication was included in the analysis.
Exclusion criteria
Excluded studies: (1) abstracts, letters, editorials, expert opinions, case reports, reviews and studies lacking control groups; (2) studies for benign lesions and gastrointestinal stromal tumor (GIST); (3) studies comparing two laparoscopic surgical approaches or comparing laparoscopic and robot-assisted gastrectomy; (4) studies including only subgroup analyses comparing LTG with OTG; and (5) repeated reports between authors, centers, and the patient community.
Outcomes of interests
Operative outcomes included operation time and intraoperative blood loss. Oncological outcomes included positive resection margins, number of retrieved lymph nodes, and proximal and distal resection margins. Postoperative recovery outcomes included time to first flatus, time to first oral intake, analgesic use and hospital stay. Outcomes for postoperative complications included overall complication rate, anastomotic leakage, anastomotic stenosis, ileus, bleeding, abdominal abscess, wound-related problems and mortality.
Data extraction and quality assessment
Two independent observers using standardized forms extracted the data. The recorded data included study characteristics, quality assessment and perioperative outcomes. The quality of the studies was assessed using the modified Newcastle-Ottawa Scale, with changes made to reflect the needs of this study[20,21]. The maximum number of stars in the selection, comparability, and outcome categories were 3, 4, and 2, respectively. Studies achieving 6 or more stars were considered high quality[22].
Statistical analysis
Meta-analysis was performed using Review Manager Version 5.0 software (The Cochrane Collaboration, Oxford, United Kingdom). For continuous variables, treatment effects were expressed as weighted mean difference (WMD) with corresponding 95% confidence interval (CI). For categorical variables, treatment effects were expressed as odds ratio (OR) with corresponding 95%CI. Heterogeneity was evaluated using the χ2 test, and a P value < 0.1 was considered significant; I2 values were used for the evaluation of statistical heterogeneity[23]. A fixed-effects model was initially calculated for all outcomes[24], but if the test rejected the assumption of homogeneity of the studies, then a random-effects analysis was performed[25]. Sensitivity analyses were performed by removing individual studies from the data set and analyzing the effect on the overall results, to identify sources of significant heterogeneity. Subgroup analyses were also undertaken by including only high quality studies to present cumulative evidence. Funnel plots based on the operation time were constructed to evaluate potential publication bias[26].
RESULTS
Description of trials included in the meta-analysis
The search strategy generated 91 relevant clinical studies, among which 19 full text articles[9,10,14-18,27-38] were identified for further investigation. Of these, four studies[18,31,35,36] were excluded for various reasons: 1 study[36], based on an administrative database, was used to assess hospital practice performance with regard to the quantity of medical care items and diet provided during hospitalization; another study[35] only compared LTG with OTG in a subgroup analysis; and two studies were repeated reports[18,31]. Finally, 15 studies[9,10,14-17,27-30,32-34,37,38] were identified for inclusion, of which two were prospective non-randomized comparative studies[14,28], the rest being retrospective comparative studies. Figure 1 shows the study selection process in our meta-analysis.
Figure 1.
Flow diagram outlining the study selection process according to PRISMA guidelines. OTG: Open total gastrectomy; LTG: Laparoscopic total gastrectomy.
Study and patient characteristics
Two thousand and twenty-two patients, 811 patients from the LTG group and 1211 patients from the OTG group, were included in the study. Eleven studies[9,10,14,16,17,28,29,32,34,37,38] included patients with both early and advanced gastric cancer, while three studies[15,30,33] only included patients with early gastric cancer; one study[27] only included patients with advanced gastric cancer. In seven studies[14,17,27,30,32,33,37], D2 lymph node dissection was exclusively performed, while D1+β was completed in three studies[9,15,28]. The remaining studies[10,16,29,34,38] reported D1+α/β and D2 dissections. All the studies were conducted in Asia and Europe, and were published between 2009 and 2013. The sample size ranged from 19 to 448 patients. From the nine studies[9,15,17,27,28,30,32,37,38] that reported data on conversion to an open procedure; LTG was converted to an open procedure in five patients in two studies[17,37]. The study characteristics (Table 1), quality assessment scoring (Table 2), perioperative outcomes of the included studies (Table 3) and the results of the meta-analysis (Table 4) have been summarized appropriately.
Table 1.
Study characteristics
| Author, year | Country | Study design | Group | No. of patients | Age(yr) | Gender(M/F) | BMI(kg/m2) | ASA (1:2:3) | Tumorsize (cm) | Tumor stage1 | Extent of LND | Population |
| Dulucq et al[28], 2005 | France | PCS | LTG | 8 | 75 ± 8 | 3/5 | NA | NA | 5.5 ± 2 | NA | D1 + β | EGC + AGC |
| OTG | 11 | 67 ± 14 | 5/6 | NA | NA | 6.1 ± 0.4 | NA | |||||
| Usui et al[9], 2005 | Japan | RCS | LTG | 20 | 66.0 ± 10.4 | 13/7 | 21.3 ± 3.1 | NA | NA | 8/10/2/0/0 | D1 + β | EGC + AGC |
| OTG | 19 | 66.2 ± 10.2 | 14/5 | 22.1 ± 2.4 | NA | NA | 10/8/1/0/0 | |||||
| Kim et al[34], 2008 | South Korea | RCS | LTG | 27 | 57.3 ± 14.2 | 16/11 | 22.6 ± 3.1 | NA | NA | NA | D1 + α/β, D2 | EGC + AGC |
| OTG | 33 | 61.6 ± 9.2 | 23/10 | 22.4 ± 2.1 | NA | NA | NA | |||||
| Mochiki et al[15], 2008 | Japan | RCS | LTG | 20 | 66 ± 2.4 | 16/4 | NA | NA | 3.6 ± 0.5 | NA | D1 + β | EGC |
| OTG | 18 | 63 ± 2.2 | 16/2 | NA | NA | 5.7 ± 0.8 | NA | |||||
| Topal et al[14], 2008 | Belgium | PCS | LTG | 38 | 68 (37-85) | 23/15 | 24 (17-30) | NA | 47 (7-180) | 0/17/7/10/4 | D2 | EGC + AGC |
| OTG | 22 | 69 (38-86) | 17/5 | 24 (17-30) | NA | 30 (10-180) | 0/7/7/6/2 | |||||
| Kawamura et al[30], 2009 | Japan | RCS | LTG | 46 | 64 ± 10.4 | 10/36 | 22.8 ± 3.0 | 15/27/4 | NA | NA | D2 | EGC |
| OTG | 35 | 65.2 ± 10.7 | 10/25 | 22.9 ± 2.4 | 14/15/6 | NA | NA | |||||
| Sakuramoto et al[16], 2009 | Japan | RCS | LTG | 30 | 63.7 ± 9.2 | 12/18 | 21.9 ± 2.7 | 9/20/1 | 4.0 ± 2.9 | 0/25/2/3/0 | D1 + β, D2 | EGC + AGC |
| OTG | 44 | 67.2 ± 9.9 | 10/34 | 22.5 ± 3.6 | 8/28/8 | 6.1 ± 3.7 | 0/15/17/12/0 | |||||
| Du et al[27], 2010 | China | RCS | LTG | 82 | 60.4 ± 18.5 | 54/28 | 22.3 ± 2.6 | NA | 5.4 ± 1.4 | 0/3/36/43/0 | D2 | AGC |
| OTG | 94 | 57.8 ± 17.2 | 61/33 | 22.5 ± 2.4 | NA | 5.9 ± 1.6 | 0/6/31/57/0 | |||||
| Kim et al[33], 2011 | South Korea | RCS | LTG | 63 | 55.9 ± 12.2 | 43/20 | 22.7 ± 2.5 | 45/15/3 | 3.8 ± 2.1 | NA | D2 | EGC |
| OTG | 127 | 57.3 ± 11.1 | 81/46 | 23.0 ± 2.9 | 86/39/2 | 3.9 ± 2.7 | NA | |||||
| Eom et al[10], 2012 | South Korea | RCS | LTG | 100 | 54.9 ± 13.5 | 57/43 | 22.7 ± 2.8 | NA | 4.3 ± 2.9 | NA | D1 + β, D2 | EGC + AGC |
| OTG | 348 | 58.7 ± 11.5 | 254/94 | 23.8 ± 2.9 | NA | 4.4 ± 3.0 | NA | |||||
| Guan et al[17], 2012 | China | RCS | LTG | 41 | 60.7 ± 9.1 | 33/8 | NA | NA | NA | 0/18/20/3/0 | D2 | EGC + AGC |
| OTG | 56 | 57.8 ± 9.9 | 40/16 | NA | NA | NA | 0/25/25/6/0 | |||||
| Siani et al[38], 2012 | Italy | RCS | LTG | 25 | 65 ± 8.5 | 15/10 | NA | NA | NA | 0/6/5/14/0 | D1 + α/β, D2 | EGC + AGC |
| OTG | 25 | 66 ± 7.8 | 18/7 | NA | NA | NA | 0/4/5/16/0 | |||||
| Kim et al[32], 2013 | South Korea | RCS | LTG | 139 | 58 (30-84) | 86/53 | 23.6 (13.6-32.4) | 85/46/8 | 3.2 (0.2, 15) | NA | D2 | EGC + AGC |
| OTG | 207 | 56 (31-84) | 134/73 | 24.1 (16.7-35.2) | 137/52/18 | 4.0 (0.3, 22) | NA | |||||
| Jeong et al[29], 2013 | South Korea | RCS | LTG | 122 | 63.2 ± 11.2 | 89/33 | 23.1 ± 3.4 | 33/80/9 | NA | NA | D1 + β, D2 | EGC + AGC |
| OTG | 122 | 62.6 ± 11.7 | 93/29 | 23.5 ± 3.2 | 43/67/12 | NA | NA | |||||
| Lee et al[37], 2013 | South Korea | RCS | LTG | 50 | 50.6 ± 22.1 | 32/18 | 23.2 ± 3.7 | 34/11/5 | NA | 0/24/13/9/4 | D2 | EGC + AGC |
| OTG | 50 | 51 ± 22.6 | 32/18 | 23 ± 3.4 | 31/16/3 | NA | 0/24/13/9/4 |
Continuous variables are presented as means ± SD or median and range.
Pathological tumor stage (0/I/II/III/IV). PCS: Prospective comparative study; RCS: Retrospective comparative study; LTG: Laparoscopic total gastrectomy; OTG: Open total gastrectomy; BMI: Body mass index; NA: Not available; ASA: American Society of Anesthesiologists; LND: Lymph node dissection; EGC: Early gastric cancer; AGC: Advanced gastric cancer; M/F: Male/female.
Table 2.
Quality assessment scoring of included studies, according to NOS criterion
| Author, year | Selection | Comparability1 | Outcome assessment | Star Score | ||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Dulucq et al[28], 2005 | * | * | * | * | * | * | ****** | |
| Usui et al[9], 2005 | * | * | * | * | * | ***** | ||
| Kim et al[34], 2008 | * | * | * | * | **** | |||
| Mochiki et al[15], 2008 | * | * | * | * | * | * | ****** | |
| Topal et al[14], 2008 | * | * | * | ** | ** | * | ******** | |
| Kawamura et al[30], 2009 | * | * | * | ** | * | * | * | ******** |
| Sakuramoto et al[16], 2009 | * | * | * | ** | * | * | ******* | |
| Du et al[27], 2010 | * | * | * | ** | * | * | ******* | |
| Kim et al[33], 2011 | * | * | * | ** | * | * | ******* | |
| Eom et al[10], 2012 | * | * | * | * | * | ***** | ||
| Guan et al[17], 2012 | * | * | * | * | * | ***** | ||
| Siani et al[38], 2012 | * | * | * | * | * | * | * | ******* |
| Kim et al[32], 2013 | * | * | * | ** | * | ****** | ||
| Jeong et al[29], 2013 | * | * | * | ** | * | * | ******* | |
| Lee et al[37], 2013 | * | * | * | ** | * | * | * | ******** |
Based on Newcastle-Ottowa Scale with maximum of *** for selection, ****for comparability, and ** for outcome assessment.
Comparability variables are (1) age, (2) sex, (3) body mass index, (4) American Society of Anesthesiologists, (5) comorbidity, (6) tumor size and (7) tumor stage. Group comparable for (1)-(3) or (4)-(7) (if yes, two stars, one star if one of these three characteristics was not reported, even if there were no other differences between the two groups and other characteristics had been controlled; no points were assigned if the two groups differed).
Table 3.
Perioperative outcomes
| Author, year | Group | Operation time (min) | Intraoperative blood loss (mL) | No. of resected lymph nodes(n) | Time to first flatus (d) | Time to first oral intake(d) | Hospital stay(d) | Analgesics use (times) | Postoperative complications (%) | In-hospitalMortality (%) |
| Dulucq et al[28], 2005 | LTG | 183 ± 48 | 81 ± 107 | 24 ± 12 | 3.6 ± 1.2 | NA | 16.9 ± 3 | NA | 0 | 0 |
| OTG | 165 ± 60 | 125 ± 95 | 20 ± 8 | 4.7 ± 1.2 | NA | 24 ± 9 | NA | 18 | 9 | |
| Usui et al[9], 2005 | LTG | 280.1 ± 45.2 | 227.5 ± 148.1 | 28.0 ± 15.1 | 2.9 ± 0.9 | 5.7 ± 2.1 | 15.5 ± 3.9 | 2.1 ± 1.3 | NA | NA |
| OTG | 266.4 ± 48.2 | 393.1 ± 173.6 | 28.9 ± 14.3 | 4.2 ± 1.4 | 8.8 ± 1.3 | 23.2 ± 4.6 | 3.4 ± 4.4 | NA | NA | |
| Kim et al[34], 2008 | LTG | 527.5 ± 95.7 | NA | 27.2 ± 15.7 | 3.6 ± 0.9 | NA | 16.2 ± 7.1 | NA | 7.4 | 0 |
| OTG | 320.9 ± 75.8 | NA | 37.2 ± 15.7 | 4.1 ± 1.3 | NA | 16.0 ± 9.3 | NA | 24.2 | 0 | |
| Mochiki et al[15], 2008 | LTG | 254 ± 10 | 299 ± 50 | 26 ± 3 | NA | NA | 19 ± 3 | NA | 25 | 0 |
| OTG | 248 ± 12 | 758 ± 78 | 35 ± 4 | NA | NA | 29 ± 3 | NA | 16.7 | 0 | |
| Topal et al[14], 2008 | LTG | 187 ± 60 | 10.0 ± 98.8 | NA | NA | NA | NA | NA | 39.5 | 2.6 |
| OTG | 152.5 ± 25 | 450.0 ± 337.5 | NA | NA | NA | NA | NA | 40.9 | 4.5 | |
| Kawamura et al[30], 2009 | LTG | 291.9 ± 59.4 | 54.9 ± 45.3 | 48.5 ± 16.3 | 4.1 ± 1.0 | NA | 15.5 ± 3.3 | 6.9 ± 5.6 | 8.7 | 0 |
| OTG | 272.1 ± 76.8 | 304.3 ± 237.3 | 47.1 ± 21.5 | 4.3 ± 1.3 | NA | 18.8 ± 6.3 | 4.0 ± 3.2 | 22.9 | 0 | |
| Sakuramoto et al[16], 2009 | LTG | 313 ± 81 | 134 ± 98 | 43.2 ± 17.2 | 2.4 ± 1.1 | 4.9 ± 1.1 | 13.5 ± 2.7 | 6.8 ± 6.4 | 16.7 | 0 |
| OTG | 218 ± 53 | 407 ± 270 | 51.2 ± 22.1 | 3.3 ± 1.0 | 6.0 ± 2.1 | 18.2 ± 9.6 | 11.8 ± 11.0 | 27.3 | 0 | |
| Du et al[27], 2010 | LTG | 275 ± 78 | 156 ± 112 | 34.2 ± 13.5 | 3.5 ± 0.8 | 3.5 ± 0.8 | NA | NA | 9.8 | 0 |
| OTG | 212 ± 51 | 339 ± 162 | 36.4 ± 19.1 | 5.3 ± 1.3 | 5.3 ± 1.3 | NA | NA | 24.5 | 2.1 | |
| Kim et al[33], 2011 | LTG | 150.8 ± 31.2 | 179.7 ± 123.8 | 38.7 ± 15.7 | 3.3 ± 0.7 | 4.3 ± 1.7 | 8.1 ± 3.8 | 5.3 ± 4.9 | 12.7 | 0 |
| OTG | 131.2 ± 21.6 | 272.7 ± 209.6 | 35.6 ± 13.1 | 3.8 ± 0.8 | 5.6 ± 4.4 | 9.6 ± 5.3 | 3.6 ± 3.9 | 18.9 | 0 | |
| Eom et al[10], 2012 | LTG | 283.7 ± 84.1 | NA | 48.3 ± 16.4 | NA | NA | 12.6 ± 15.5 | NA | 27 | 1 |
| OTG | 198.5 ± 59.7 | NA | 49.8 ± 18.4 | NA | NA | 14.3 ± 16.7 | NA | 23.6 | 0.9 | |
| Guan et al[17], 2012 | LTG | 235.7 ± 38.5 | 104.2 ± 42.9 | 23.1 ± 8.0 | 3 ± 0.7 | 2.2 ± 0.9 | 9.7 ± 2.2 | NA | 4.9 | 0 |
| OTG | 211.5 ± 33.2 | 355.6 ± 51.3 | 24.2 ± 7.5 | 3.3 ± 0.4 | 3.1 ± 0.5 | 13.6 ± 3.6 | NA | 5.4 | 0 | |
| Siani et al[38], 2012 | LTG | 211 ± 23 | 250 ± 150 | 35 ± 18 | 2.1 ± 0.9 | NA | 10.5 ± 1.5 | NA | 16 | 0 |
| OTG | 185 ± 19 | 495 ± 190 | 40 ± 16 | 4.1 ± 1.5 | NA | 14.5 ± 3.1 | NA | 4 | 0 | |
| Kim et al[32], 2013 | LTG | 144 ± 104.3 | NA | 37 ± 24 | 3 ± 2 | 3 ± 12.3 | 7 ± 19.3 | 3 ± 24.5 | 10 | 0 |
| OTG | 137 ± 105 | NA | 34 ± 18.8 | 4 ± 2.3 | 5 ± 10 | 8 ± 9 | 4 ± 9.3 | 21.7 | 0 | |
| Jeong et al[29], 2013 | LTG | 289 ± 89 | 249 ± 204 | 42 ± 15 | 2.9 ± 0.8 | 3.9 ± 4.4 | 11.8 ± 11.8 | NA | 23.8 | 1.6 |
| OTG | 203 ± 78 | 209 ± 157 | 46 ± 17 | 3.0 ± 0.8 | 3.6 ± 3.3 | 10.8 ± 7.0 | NA | 17.2 | 0.9 | |
| Lee et al[37], 2013 | LTG | 258 ± 54 | 167.3 ± 135.2 | 48.4 ± 18.4 | 4 ± 1.2 | 5 ± 1.7 | 9.3 ± 4.2 | NA | 24 | 0 |
| OTG | 198 ± 57 | 178.4 ± 107 | 54.3 ± 20.5 | 4.5 ± 1.5 | 6.1 ± 2.5 | 11.7 ± 7.3 | NA | 32 | 0 |
LTG: Laparoscopic total gastrectomy; OTG: Open total gastrectomy; NA: Not available.
Table 4.
Results of meta-analysis comparing laparoscopic total gastrectomy vs open total gastrectomy
| Outcome of interest | No. of studies | No. of patients | OR/WMD | 95%CI | P value | Heterogeneity P value | I2 |
| Operative outcomes | |||||||
| Operation time (min) | 15 | 2022 | 48.25 | 31.15-65.35 | < 0.00001 | < 0.00001 | 93% |
| Intraoperative blood loss (mL) | 12 | 1168 | -201.19 | -296.50--105.87 | < 0.0001 | < 0.00001 | 98% |
| Postoperative recovery | |||||||
| Time to first flatus (d) | 12 | 1412 | -0.82 | -1.18--0.45 | < 0.0001 | < 0.00001 | 90% |
| Time to first oral intake (d) | 8 | 1266 | -1.3 | -1.84--0.75 | < 0.00001 | < 0.00001 | 82% |
| Hospital stay (d) | 13 | 1786 | -3.55 | -5.13--1.96 | < 0.0001 | < 0.00001 | 86% |
| Analgesics use (times) | 5 | 730 | -0.09 | -2.39-2.20 | 0.94 | 0.0008 | 79% |
| Postoperative complications | |||||||
| Overall complication | 14 | 1983 | 0.73 | 0.57-0.92 | 0.009 | 0.08 | 37% |
| Anastomotic leakage | 14 | 1983 | 1.6 | 0.88-2.91 | 0.12 | 0.68 | 0% |
| Anastomotic stenosis | 13 | 1923 | 1.22 | 0.68-2.21 | 0.50 | 0.95 | 0% |
| Ileus | 13 | 1923 | 1.26 | 0.69-2.30 | 0.46 | 0.85 | 0% |
| Bleeding | 13 | 1923 | 1.42 | 0.70-2.87 | 0.33 | 0.26 | 23% |
| Abdominal abscess | 13 | 1923 | 0.53 | 0.28-1.03 | 0.06 | 0.37 | 8% |
| Wound problems | 13 | 1923 | 0.39 | 0.21-0.72 | 0.002 | 0.75 | 0% |
| Oncological outcomes | |||||||
| Positive resection margins | 5 | 698 | 0.57 | 0.03-9.55 | 0.69 | - | - |
| No. of resected lymph nodes | 14 | 1962 | -2.49 | -5.18-0.21 | 0.07 | < 0.00001 | 74% |
| Proximal resection margin (cm) | 4 | 1160 | -0.26 | -0.54-0.01 | 0.06 | 0.65 | 0% |
| Distal resection margin (cm) | 4 | 1160 | 0.32 | -0.05-0.68 | 0.09 | 0.22 | 32% |
Operative outcomes
“Operation time” was reported in all studies. The analysis showed that the LTG group had a significantly longer operation time compared with the OTG group (WMD 48.25 min, 95%CI: 31.15-65.35, P < 0.00001), albeit with a significant heterogeneity (I2 = 93%). Data from 12 studies[9,14-17,27-29,31,33,37,38] were pooled together to obtain the mean intraoperative blood loss in the two groups. LTG was associated with a significantly lower intraoperative blood loss compared with OTG (WMD -201.19 mL, 95%CI: -296.50--105.87 mL, P < 0.0001), with a significant heterogeneity (I2 = 98%). Forest plots for operative outcomes are shown in Figure 2.
Figure 2.
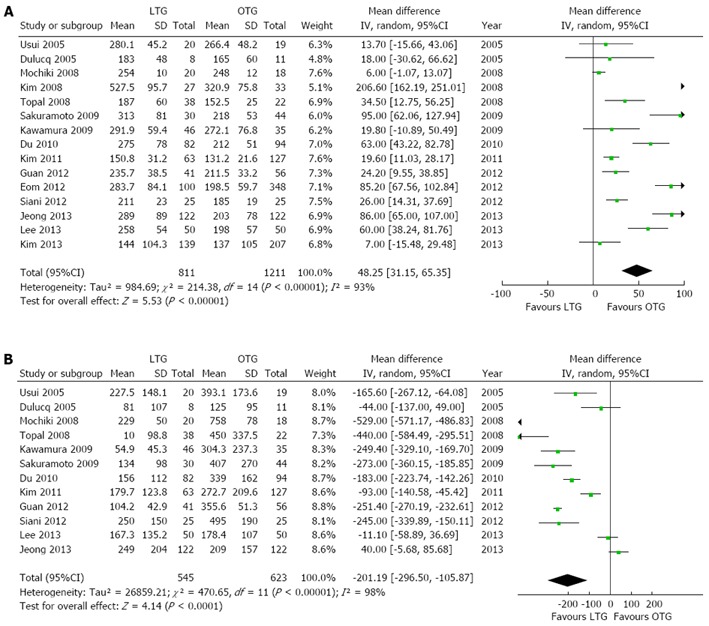
Forest plots illustrating results of operative outcomes in the form of a meta-analysis comparing laparoscopic total gastrectomy vs open total gastrectomy for gastric cancer. Pooled weighted mean difference (WMD) with 95%CI was calculated using the random effects model. A: Operation time; B: Intraoperative blood loss. LTG: Laparoscopic total gastrectomy; OTG: Open total gastrectomy.
Postoperative recovery
Twelve studies[9,16,17,27-30,32-34,37,38] reported the time to first flatus and eight studies[9,16,17,27,29,32,33,37] reported data on oral intake post-surgery. Our analyses showed that patients undergoing LTG had a quicker recovery of intestinal motility compared with the OTG group. The time to first flatus (WMD -0.82, 95%CI: -1.18--0.45, P < 0.0001) and the time to first oral intake (WMD -1.30, 95%CI: -1.84--0.75, P < 0.00001) were significantly shorter in the LTG group compared with the OTG group. Analysis of the 13 studies[9,10,15-17,28-30,32-34,37,38] that reported the duration of hospital stay indicated that LTG was associated with a significantly shorter postoperative hospital stay compared with OTG (WMD -3.55, 95%CI: -5.13--1.96, P < 0.0001). However, there was no statistically significant difference between the two groups in the use of analgesics post-surgery (WMD -0.09, 95%CI: -2.39-2.20, P = 0.94). Forest plots for postoperative recovery outcomes are shown in Figure 3.
Figure 3.
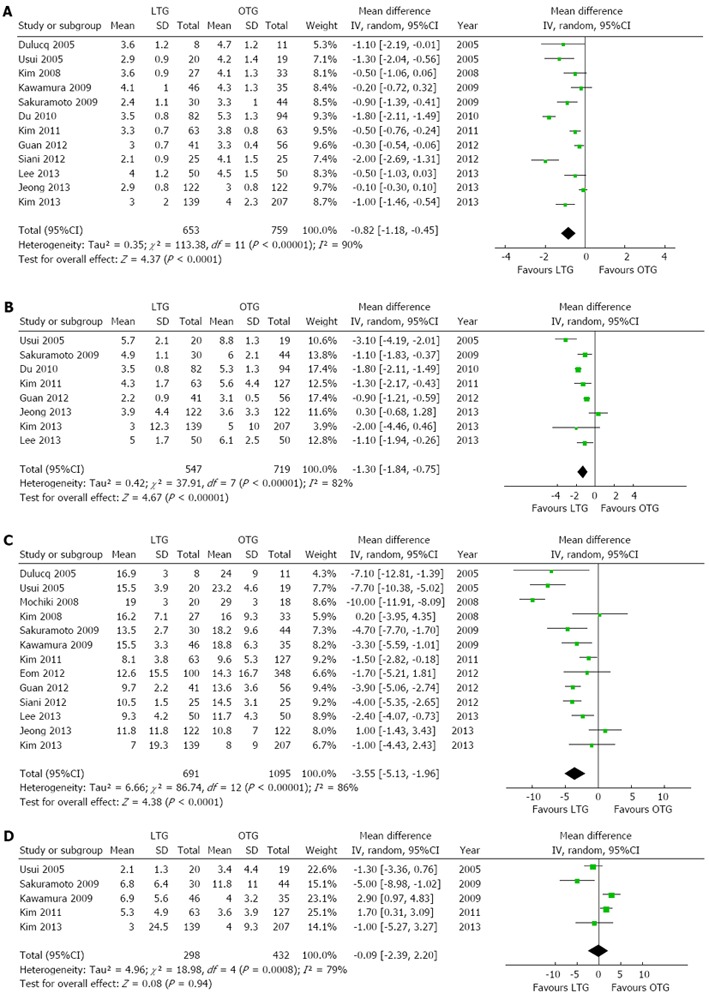
Forest plots illustrating results of postoperative recovery in the form of a meta-analysis comparing laparoscopic total gastrectomy vs open total gastrectomy for gastric cancer. Pooled weighted mean difference (WMD) with 95%CI was calculated using the random-effects model. A: Time to first flatus; B Time to first oral intake; C: Hospital stay; D: Analgesic use. LTG: Laparoscopic total gastrectomy; OTG: Open total gastrectomy.
Postoperative complications
A pooled analysis of 14 studies[10,14-17,27-30,32-34,37,38] indicated that the overall complication rate was significantly lower in the LTG group compared with the OTG group (OR = 0.73, 95%CI: 0.57-0.92, P = 0.009). Also, the analysis of 13 studies[10,15-17,27-30,32-34,37,38] suggested that patients in the LTG group had significantly fewer wound-related complications compared with the OTG group (OR = 0.39, 95%CI: 0.21-0.72, P = 0.002). However, there were no significant differences in the rate of anastomotic leak (OR = 1.6, 95%CI: 0.88-2.91, P = 0.12), anastomotic stenosis (OR = 1.22, 95%CI: 0.68-2.21, P = 0.50), ileus (OR 1.26, 95%CI: 0.69,2.30; P = 0.46), bleeding (OR = 1.42, 95%CI: 0.70-2.87; P = 0.33), abdominal abscess (OR = 0.53, 95%CI: 0.28-1.03, P = 0.06) or mortality (OR = 0.74, 95%CI: 0.24-2.31, P = 0.61) between the two groups. Forest plots for postoperative outcomes are shown in Figure 4.
Figure 4.
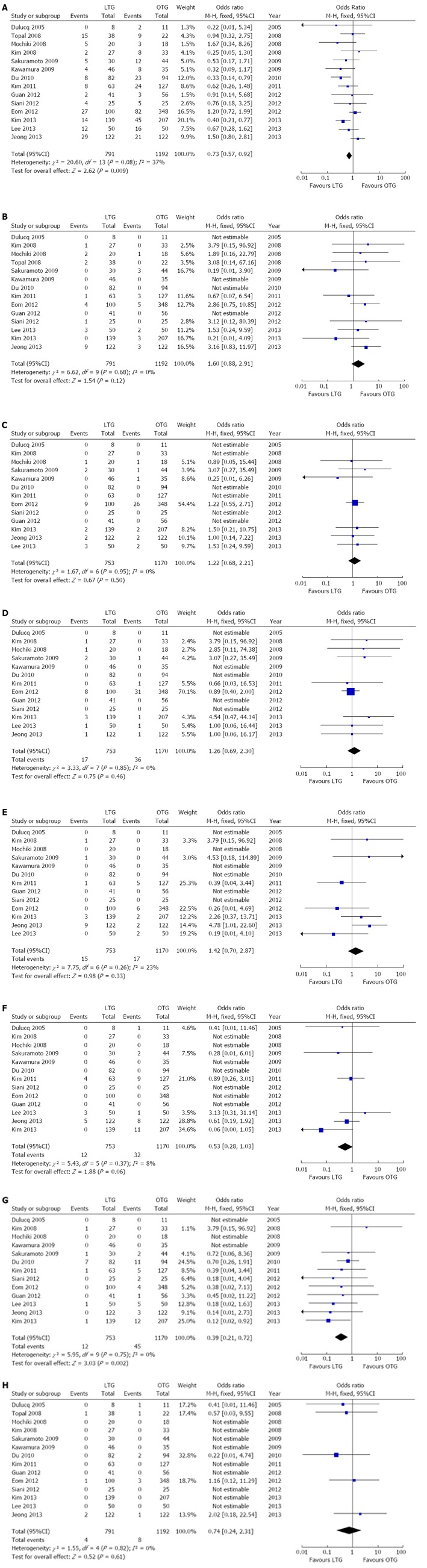
Forest plots illustrating results of postoperative complications in the form of a meta-analysis comparing laparoscopic total gastrectomy vs open total gastrectomy for gastric cancer. Pooled odds ratio (OR) with 95%CI was calculated using the fixed-effects model. A: Overall complication rate; B: Anastomotic leak; C: Anastomotic Stenosis; D: Ileus; E: Bleeding; F: Abdominal abscess; G: Wound-related complications; H: Mortality. LTG: Laparoscopic total gastrectomy; OTG: Open total gastrectomy.
Oncological outcomes
All included studies reported data on the number of lymph nodes retrieved; there was no significant difference between the two groups (WMD -2.49, 95%CI: -5.18-0.21, P = 0.07), albeit with a significant heterogeneity in the result (I2 = 74%). Five studies[14,17,27,28,32] reported data on positive resection margins; in only one study[14], resection margins were found to be positive in one patient each from the LTG and OTG groups and with no significant difference between the two groups (OR = 0.57, 95%CI: 0.03-9.55, P = 0.69). There were also no significant differences in the lengths of the proximal resection margin (WMD -0.26, 95%CI: -0.54-0.01, P = 0.06) and distal resection margin (WMD 0.32, 95%CI: -0.05-0.68, P = 0.09) between the two groups when data from four studies[10,27,32,33] were pooled. Seven studies reported data on long-term survival following the two procedures[10,15,16,27,28,37,38]. Lee et al[37] reported no significant difference in the disease-specific survival rate between the LTG and OTG groups at a median follow-up of 50 months; there were also no significant differences reported in the disease-free survival rate (100% vs 90.9%, P = 0.5) and the cumulative survival rate (91.5% vs 95.2%, P = 0.618) in patients with stage I cancer (TNM) between the LTG and OTG groups. Eom et al[10] reported no significant difference in the disease-free survival rates between the LTG and OTG groups, after adjustment for five variables (age, tumor size, Lauren classification, depth of invasion and lymph node metastasis). Mochiki et al[15] reported no significant difference in the cumulate 5-year or disease-specific survival rates between the LTG and OTG groups, while Siani et al[38] reported 5-year overall and disease free survival rates of 55.7% and 54.2% in the LTG group and 52.9% and 52.1% in the OTG group respectively, with no statistically significant differences. However, as the duration of follow-up varied between studies, it was difficult to compare the survival rates. Forest plots for oncological outcomes are shown in Figure 5.
Figure 5.
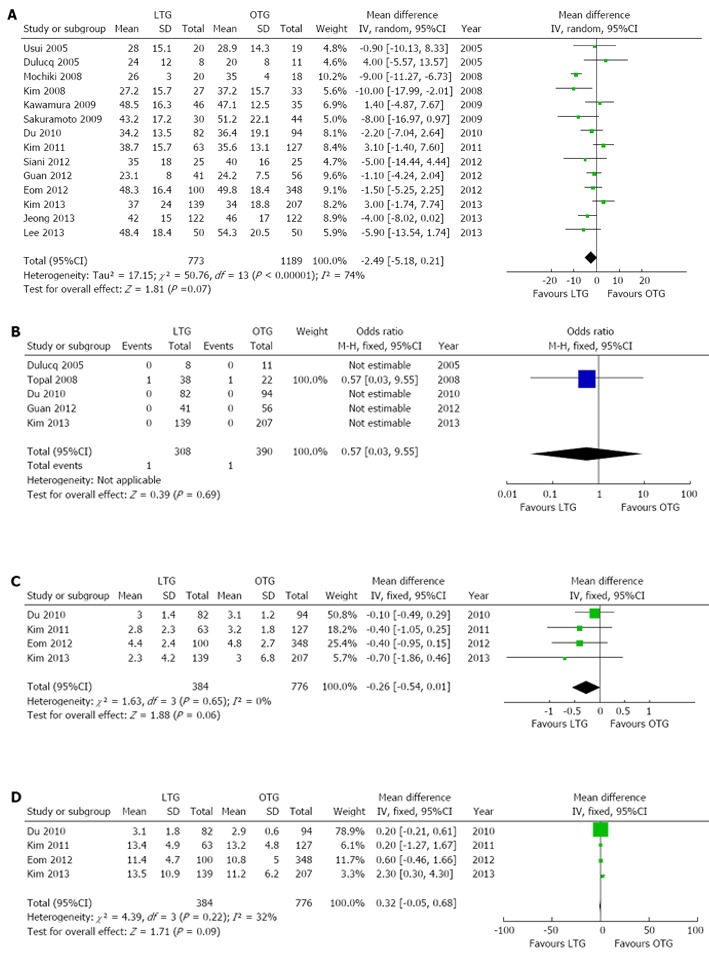
Forest plots illustrating results of oncological outcomes in the form of a meta-analysis comparing laparoscopic total gastrectomy vs open total gastrectomy for gastric cancer. Pooled weighted mean difference (WMD) or odds ratio (OR) with 95%CI were calculated using the fixed or random-effects model. A: No. of resected lymph nodes; B: Positive resection margins; C: Proximal resection margin; D: Distal resection margin. LTG: Laparoscopic total gastrectomy; OTG: Open total gastrectomy.
Sensitivity and subgroup analysis
Sensitivity analyses were performed by removing individual studies from the data and analyzing the effect on the overall results to identify sources of significant heterogeneity. These exclusions did not alter the results obtained from the cumulative analyses. Subgroup analyses were undertaken for all outcome measures by including only high quality studies. Analysis of the high-quality studies showed that there were no significant differences for any of the outcomes. These are shown in Figure 6.
Figure 6.
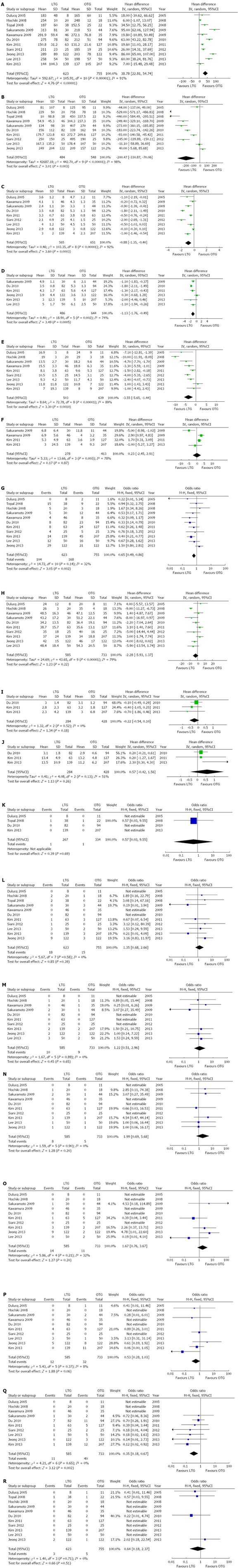
Forest plots illustrating results of all outcomes in the form of a meta-analysis comparing laparoscopic total gastrectomy vs open total gastrectomy for gastric cancer. Pooled weighted mean difference (WMD) or odds ratio (OR) with 95%CI were calculated using the fixed or random-effects model. A: Operation time; B: Intraoperative blood loss; C: Time to first flatus; D: Time to first oral intake; E: Hospital stay; F: Analgesics use; G: Postoperative complications; H: No. of resected lymph nodes; I: Proximal resection margin; J: Distal resection margin; K: Positive resection margins; L: Anastomotic leakage; M: Anastomotic Stenosis; N: Ileus; O: Bleeding; P: Abdominal abscess; Q: Wound-related complications; R: Mortality. LTG: Laparoscopic total gastrectomy; OTG: Open total gastrectomy.
Publication bias
The funnel plot based on the operation time is shown in Figure 7. There was no broad evidence of publication bias, as none of the studies lay outside the 95%CI limits.
Figure 7.
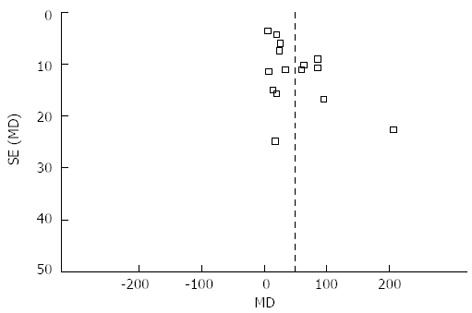
Funnel plot of operation time of all included studies.
DISCUSSION
Laparoscopic surgery is being used increasingly to treat gastric cancer, and has been shown to have many advantages over open surgery. However, LTG is less widely practiced compared with laparoscopic distal gastrectomy because of the technical challenges it poses and the absence of compelling evidence to substantiate its use[9]. Technical advances, better instrumentation and increasing surgical experience in the procedure are aiding its increasing application to treat of early and advanced gastric cancer. The aim of the current study was to inform future surgical practice by comparing the technical feasibility, effectiveness, and safety of LTG with OTG in the treatment of early and advanced gastric cancer, using a systematic review and meta-analysis of published comparative studies.
Our analyses indicated that the operation time was significantly longer in the LTG group than in the OTG group. This may be because LTG is more technically demanding than OTG and may result from the learning curve associated with the procedure[9,10,35]. While adequate training in laparoscopic techniques is necessary, it was concluded that an experienced laparoscopic surgeon would not require any more time to perform LTG compared with OTG[15]. In one study, the operation time for LTG in the later period was significantly shorter than in the early period; this related to the experience gained by the surgeon over the period of the study[34]. Further development in surgical techniques, especially for anastomosis and new instruments, may further decrease the operation time for LTG[10]. In our study, LTG was associated with a significantly lower intraoperative blood loss, which depends considerably on a surgeon’s skill and experience[34].
The times to first flatus and to first oral intake were significantly shorter in the LTG group compared with the OTG group, which suggests that intestinal motility recovered more quickly in the LTG group. Also, the period of hospital stay was significantly shorter in the LTG group. LTG is a less invasive procedure and is associated with less surgical trauma. This results in a reduced inflammatory response and better glucose tolerance, which may aid a quicker recovery[19,30]. Pain following LTG subsides earlier when compared to OTG[18]. However, our study showed no significant difference in the postoperative use of analgesics between the two groups. A quicker recovery and shorter hospital stay have important cost and quality of life implications for the wider use of LTG in the treatment of gastric cancer.
Total gastrectomy has often been described as high-risk[39,40] and LTG is technically demanding[9,10]. Common postoperative complications associated with LTG include anastomotic leak, anastomotic stenosis and luminal bleeding[37]. The anastomotic complications could be caused by excessive traction applied on the esophagus and jejunal limb mobilization[10] or may reflect the learning curve associated with LTG[37]. In our study, the overall complication rate was significantly lower in the LTG group compared with the OTG group. Also, there were significantly fewer wound-related complications in the LTG group. However, there were no significant differences in rate of anastomotic leak, anastomotic stenosis, bleeding, abdominal abscess and postoperative mortality in the two groups. These results indicate that LTG is a safe procedure.
While lymph node metastasis is associated with a poor prognosis in gastric cancer, the extent of lymph node dissection required is open to debate. Many surgeons believe that D1+α or β dissection is adequate for early gastric cancer, and D2 dissection is optimal for advanced gastric cancer, although this remains controversial[41,42]. Surgical removal of at least 15 lymph nodes is advocated in gastric cancer[43]. The mean number of harvested lymph nodes in all included studies was more than 15. The surgical approach did not appear to influence the lymph node yield; however, LTG with extended lymph node dissection may require further refinement of the operative technique and improved instrumentation, and should be performed with caution by surgeons with adequate experience in laparoscopic gastrectomy[29]. Another major concern of laparoscopic resection for gastric cancer is obtaining clear proximal esophageal and distal duodenal margins[17]. Five included studies reported tumor margins, but only one study reported positive resection margins in one patient each in LTG and OTG, respectively; there was no statistically significant difference between the two groups. Our analyses also showed that there was no significant difference in the lengths of the proximal and distal resection margins between the two groups. Seven studies reported data on long-term survival following the two procedures. However, as the duration of follow-up varied between studies, it was difficult to compare them.
Our study has some limitations. Firstly, all the studies included were non-randomized, because of a lack of randomized controlled trials. Secondly, there was significant heterogeneity in the studies with respect to the extent of lymph node dissection, tumor staging and surgical anastomosis techniques. Also, there were differences in the number of patients in the two groups and between studies.
In conclusion, compared with OTG, LTG with regional lymph node dissection for early and advanced gastric cancer is safe and effective; with comparable short-term oncological outcomes; lower intraoperative blood loss and overall complication rates; fewer wound-related complications; quicker recovery of gastrointestinal motility and a shorter hospital stay, albeit with a longer operating time. However, there is a need to develop well-designed, adequately powered, prospective, multicenter, randomized controlled trials, investigating LTG with adequate long-term follow-up, before recommending its wider use in surgical practice.
COMMENTS
Background
Since laparoscopic total gastrectomy (LTG) was first reported in 1999, it has been used increasingly to treat gastric cancer as result of technical advances and improved instrumentation. However, compared with conventional open total gastrectomy (OTG), the safety and efficacy of LTG is not known.
Research frontiers
To conduct a meta-analysis comparing the safety and effectiveness of LTG with OTG in patients with gastric cancer; the available perioperative and oncological outcomes were included in this study.
Innovations and breakthroughs
Based on this meta-analysis, when compared with OTG, LTG for early and advanced gastric cancer is safe and effective; with comparable short-term oncological outcomes; lower intraoperative blood loss and overall complication rates; fewer wound-related complications; quicker recovery of gastrointestinal motility and a shorter hospital stay, albeit with a longer operating time.
Applications
LTG is safe, effective and offers some advantages over OTG in the treatment of early and advanced gastric cancer. However, well-designed prospective multicenter, randomized controlled trials investigating the advantage of LTG with adequate long-term follow-up need to be performed before recommending its wider use in surgical practice.
Peer review
In the future, LTG will be rapidly developed in the field of abdominal minimally invasive surgery. This is a well-written study that clarifies some advantages of LTG in the treatment of patients with early and advanced gastric cancer. This study may be interesting for gastrointestinal surgeons worldwide.
Footnotes
Supported by UK/China Postgraduate Scholarships for Excellence, an NIHR Translational Research Fellowship and a Royal College of Surgeons of England-Ethicon Research Fellowship grant
P- Reviewer: Nishiyama M S- Editor: Wen LL L- Editor: Stewart GJ E- Editor: Zhang DN
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kim JP. Current status of surgical treatment of gastric cancer. J Surg Oncol. 2002;79:79–80. doi: 10.1002/jso.10050. [DOI] [PubMed] [Google Scholar]
- 3.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 4.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 5.Ryu KW, Kim YW, Lee JH, Nam BH, Kook MC, Choi IJ, Bae JM. Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann Surg Oncol. 2008;15:1625–1631. doi: 10.1245/s10434-008-9845-x. [DOI] [PubMed] [Google Scholar]
- 6.Lee SE, Kim YW, Lee JH, Ryu KW, Cho SJ, Lee JY, Kim CG, Choi IJ, Kook MC, Nam BH, et al. Developing an institutional protocol guideline for laparoscopy-assisted distal gastrectomy. Ann Surg Oncol. 2009;16:2231–2236. doi: 10.1245/s10434-009-0490-9. [DOI] [PubMed] [Google Scholar]
- 7.Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 8.Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230–234. doi: 10.1007/s101200050069. [DOI] [PubMed] [Google Scholar]
- 9.Usui S, Yoshida T, Ito K, Hiranuma S, Kudo SE, Iwai T. Laparoscopy-assisted total gastrectomy for early gastric cancer: comparison with conventional open total gastrectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:309–314. doi: 10.1097/01.sle.0000191589.84485.4a. [DOI] [PubMed] [Google Scholar]
- 10.Eom BW, Kim YW, Lee SE, Ryu KW, Lee JH, Yoon HM, Cho SJ, Kook MC, Kim SJ. Survival and surgical outcomes after laparoscopy-assisted total gastrectomy for gastric cancer: case-control study. Surg Endosc. 2012;26:3273–3281. doi: 10.1007/s00464-012-2338-9. [DOI] [PubMed] [Google Scholar]
- 11.Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010;211:e25–e29. doi: 10.1016/j.jamcollsurg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Usui S, Nagai K, Hiranuma S, Takiguchi N, Matsumoto A, Sanada K. Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument “Endo-PSI (II)” and circular stapler. Gastric Cancer. 2008;11:233–237. doi: 10.1007/s10120-008-0481-8. [DOI] [PubMed] [Google Scholar]
- 13.Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc. 2009;23:2624–2630. doi: 10.1007/s00464-009-0461-z. [DOI] [PubMed] [Google Scholar]
- 14.Topal B, Leys E, Ectors N, Aerts R, Penninckx F. Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg Endosc. 2008;22:980–984. doi: 10.1007/s00464-007-9549-5. [DOI] [PubMed] [Google Scholar]
- 15.Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, Aihara R, Asao T, Kuwano H. Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc. 2008;22:1997–2002. doi: 10.1007/s00464-008-0015-9. [DOI] [PubMed] [Google Scholar]
- 16.Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, Yamashita K, Watanabe M. Laparoscopy-assisted pancreas- and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc. 2009;23:2416–2423. doi: 10.1007/s00464-009-0371-0. [DOI] [PubMed] [Google Scholar]
- 17.Guan G, Jiang W, Chen Z, Liu X, Lu H, Zhang X. Early results of a modified splenic hilar lymphadenectomy in laparoscopy-assisted total gastrectomy for gastric cancer with stage cT1-2: a case-control study. Surg Endosc. 2013;27:1923–1931. doi: 10.1007/s00464-012-2688-3. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura H, Homma S, Yokota R, Watarai H, Yokota K, Kondo Y. Assessment of pain by face scales after gastrectomy: comparison of laparoscopically assisted gastrectomy and open gastrectomy. Surg Endosc. 2009;23:991–995. doi: 10.1007/s00464-008-0090-y. [DOI] [PubMed] [Google Scholar]
- 19.Natsume T, Kawahira H, Hayashi H, Nabeya Y, Akai T, Horibe D, Shuto K, Akutsu Y, Matsushita K, Nomura F, et al. Low peritoneal and systemic inflammatory response after laparoscopy-assisted gastrectomy compared to open gastrectomy. Hepatogastroenterology. 2011;58:659–662. [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Athanasiou T, Al-Ruzzeh S, Kumar P, Crossman MC, Amrani M, Pepper JR, Del Stanbridge R, Casula R, Glenville B. Off-pump myocardial revascularization is associated with less incidence of stroke in elderly patients. Ann Thorac Surg. 2004;77:745–753. doi: 10.1016/j.athoracsur.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, Antoniou A. Laparoscopic versus open hepatic resections for benign and malignant neoplasms--a meta-analysis. Surgery. 2007;141:203–211. doi: 10.1016/j.surg.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6:341–350. doi: 10.1002/sim.4780060325. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du J, Zheng J, Li Y, Li J, Ji G, Dong G, Yang Z, Wang W, Gao Z. Laparoscopy-assisted total gastrectomy with extended lymph node resection for advanced gastric cancer--reports of 82 cases. Hepatogastroenterology. 2010;57:1589–1594. [PubMed] [Google Scholar]
- 28.Dulucq JL, Wintringer P, Stabilini C, Solinas L, Perissat J, Mahajna A. Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc. 2005;19:933–938. doi: 10.1007/s00464-004-2172-9. [DOI] [PubMed] [Google Scholar]
- 29.Jeong O, Jung MR, Kim GY, Kim HS, Ryu SY, Park YK. Comparison of short-term surgical outcomes between laparoscopic and open total gastrectomy for gastric carcinoma: case-control study using propensity score matching method. J Am Coll Surg. 2013;216:184–191. doi: 10.1016/j.jamcollsurg.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura H, Yokota R, Homma S, Kondo Y. Comparison of invasiveness between laparoscopy-assisted total gastrectomy and open total gastrectomy. World J Surg. 2009;33:2389–2395. doi: 10.1007/s00268-009-0208-y. [DOI] [PubMed] [Google Scholar]
- 31.Kawamura H, Yokota R, Homma S, Kondo Y. Comparison of respiratory function recovery in the early phase after laparoscopy-assisted gastrectomy and open gastrectomy. Surg Endosc. 2010;24:2739–2742. doi: 10.1007/s00464-010-1037-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and open total gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013;23:323–331. doi: 10.1089/lap.2012.0389. [DOI] [PubMed] [Google Scholar]
- 33.Kim MG, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS. The effects of laparoscopic assisted total gastrectomy on surgical outcomes in the treatment of gastric cancer. J Korean Surg Soc. 2011;80:245–250. doi: 10.4174/jkss.2011.80.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SG, Lee YJ, Ha WS, Jung EJ, Ju YT, Jeong CY, Hong SC, Choi SK, Park ST, Bae K. LATG with extracorporeal esophagojejunostomy: is this minimal invasive surgery for gastric cancer? J Laparoendosc Adv Surg Tech A. 2008;18:572–578. doi: 10.1089/lap.2007.0106. [DOI] [PubMed] [Google Scholar]
- 35.Kunisaki C, Makino H, Kosaka T, Oshima T, Fujii S, Takagawa R, Kimura J, Ono HA, Akiyama H, Taguri M, et al. Surgical outcomes of laparoscopy-assisted gastrectomy versus open gastrectomy for gastric cancer: a case-control study. Surg Endosc. 2012;26:804–810. doi: 10.1007/s00464-011-1956-y. [DOI] [PubMed] [Google Scholar]
- 36.Kuwabara K, Matsuda S, Ishikawa KB, Horiguchi H, Fujimori K. Association of operating time and gastrectomy with initiation of postoperative oral food intake. Dig Surg. 2011;28:157–162. doi: 10.1159/000323626. [DOI] [PubMed] [Google Scholar]
- 37.Lee MS, Lee JH, Park do J, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013;27:2598–2605. doi: 10.1007/s00464-013-2796-8. [DOI] [PubMed] [Google Scholar]
- 38.Siani LM, Ferranti F, De Carlo A, Quintiliani A. Completely laparoscopic versus open total gastrectomy in stage I-III/C gastric cancer: safety, efficacy and five-year oncologic outcome. Minerva Chir. 2012;67:319–326. [PubMed] [Google Scholar]
- 39.Bittner R, Butters M, Ulrich M, Uppenbrink S, Beger HG. Total gastrectomy. Updated operative mortality and long-term survival with particular reference to patients older than 70 years of age. Ann Surg. 1996;224:37–42. doi: 10.1097/00000658-199607000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 41.Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 42.McCulloch P, Niita ME, Kazi H, Gama-Rodrigues JJ. Gastrectomy with extended lymphadenectomy for primary treatment of gastric cancer. Br J Surg. 2005;92:5–13. doi: 10.1002/bjs.4839. [DOI] [PubMed] [Google Scholar]
- 43.Kwon SJ. Evaluation of the 7th UICC TNM Staging System of Gastric Cancer. J Gastric Cancer. 2011;11:78–85. doi: 10.5230/jgc.2011.11.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]


