Abstract
Myosin description in human laryngeal muscles is incomplete, but evidence suggests the presence of type I, IIA, IIX, and tonic myosin heavy chain (MHC) fibers. This study describes the unloaded shortening velocity (V0) of chemically skinned laryngeal muscle fibers measured by the slack test method in relation to MHC content. Skeletal fibers from human laryngeal and limb muscle biopsy specimens were obtained for determination of V0, and subsequently, glycerol–sodium dodecyl sulfate–polyacrylamide gel electrophoresis was used to determine the MHC isoform content. The fibers from human limb muscle had shortening speeds similar to those in previous reports on human skeletal fibers. Type I, IIA, and IIX fibers of laryngeal muscle had shortening speeds similar to those of fibers from limb muscle, but laryngeal fibers with heterogeneous MHC expression had a wide range of shortening speeds, some being nearly twice as fast as limb fibers. In addition. MHC isoform bands from human extraocular muscle comigrated with some bands from laryngeal muscle — a finding suggesting that extraocular myosin may also be expressed.
Keywords: laryngeal muscle fibers, myosin heavy chain, unloaded shortening velocity
INTRODUCTION
The shortening velocity of mammalian skeletal muscle fibers is principally determined by variations in isoforms of the protein myosin. Myosin is a hexomeric molecule that has 3 basic functions: adenosine triphosphatase (ATPase) enzymatic activity, binding to actin, and sarcomeric thick filament formation. The speed of fiber shortening is correlated to enzymatic activity of the thick filament,1 and myofibrillar ATPase activity is isolated to the myosin heavy chain (MHC) portion of the myosin molecule.2 As such, there are many reports that positively correlate the type and amount of MHC isoform content to cellular shortening speed. The MHC isoforms typically found in somatic skeletal muscle include types I, IIA, IIB, and IIX. The relative shortening speeds of fibers that homogeneously express 1 isoform are IIB > IIX > IIA > I. However, when tested in various muscles and species, this structure-function correlation is often variable, especially where type II isoforms are concerned. Also, MHC isoform coexpression in somatic muscle fibers is relatively common,3 with isoforms usually seen in combinations of I–IIA, IIA–IIX, and IIX–IIB. The contractile velocity in coexpressing fibers may span the entire range of both isoforms, such that correlation of shortening speed to the type and amount of MHC isoforms is further complicated. Some cranial muscles express novel MHC isoforms, which correlate to distinctive shortening speeds relative to somatic fibers — so much so that in theory these myosins evolved to serve highly specialized locomotor functions.4,5 The jaw-closing muscles of all carnivores and primates investigated to date (except the lesser panda and human) express a type II masticatory MHC (IIM) that has a twitch contraction speed faster than that of fast limb muscles. Extraocular muscles are even faster than chewing muscles, with twitch contraction speeds as fast as about 7.5 ms.6 Mammalian extraocular muscles also have a unique MHC termed extraocular myosin (EOM). Unlike chewing muscle fibers, which usually homogeneously express type IIM to achieve distinctively fast contraction, extraocular fibers coexpress EOM with other isoforms.
One of the last cranial muscle groups to be investigated for structural-functional relationships of myosin were the intrinsic laryngeal muscles. DelGaudio et al7 were the first to report an “atypical” MHC isoform expressed in rat posterior cricoarytenoid (PCA) and thyroarytenoid (TA) muscles that was identified by a distinctive migration pattern on glycerol–sodium dodecyl sulfate–polyacrylamide gel electrophoresis (glycerol-SDS-PAGE). This isoform was termed “type IIL” when present in laryngeal muscle, even though it comigrated with the EOM species from rat lateral rectus muscle. Lucas et al8 subsequently demonstrated the presence of EOM expression in rabbit TA muscle by tissue staining with monoclonal antibody specific for EOM. Preliminary evidence also suggested that transcripts from a novel MHC gene were expressed in rat TA muscle,9 but further study determined that these transcripts were from the EOM gene, and not a novel laryngeal myosin.10 Finally, electrophoretic analyses on dogs11 and humans12 were unable to identify additional MHC species comparable to “IIL” or EOM MHC. Two reports, however. leave open the possibility that atypical or novel MHC isoforms may exist in human intrinsic laryngeal muscles. Shiotani et al13 have identified typical MHCs I, IIA, and IIB by electrophoresis in human laryngeal muscle and, in addition, a protein band with mobility similar to that of the rat IIL reported by Del-Gaudio et al that does not react with EOM antibodies. A subsequent report found a similar band on glycerol gels in TA and PCA muscles of a 7-month-old infant, but adults 40 years and older did not contain this protein species.14
In whole muscle preparations, evaluation of isometric twitch contraction speed demonstrates shortening for TA muscle as fast as 6.5 to 14 ms in rabbits.15 dogs,16 primates,17 and cats.18 In cats18 and dogs,16 isometric contraction speeds in PCA muscles range from 22 to 40 ms, which are comparable to speeds of fast-contracting limb muscles such as the rabbit tibialis anterior (24 to 28.5 ms)15 and the cat extensor digitorum longus (40 ms).6 Because whole muscle preparations that are comparable to isometric contraction experiments in animals are impossible to perform on humans, investigators have relied upon indirect assessment of contraction speeds. A study of changes in human vocal fold vibration after electrical stimulation of laryngeal muscle estimated TA contraction speed to be about 35 ms.19 More recently. Luschei et al20 attempted to determine the speed of laryngeal muscle motor unit recruitment by measuring interspike interval variability during phonation. According to their data, the contraction speed of human laryngeal muscle is not faster than that of limb muscles such as the tibialis anterior or biceps. An immunohistochemical analysis has determined that a tonic MHC isoform is expressed in human TA muscle fibers,21 indicating that these fibers should be optimally suited for very slow contraction speeds. Collectively, these recent findings from humans estimate that the shortening speeds in laryngeal muscle are about the same as or even slower than limb muscles and, hence, very different from the findings of direct work on other mammalian larynges.
In summary, investigations to determine structure-function relationships of myosin in human laryngeal muscles are lacking. Further, most of the recent evidence on human laryngeal muscle suggests that their shortening speeds are not comparable to those of other mammals. Accordingly, the present study attempts to determine the relationship between myosin isoform content and unloaded shortening velocity (V0) in single fibers from human laryngeal muscles.
MATERIALS AND METHODS
Materials
Samples of TA and PCA muscles were obtained from 4 patients who underwent laryngectomy as a treatment for cancer. Presurgical diagnostic laryngeal evaluation by flexible laryngoscopy was done to ensure that normal neuromotor laryngeal function (ie, normal breathing, swallowing, and phonation) was present in these subjects. No individuals with vocal fold paralysis were included. Excision of 3 TA muscles and 2 PCA muscles from uninvolved areas of the larynx was performed immediately after laryngectomy and before pathology submission. The subject population was of mixed race and sex and had an average age of 67 years (range, 50 to 78 years). Two control muscle biopsy samples were obtained from vastus muscles (24-year-old woman and 46-year-old man), I sample was obtained from a tibialis muscle (33-year-old man), and 1 sample was obtained from an external oblique muscle (57-year-old woman). The control muscles were functionally normal and were obtained from a population being treated by an orthopedic oncology surgeon for removal of a tumor that did not involve the sampled muscle. The samples for this study were obtained according to the guidelines established by the university's Internal Review Board for Human Subjects.
Biochemistry
The samples of whole muscle were minced and then homogenized in buffer containing 0.1 mol/L potassium chloride, 5 mmol/L ethylenediaminetetraacetic acid (EDTA), 5 mmol/L ethyleneglycoltetraacetic acid (EGTA), and 1 mmol/L dithiothreitol, pH 7.0, and the contractile proteins were sedimented by centrifugation.22 The final pellet was resuspended in electrophoresis sample buffer (0.15 mol/L Tris–hydrochloric acid [HCl], 4% SDS, 20% glycerol, 10% β-mercaptoethanol, and 0.002% bromophenol blue, pH 6.8). These protein samples were vortexed, heated to 100°C for 5 minutes, and then stored at −70°C until electrophoresis was carried out. Individual segments of single fibers that were used for V0 measurements were placed in sample buffer, ultrasonicated for 15 minutes, and then heated and frozen.
Discontinuous glycerol-SDS-PAGE was conducted on 0.75-mm-thick separating gels of 9% (wt/vol) acrylamide cross-linked with bisacrylamide (200:1.0, acrylamide:bisacrylamide), 10% SDS, and 6.0% glycerol, buffered with 0.75 mol/L Tris-HCl at pH 8.8. The stacking gel contained 4% acrylamide cross-linked with bisacrylamide (20:1.0, acrylamide:bisacrylamide) with 0.5 mol/L Tris buffer at pH 6.8. The running buffer consisted of 0.3% Tris-HCl, 1.44% glycine, and 0.1% SDS. The upper electrode running buffer also contained 0.1% β-mercaptoethanol. Proteins were electrophoresed at 4°C with a constant current of 25 mA for about 2 hours to allow the samples to pass from the stacking gel into the separating gel. The current was increased to 40 mA, and electrophoresis continued until 8,000 volt-hours (approximately 22 hours of total running time). The gels were stained with silver according to the method of Merril et al.23 Densitometric analyses were done on the stained gels in an AGFA DuoScan with Quantity 1 analysis software (BioRad Corp, Hercules, California).
Immunoblotting
Protein extracts from chemically skinned fibers or muscle homogenates placed in sample buffer as described above were applied to nitrocellulose membrane under a gentle vacuum with a Hoeffer PR600 slot blot apparatus. The blotted membranes were blocked with powdered milk in phosphate-buffered saline solution and subsequently incubated in the primary anti-MHC antibodies BA-F8 (anti–type I), SC-71 (anti–type IIA). BF-F3 (anti–type IIB), and MY-32 (anti-type II). The antibody reactivity in the fibers was detected after incubation in secondary antibodies by the indirect immunoperoxidase method.5
Physiology
Portions of the muscle samples were microdissected into fiber bundles 2 to 3 mm in diameter and placed into a pre-skinning solution. This solution was prepared by adding 0.1 mmol/L phenylmethanesulfonyl fluoride (a serine protease inhibitor) to a relaxing solution (see below). The bundles were gently agitated for 4 to 6 hours, during which the pre-skinning solution was changed 2 or 3 times until free from discoloration. The bundles were then placed in a 50/50 pre-skinning–glycerol solution and gently agitated for approximately 12 hours at 4°C before being stored in the same solution at −30°C for up to 2 months. On the day of the experiment, single fibers were isolated in relaxing solution by pulling from one end of the bundle. The relaxing solution contained (mmol/L): 20 N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (Bes), 7 potassium-EGTA, 4 sodium–adenosine triphosphate, 14.5 creatine phosphate, 1 free magnesium ion, and sufficient potassium propionate to adjust the ionic strength to 180 mmol/L. Propionate was the major anion in all solutions. The free calcium ion concentrations were 10−9 for the relaxing solution and 10−4 for the activating solution, respectively. Free bivalent cation and metal-ligand complex concentrations were estimated with the computer program “Winmaxc,”24 obtained from URL http://www.stanford.edu/~cpatton/maxc. html. Single fibers (1.5 to 5 mm long) were mounted via small stainless steel clamps between a force transducer and the linear motor of the physiological test rig (Scientific Instruments, Heidelberg, Germany) in relaxing solution. The test rig was interfaced to a personal computer that controlled the experimental protocol–acquired data via dedicated software (SKI. Scientific Instruments). All experiments were performed at 15°C. A 670-nm helium-lanthanum laser beam was directed onto the fiber, and the resulting diffraction pattern was focused onto a ground glass screen. The initial sarcomere length was adjusted to approximately 2.5 μm, as calculated from the first deflection distance of the diffraction pattern produced. Before activation, the relaxing solution was exchanged for a pre-activating solution, with reduced calcium ion-EGTA buffering capacity, for 20 to 30 seconds. The pre-activating solution was identical to the relaxing solution, except that the potassium-EGTA was reduced to 0.5 mmol/L; this reduction resulted in a faster rise to steady tension after activation and improved the maintenance of cross-striation uniformity during activation.25 The fiber was then induced to contract in a maximal activating solution (pCa 4.0) until a stable force was recorded. A slack test was then conducted to determine the maximum velocity of unloaded shortening of skinned fibers (also termed V0).26 For the slack test, the fiber was allowed to shorten by a series of 5 incremental length changes, typically 10% to 15% of resting length for up to 3.0 seconds' duration, after which resting length was restored. For each length change (ΔL), the time it took the fiber to shorten and just redevelop force (ΔT) was measured. The graph of ΔL versus ΔT was fitted with a straight line by least squares regression, and V0 was taken as the slope of this line (Fig 1). Data with an r2 value greater than 0.98 were rejected.
Fig 1.
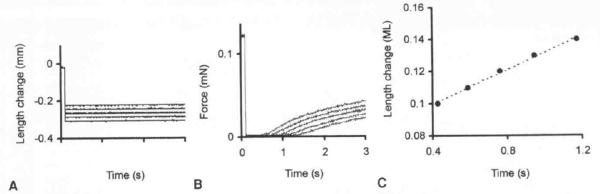
Determination of unloaded shortening velocity (V0) in skinned skeletal muscle fibers by slack test protocol. A) During maximal activation of fiber, series of step reductions in length were performed (superimposed). B) Shortening times necessary for fiber to remove stack were measured (superimposed). mN — millinewtons. C) Slope of relationship between length change and shortening time determined V0 in muscle lengths (ML) per second.
RESULTS
Electrophoresis
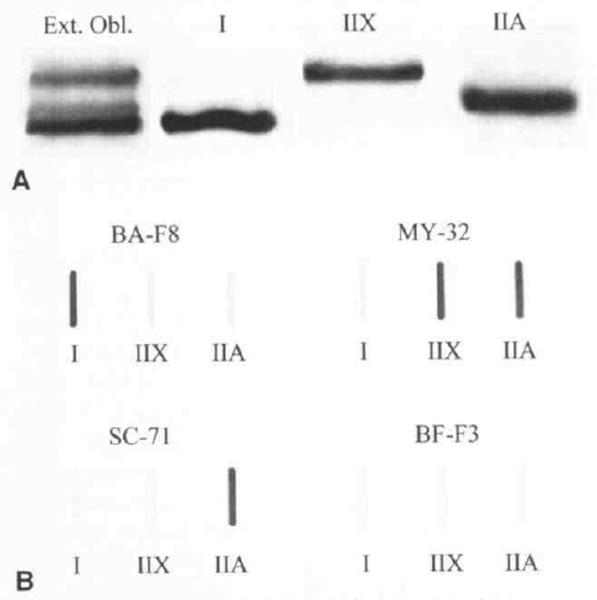
Using whole muscle and single fiber samples from external oblique and vastus muscle biopsy specimens, we were able to establish MHC control standards for type I, IIA, and IIX isoforms (Fig 2). The fastest-migrating band on gels stained positively for the type I antibody (BA-F8). but was negative for all of the fast antibodies on slot blots. The intermediately migrating band on gels stained positively for the type IIA antibody (SC-71) and the general fast antibody (MY-32), but not the IIB antibody (BF-F3). The slowest-migrating band stained positively only for the general fast antibody. Thus, electrophoretic standards whose relative mobilities correspond to protein species I > IIA > IIX were then used to identify MHC isoforms from whole muscle and single fiber preparations in the TA and PCA muscles. Whole muscle TA and PCA samples contained variable amounts and types of MHC isoforms. Some biopsy samples contained only variable amounts of the typical isoforms I, IIA, and IIX, but others contained 2 additional fast-migrating bands (Fig 3). Given evidence from animal studies of additional myosin isoforms present in some species, we also investigated the pattern of isoform banding from human inferior oblique (extraocular) muscle, fetal muscle, and atrium (cardiac) muscle. The inferior oblique muscle contained type I, IIA, and IIX MHCs, but 3 additional fast-migrating bands were also present. The band with a migration speed slightly faster than that of type I comigrated with the neonatal MHC from fetal muscle. The additional 2 bands comigrated with the additional bands seen in laryngeal muscle. The α-MHC band from the atrial sample migrated between the type IIX and IIA bands, and was not detected in any of the skeletal muscle samples.
Fig 2.
Identification of myosin heavy chain isoforms present in human limb muscle, from whole muscle biopsy homogenates and single fibers. A) Mobilities of proteins from biopsy homogenate of external oblique (lane 1) corresponded to type I, IIA, and IIX isoforms from single fibers that expressed only 1 MHC (lanes 2, 3, and 4). B) Identification of MHC isoforms from standard single fibers were confirmed in immunologic slot blots by means of BA-F8 (anti–type I), MY-32 (general anti-fast), SC-71 (anti–type IIA), and BF-F3 (anti–type IIB) antibodies. No antigenic activity for IIB antibodies was detected in single fibers.
Fig 3.
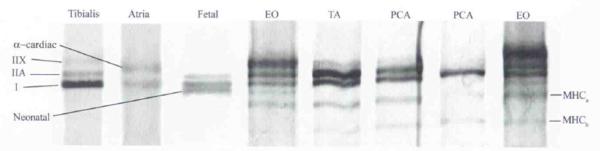
Glycerol–sodium dodecyl sulfate–polyacrylamide gel electrophoresis of whole muscle biopsy homogenates of human tibialis, atria, fetal, extraocular (EO), thyroarytenoid (TA), and posterior cricoarytenoid (PCA) muscles. Relative mobilities of known protein species were neonatal > I > IIA > α-cardiac > IIX. Two additional bands (labeled MHCa and MHCb) were always present in EO muscle and sometimes present in TA and PCA muscles.
Physiology
We determined both the V0 (using the slack-test method) and the MHC isoform content (using glycerol-SDS-PAGE) for 38 control fibers, 28 PCA fibers, and 29 TA fibers. The vastus, tibialis, and external oblique fiber physiology results were grouped together as control data to determine whether the results of our techniques were similar to those of previous reports of V0 in human skeletal muscle. The V0 values for fibers that homogeneously expressed MHC in control muscles were (in muscle lengths per second) type I, 0.508 ± 0.089 (mean ± SEM, n = 18); type IIA, 0.783 ± 0.072 (n = 10): and type IIX, 2.218 (n = 1; see Table and Fig 4). Fibers with heterogeneous myosin expression were grouped together, producing a V0 value of 0.726 ± 0.127 (n = 9). These results were comparable in 2 respects to those of previous reports on human skeletal muscles.25 The V0 generally increased in homogeneous fibers in the order I, IIA, IIX. Second, the speed of shortening in hybrid fibers correlated with the type and amount of MHC as determined by glycerol-SDS-PAGE and densitometry. In the PCA muscle, type I fibers had V0 values of 0.491 ± 0.056 (n = 23), and type IIA, 1.255 (n = 2). Fibers with heterogeneous myosin expression in the PCA muscle had a V0 value of 2.898 ± 1.45 (n = 3). In the TA muscle, the V0 values for fibers that homogeneously expressed MHC were type I, 0.787 ± 0.115 (n = 3); type IIA, 0.851 ± 0.39 (n = 6); and type IIX, 1.36 ± 0.729 (n = 3). Fibers heterogeneous for MHC expression had a V0 value of 1.140 ± 0.28 (n = 17; Fig 4).
FIBER UNLOADED SHORTENING VELOCITY
| Fiber Type | Control Muscle | PCA Muscle | TA Muscle |
|---|---|---|---|
| I | 0.508 ± 0.089 | 0.491 ± 0.056 | 0.787 ± 0.115 |
| (18) | (23) | (3) | |
| IIA | 0.783 ± 0.072 | 1.255 | 0.851 ± 0.39 |
| (10) | (2) | (6) | |
| IIX | 2.218 | 1.360 ± 0.729 | |
| (1) | (3) | ||
| Heterogeneous | 0.726 ± 0.127 | 2.898 ± 1.45 | 1.140 ± 0.28 |
| (9) | (3) | (17) | |
| Data are mean ± SEM (n in parentheses). | |||
| PCA — posterior cricoarytenoid, TA — thyroarytenoid. | |||
Fig 4.
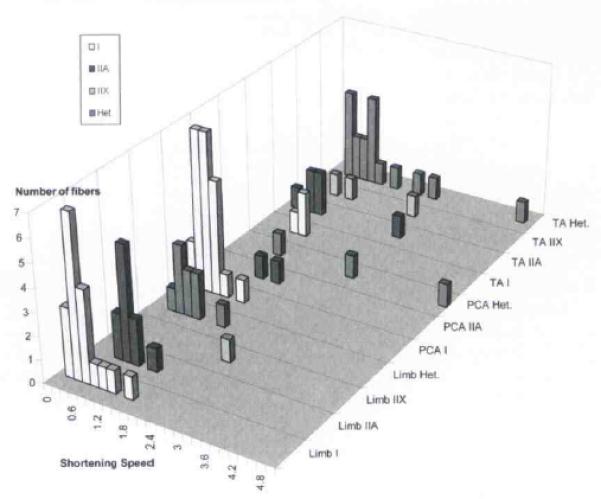
Shortening speed assessed by grouping fibers according to muscle and fiber type. Het. — heterogeneous.
DISCUSSION
In this study, we present preliminary evidence that the V0 in single chemically skinned fibers from human PCA and TA muscles spans a wide range of speeds. Type I, IIA, and IIX laryngeal fibers were comparable in V0 to control fibers from human limb muscle. Some of the laryngeal fibers, however, shortened nearly twice as rapidly as their counterparts from fast-contracting limb muscle. The fastest-contracting fibers from laryngeal muscle always had heterogeneous MHC expression. To our knowledge, this is the first examination of the physiological properties of individual skeletal fibers dissected from human laryngeal muscles.
Myosin Expression in Human Laryngeal Muscle
Ten MHC isoforms have been identified in mammalian muscle, which may be subdivided into the following groups: 1) developmental isoforms (embryonic and neonatal); 2) slow isoforms (I or slow twitch contraction, and tonic or slow tonic contraction); 3) fast isoforms (IIA, IIB, and IIX); 4) very fast cranial isoforms (IIM in masticatory muscles and EOM in extraocular and laryngeal muscles); and 5) cardiac isoforms (α and β; β is the same isoform as type I in skeletal muscle; for a general review, see Schiaffino and Reggiani3). All of these isoforms have been identified in human skeletal muscle, with the exception of type IIB. Fibers formally classified as IIB with myofibrillar ATPase histochemistry have more recently been determined to contain a homolog of the type IIX protein isoform, rather than IIB.27 Although IIB protein has not as yet been positively identified in human muscle, the MHC-IIB gene has been sequenced28 — a finding suggesting that a gene message may be present.
The first reports on myosin expression in human laryngeal muscle were based on histochemical fiber typing, which described differences in type I versus type II fiber populations. Most of these reports concluded that TA muscle contains the most fast fibers (about 65%) and that the PCA muscle contains roughly equivalent proportions of type I and type II fibers.29,30 A recent histochemical report confirms these earlier results on human TA muscle.31 Immunohistochemical identification of myosin isoforms has only recently been investigated, and shows that antibodies reactive for type I MHC, general fast type II MHCs,32 tonic MHCs,21 and neonatal MHC do react with fibers in human TA muscles. The neonatal MHC is thought to be expressed in regenerating fibers associated with age-related muscle changes,33 rather than as part of normal protein expression. A comprehensive immunohistochemical and histochemical fiber type classification scheme on TA and other human laryngeal muscles has yet to be developed.
Biochemical isolation of MHC isoforms by electrophoresis is made possible by modifying the SDS-PAGE technique. By variation of the proportions of acrylamide and bisacrylamide and addition of glycerol to the gel, isoforms of myosin were shown to be separated by properties other than molecular weight.34 Although not yet directly tested, this technique most likely relies on variations in post-transcriptional modifications such as protein glycosylation. Since this separation is not based on protein weight, MHC migration speeds relative to each other sometimes vary by species rather than by isoform. For example, in rats, IIA and IIX isoforms do not always separate from each other,7 but in humans, IIA and IIX isoforms are consistently resolved as distinct bands.22 Hence, it may not always be possible to determine isoform identity by comparing the myosin banding pattern in one species to that in another. In rats, EOM can be separated from other fast MHC isoforms in the extrinsic eye muscles and laryngeal muscles.7 In addition to this present study, 3 reports have described MHC banding by glycerol-SDS-PAGE in humans. One of these reports identifies only type I, IIA, and IIX isoforms.12 The 2 other reports have identified an additional myosin band that has yet to be positively identified.13,14
The samples from human PCA and TA muscles analyzed here contained either 1 or 2 additional MHC bands that, in comparison to whole muscle homogenates of limb control muscle samples, were not type I, IIA, or IIX isoforms. Homogenates from human atrium, known to contain α MHC, and from fetal muscle, known to contain neonatal MHC, were also investigated to determine whether the additional bands in laryngeal muscle were these isoforms. The α isoform consistently migrated intermediately between the IIA and IIX bands, and the neonatal isoform migrated slightly faster than the type I band. The additional protein species in human laryngeal muscle did not comigrate with α or neonatal MHC. Since extraocular muscle is thought to express at least 6 MHC genes,35 samples of human inferior oblique muscle were obtained. The extraocular muscle contained types I, IIA, and IIX, as found in control limb muscle, and neonatal MHC, as found in fetal muscle. Two additional bands in extraocular muscle comigrated with the additional bands seen in laryngeal muscle. From our control data, we excluded type IIX, α, IIA, I, and neonatal MHCs, and suggest that one of these additional bands is human EOM and the other is either tonic or embryonic MHC.
VO and MHC Composition
The shortening speeds of limb muscle fibers were obtained to compare the results of our slack test protocol to those previously reported for human muscle. In a study that included relatively large numbers of soleus and quadriceps fibers, type I, IIA, and IIX fibers had respective shortening speeds of 0.3, 1.0, and 3.1 muscle lengths per second.25 The range of speeds for these fibers was inclusive of the mean data of our results for I, IIA, and IIX fibers (0.508, 0.783, and 2.218 muscle lengths per second, respectively) from human vastus, triceps, and external oblique muscles. Also, as reported previously, the shortening speed of chemically skinned fibers correlated to the type of MHC isoform present. Type I, IIA, and IIX fibers from TA and PCA muscles also had shortening speeds within the anticipated range for human limb fibers. Some of the fibers with heterogeneous expression of MHCs, however, had unusually fast contraction times, approaching twice the speed of fast-contracting type II fibers. These fibers were identified in both the TA and PCA muscles sampled. Gels from these fibers showed only type I, IIA, and IIX MHCs, and not the additional bands found in whole muscle homogenates of PCA and TA muscles. Why some fibers that heterogeneously express MHCs from TA and PCA muscles have unusually fast shortening speeds remains to be determined. Extraocular muscle fibers with comparably fast contraction speeds are known to coexpress EOM along with other isoforms. Our heterogeneous fibers may have small amounts of EOM that are beyond the limits of detection with the glycerol-SDS-PAGE technique. Another possible explanation for fast shortening speeds may be that factors other than MHC composition affect these properties. Further descriptions of cellular kinetics in relation to the type and amount of contractile and regulatory proteins are necessary in order for us to understand how physiological activity is determined in laryngeal fibers. Ultimately, direct assessment of cellular activity should be compared to indirect clinical assessments of human laryngeal muscle function, to elucidate further the functioning of this organ. The findings in this study of diverse laryngeal muscle fiber types (and varying contraction speed) could be explained by review of the muscle activities and functions of the larynx. Laryngeal functions involve voice, respiration, and swallowing. Respiratory activities of the larynx require periodic, frequent rapid firing of the PCA muscle to abduct the vocal folds. This muscle activity is probably quite similar to the TA muscle activities during initiation and variation of speech and singing (rapid muscle contraction). However, laryngeal muscle activities also require constant and prolonged muscle contraction, notably during sustained phonation (both TA and PCA muscles).
Preliminary investigations of the individual laryngeal muscles and their activities have found that there are different subgroups of laryngeal muscles with unique functions. This specialization is most recognized in the PCA muscle. There are 2 bellies of the PCA muscle: horizontal and vertical. The former appears to be involved in vocal fold abduction for respiration, and the latter in maintenance of vocal fold tone, bulk, and possibly lengthening. There have been similar anatomic and functional subcompartments proposed for the TA muscle.36 Given the disparate findings of the muscle fiber types and properties found in this study, we hypothesize that our random muscle sampling involved different areas of the laryngeal muscles under investigation. Future studies are planned that would use a reliable method of muscle harvesting from similar subregions of the TA and PCA muscles.
ACKNOWLEDGMENTS
We are grateful to Dr Eugene Myers and Dr Jonas Johnson in the Department of Otolaryngology, School of Medicine, and Dr Leon Barnes, Department of Pathology, School of Medicine, for assistance in obtaining biopsy specimens of laryngeal muscle. We are also grateful to Dr Kenneth M. Yaw, Department of Orthopedic Surgery, School of Medicine, for assistance in obtaining biopsy specimens of limb muscle.
Footnotes
Copyright of Annals of Otology, Rhinology & Laryngology is the property of Annals Publishing Company and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
REFERENCES
- 1.Barany M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50(suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire JM. Muscle filament structure and muscle contraction. Annu Rev Biophys Bioeng. 1975;4:137–63. doi: 10.1146/annurev.bb.04.060175.001033. [DOI] [PubMed] [Google Scholar]
- 3.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 4.Rowlerson A, Pope B, Murray J, Whalen RG, Weeds AG. A novel myosin present in cat jaw closing muscles. J Muscle Res Cell Motil. 1981;2:415–38. [Google Scholar]
- 5.Sciote JJ, Rowlerson A. Skeletal fiber types and spindle distribution in limb and jaw muscles of the adult and neonatal opossum. Monodelphis domestica. Anal Rec. 1998;251:548–62. doi: 10.1002/(SICI)1097-0185(199808)251:4<548::AID-AR10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Cooper S, Eccles JC. The isometric responses of mammalian muscles. J Physiol. 1930;69:377–85. doi: 10.1113/jphysiol.1930.sp002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DelGaudio JM, Sciote JJ, Carroll WR, Esclamado RM. Atypical myosin heavy chain in rat laryngeal muscle. Ann Otol Rhinol Laryngol. 1995;104:237–45. doi: 10.1177/000348949510400310. [DOI] [PubMed] [Google Scholar]
- 8.Lucas CA, Rughani A, Hoh JFY. Expression of extraocular myosin heavy chain in rabbit laryngeal muscle. J Muscle Res Cell Motil. 1995;16:368–78. doi: 10.1007/BF00114502. [DOI] [PubMed] [Google Scholar]
- 9.Merati AL, Bodine SC, Bennett T, Junu H-H, Furuta H, Ryan AF. Identification of a novel myosin heavy chain gene expressed in the rat larynx. Biochim Biophys Acta. 1996;1306:153–9. doi: 10.1016/0167-4781(95)00237-5. [DOI] [PubMed] [Google Scholar]
- 10.Jung HH, Lieber RL, Ryan AF. Quantification of myosin heavy chain mRNA in somatic and branchial arch muscles using competitive PCR. Am J Physiol. 1998;275:C68–C74. doi: 10.1152/ajpcell.1998.275.1.C68. [DOI] [PubMed] [Google Scholar]
- 11.Wu YZ, Baker MJ, Crumley RL, Blanks RHI, Caiozzo VJ. A new concept in laryngeal muscle: multiple myosin isoform types in single muscle fibers of the lateral cricoarytenoid. Otolaryngol Head Neck Surg. 1998;118:86–94. doi: 10.1016/S0194-5998(98)70380-8. [DOI] [PubMed] [Google Scholar]
- 12.Wu YZ, Crumley RL, Armstrong WB, Caiozzo VJ. New perspectives about human laryngeal muscle: single-fiber analyses and interspecies comparisons. Arch Otolaryngol Head Neck Surg. 2000;126:857–64. doi: 10.1001/archotol.126.7.857. [DOI] [PubMed] [Google Scholar]
- 13.Shiotani A, Westra WH, Flint PW. Myosin heavy chain composition in human laryngeal muscles. Laryngoscope. 1999;109:1521–4. doi: 10.1097/00005537-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Perie S, Agbulut O, St Guily JL, Buller-Browne GS. Myosin heavy chain expression in human laryngeal muscle fibers. A biochemical study. Ann Otol Rhinol Laryngol. 2000;109:216–20. doi: 10.1177/000348940010900218. [DOI] [PubMed] [Google Scholar]
- 15.Hall-Craggs EC. The contraction times and enzyme activity of two rabbit laryngeal muscles. J Anal. 1968;102:241–55. [PMC free article] [PubMed] [Google Scholar]
- 16.Martensson A, Skoglund CR. Contraction properties of intrinsic laryngeal muscles. Acta Physiol Scand. 1964;60:318–36. doi: 10.1111/j.1748-1716.1964.tb02895.x. [DOI] [PubMed] [Google Scholar]
- 17.Hast MH. The primate larynx. A comparative physiological study of intrinsic muscles. Acta Otolaryngol (Stockh) 1969;67:84–92. doi: 10.3109/00016486909124371. [DOI] [PubMed] [Google Scholar]
- 18.Hirose H, Ushijima T, Kobayashi T, Sawashima M. An experimental study of the contraction properties of the laryngeal muscles in the cat. Ann Otol Rhinol Laryngol. 1969;78:297–306. doi: 10.1177/000348946907800209. [DOI] [PubMed] [Google Scholar]
- 19.Larson CR, Kempster GB, Kistlcr MK. Changes in voice fundamental frequency following discharge of single motor units in cricothyroid and thyroarytenoid muscles. J Speech Hear Res. 1987;30:552–8. doi: 10.1044/jshr.3004.552. [DOI] [PubMed] [Google Scholar]
- 20.Luschei ES, Ramig LO, Baker KL, Smith ME. Discharge characteristics of laryngeal single motor units during phonation in young and older adults and in persons with Parkinson disease. J Neurophysiol. 1999;81:2131–9. doi: 10.1152/jn.1999.81.5.2131. [DOI] [PubMed] [Google Scholar]
- 21.Han Y, Wang J, Fischman DA, Biller HF, Sanders I. Slow tonic muscle fibers in the thyroarytenoid muscles of human vocal folds: a possible specialization for speech. Anat Rec. 1999;256:146–57. doi: 10.1002/(SICI)1097-0185(19991001)256:2<146::AID-AR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Sciote JJ, Rowlerson AM, Hopper C, Hunt NP. Fibre type classification and myosin isoforms in the human masseter muscle. J Neurol Sci. 1994;126:15–24. doi: 10.1016/0022-510x(94)90089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merril CR, Goldman D, Sedman SA, Ebert MH. Ultra-sensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981;211:1437–8. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- 24.Bers D, Patton C, Nuccitelli R. A practical guide to the preparation of Ca buffers. In: Patton C, editor. A practical guide to the study of Ca2+ in living cells. Vol. 3. Academic Press; San Diego. Calif: 1994. p. 29. [Google Scholar]
- 25.Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edman KAP. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in verte-brate muscle fibres. J Physiol. 1979;291:143–59. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sant'ana Pereira JAA, Wessels A, Nijtmans L, Moorman AFM, Sargeant AJ. New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid fibre types. J Muscle Res Cell Motil. 1995;16:21–34. doi: 10.1007/BF00125307. [DOI] [PubMed] [Google Scholar]
- 28.Weiss A, Schiaffino S, Leinwand LA. Comparative sequence analysis of the complete human sareomeric myosin heavy chain family: implications for functional diversity. J Mol Biol. 1999;290:61–75. doi: 10.1006/jmbi.1999.2865. [DOI] [PubMed] [Google Scholar]
- 29.Sadeh M, Kronenberg J, Galon E. Histochemistry of human laryngeal muscles. Cell Viol Biol. 1981;27:643–8. [PubMed] [Google Scholar]
- 30.Teig E, Dahl HA, Thorkelsen H. Actoinyosin ATPase activity of human laryngeal muscles. Acta Otolaryngol (Stockh) 1978;85:272–81. doi: 10.3109/00016487809111935. [DOI] [PubMed] [Google Scholar]
- 31.Guida HL, Zorzetto NL. Morphometric and histochemical study of the human vocal muscle. Ann Otol Rhinol Laryngol. 2000;109:67–71. doi: 10.1177/000348940010900113. [DOI] [PubMed] [Google Scholar]
- 32.Malmgren LT, Fisher PJ, Bookman LM, Uno T. Age-related changes in muscle fiber types in the human thyroarytenoid muscle: an immunohistochemical and stereological study using confocal laser scanning microscopy. Otolaryngol Head Neck Surg. 1999;121:441–51. doi: 10.1016/S0194-5998(99)70235-4. [DOI] [PubMed] [Google Scholar]
- 33.Malmgren LT, Lovice DB, Kaufman MR. Age-related changes in muscle fiber regeneration in the human thyroarytenoid muscle. Arch Otolaryngol Head Neck Surg. 2000;126:851–6. doi: 10.1001/archotol.126.7.851. [DOI] [PubMed] [Google Scholar]
- 34.Carraro U, Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun. 1983;116:793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- 35.Wieczorek DF, Periasamy M, Butler-Brown GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–29. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders I, Rai S, Han Y, Biller HF. Human vocalis contains distinct superior and inferior subcompartments: possible candidates for the two masses of vocal fold vibration. Ann Otol Rhinol Laryngol. 1998;107:826–33. doi: 10.1177/000348949810701003. [DOI] [PubMed] [Google Scholar]