Abstract
Purpose: The aim of this study was to design, formulate and physicochemically evaluate effervescent ranitidine hydrochloride (HCl) tablets since they are easily administered while the elderly and children sometimes have difficulties in swallowing oral dosage forms. Methods: Effervescent ranitidine HCl tablets were prepared in a dosage of 300 mg by fusion and direct compression methods. The powder blend and granule mixture were evaluated for various pre-compression characteristics, such as angle of repose, compressibility index, mean particle size and Hausner's ratio. The tablets were evaluated for post-compression features including weight variation, hardness, friability, drug content, dissolution time, carbon dioxide content, effervescence time, pH, content uniformity and water content. Effervescent systems with appropriate pre and post-compression qualities dissolved rapidly in water were selected as the best formulations. Results: The results showed that the flowability of fusion method is more than that of direct compression and the F5 and F6 formulations of 300 mg tablets were selected as the best formulations because of their physicochemical characteristics. Conclusion: In this study, citric acid, sodium bicarbonate and sweeteners (including mannitol, sucrose and aspartame) were selected. Aspartame, mint and orange flavors were more effective for masking the bitter taste of ranitidine. The fusion method is the best alternative in terms of physicochemical and physical properties.
Keywords: Effervescent tablet, Ranitidine HCl, Fusion method, Direct compression method
Introduction
Oral dosage forms of drugs are the main popular routes in spite of some disadvantages such as slow absorption and delayed onset of action. On the other hand, liquid forms of drugs are not stable enough and slow release dosage forms have longer routes for changing throughout the gastrointestinal tract. These two forms are thus limited in applications. Hence, effervescent tablets seem to be an appropriate alternative for oral dosage forms.1
Effervescent tablets are designed to be dissolved or dispersed in water before administration.2 The tablet is promptly broken apart by internal release of CO2 in water and the CO2 reaction is created by an interaction of tartaric acid and citric acid with alkali metal carbonates or bicarbonates in the presence of the water. Effervescent tablets are uncoated tablets that usually consist of acids and bicarbonates or carbonates.3,4 Some products are useful for pharmaceuticals that damage the stomach or those which are susceptible to stomach pH. In addition, the drugs prescribed commonly in high doses may be used in the form of effervescent tablets.3,5
Moreover, since effervescent tablets are administrated in liquid form, they are easily swallowed so they are preferred over tablets or capsules with a difficult consumption for some patients. On the other hand, one dose of effervescent tablet is often dissolved in 3-4 ounces of water. Being previously dissolved in a buffer solution, effervescent products do not get in direct contact with the gastrointestinal tract. They can thus be tolerated in stomach and intestine well due to reduced gastrointestinal irritation.
Another advantage relating to effervescent tablet is that when they are taken by the patient, exactly the taken amount enters the stomach. In fact, the CO2 produced in an effervescence reaction increases the penetration of active substances into the paracellular pathway and consequently their absorption.6,7
These products contain active ingredients, mixtures of acids/acid salts (citric, tartaric and malic acids or any other suitable acid or acid anhydride), and bicarbonate or carbonate salts (sodium, potassium or any other carbonate or bicarbonate relating to alkali metals) and they all release CO2 when mixed with water.3 Effervescent tablets also contain other materials such as fillers, binders, sweeteners, flavors and lubricants. Water soluble lubricants are used to prevent the adhesion of the tablet to the device and formation of insoluble scum on water surface. Sweeteners are also essential in these formulations. Since sucrose is hygroscopic and it leads to an increase tablet bulk, therefore other sweeteners such as aspartame, maltitol and sucralose are frequently used.1,8
Various methods including wet granulation, fusion method, fluid-bed granulation and direct compression are employed in producing the effervescent tablets. Controlled environmental conditions are very important in producing the effervescent tablets. Since these products are sensitive to moisture and temperature, a relative humidity (RH) of 25% or less and moderate temperatures (25 °C) are essential in manufacturing areas to prevent granulation or adhesion of tablets to the machinery as a result of absorbed moisture.2,5 Currently, the most commonly used effervescent tablet is aspirin tablet.3
Ranitidine is a potent histamine H2 receptor antagonist extensively used in the treatment of conditions like duodenal and gastric ulceration, reflux esophagitis and Zollinger-Ellison syndrome. It is also used in postoperative prophylaxis and in the treatment of allergic and inflammatory conditions related to histamine receptors.9 Ranitidine is more effective than omeprazole in treating gastric ulcer among the children who develop this condition two weaks after taking non-steroidal anti-inflammatory drugs (NSAIDs).10 Ranitidine has both oral (tablets, capsules and syrups) and injectable dosage forms.
The aim of this study was to design, prepare and physicochemically evaluate effervescent tablets of ranitidine HCl. Ranitidine effervescent tablets are of a faster action onset and a more effective treatment for gastrointestinal diseases. Ranitidine of 300 mg effervescent tablets aren't available. The advantages of formulations prepared in this study are their equal properties with other effervescent tablets i.e suitable flavor and weight. Since the weight of effervescent tablets in this study are about half that of other effervescent tablets, so they are economical for pharmaceutical industries. Effervescent tablets are more suitable for the children due to their better flavor and acceptability. Patients' compliance to the drug can be increased due to the appearance of this product during effervescence, convenience of usage and use of attracting colors and flavors in these products.
Materials and Methods
Chemicals
The pharmaceuticals including ranitidine HCl was purchased from Saraca (India). Citric acid, tartaric acid, sodium bicarbonate mannitol, sorbitol, sucrose, povidone k-30 (PVP), polyethylene glycol 6000 (PEG 6000), sodium benzoate, and aspartame were obtained from Merck (Germany). Flavoring agents were gifted by Farabi Pharmaceutical Company (Isfahan, Iran).
Spectrophotometeric Analysis
Different aliquots (1.0-7.0 ml) of a standard 100 µg/ml drug solution were transferred into a series of 10 ml volumetric flasks. Adequate purified water was then added to fill the flasks. The amount of ranitidine HCl was determined by measuring the drug absorbance at 315.3 nm using a Shimadzu UV-1240 model UVmini-visible spectrophotometer.
Determination of Effervescent Components
The effervescent components and the ratios between them were determined according to the neutralization of acids and alkali and the allowed amount of each component. All components were then mixed with ranitidine HCl. Afterwards, the effects of citric acid and tartaric acid on solubility, effervescence time and pH were investigated using changing the acid amounts as follows: 0.5, 0.75 1, 1.5 and 2 times. The same experiment was repeated for sodium bicarbonate (Table 1).
Table 1. Determination of effervescent components based on ratio of effervescent materials (Mean ± SD).
| Code | Citric Acid (mg) | Tartaric Acid(mg) | Sodium bicarbonate(mg) | Effervescent time (s) | *Solubility | pH |
| P1 | 85.9 | 171.8 | 292.2 | 55± 2.08 | 2 | 5.35± 0.01 |
| P2 | 85.9 | 85.9 | 292.2 | 50± 3.21 | 3 | 6.30± 0.1 |
| P3 | 85.9 | 128.8 | 292.2 | 67± 1.53 | 2 | 6.11± 0.07 |
| P4 | 85.9 | 257.7 | 292.2 | 73± 3.51 | 1 | 3.51± 0.05 |
| P5 | 85.9 | 343.6 | 292.2 | 75± 1.83 | 1 | 2.72± 0.04 |
| P6 | 171.8 | 171.8 | 292.2 | 67± 2.87 | 2 | 3.45± 0.06 |
| P7 | 42.9 | 85.9 | 292.2 | 53± 1.52 | 3 | 6.74± 0.04 |
| P8 | 64.4 | 85.9 | 292.2 | 60± 2.31 | 3 | 6.47± 0.03 |
| P9 | 128.8 | 85.9 | 292.2 | 67± 1 | 3 | 6.13± 0.02 |
| P10 | 171.8 | 85.9 | 292.2 | 80± 2.08 | 3 | 5.37± 0.08 |
| P11 | - | 85.9 | 292.2 | 68± 2.52 | 2 | 6.48± 0.1 |
| P12 | 85.9 | - | 292.2 | 75± 1.15 | 5 | 6.57± 0.05 |
| P13 | 171.8 | - | 292.2 | 77± 2 | 5 | 6.10± 0.02 |
| P14 | 128.8 | - | 292.2 | 58± 1.53 | 5 | 6.42± 0.05 |
| P15 | 128.8 | - | 146.1 | 60± 2.50 | 5 | 5.59± 0.07 |
| P16 | 128.8 | - | 219.2 | 67± 1.53 | 5 | 6.23± 0.09 |
| P17 | 128.8 | - | 438.3 | 65± 2.31 | 3 | 6.73± 0.04 |
| P18 | 128.8 | - | 584.4 | 65± 3.64 | 3 | 6.75± 0.06 |
| *Solubility of formulations using a standard table15 (1=insoluble; 2=slightly soluble; 3=sparingly soluble; 4=soluble; 5=freely soluble) | ||||||
Since ranitidine HCl has a bitter taste and a sulfur-like smell, using sweeteners and flavoring agents is necessary. We used different sweeteners at different levels in F1 formulation. After adding the sweeteners and flavors, formulations were surveyed by the Latin square design.11 The formulation with the highest mean score was selected as the best formulation (Table 2).
Table 2. Panel test for sweeteners and flavors by Latin Square method (on 40 volunteers).
| Ingredients(mg) | Formulations | |||||||||||||
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | |
| Ranitidine | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 336 |
| Citric acid | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 | 171.8 |
| Na bicarbonate | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 | 292.2 |
| Mannitol | 80 | – | 100 | 100 | – | 100 | 100 | 150 | 150 | 150 | 150 | 150 | 200 | 150 |
| Sorbitol | – | 50 | 50 | 80 | – | – | 80 | 80 | 100 | 100 | 100 | 100 | 100 | 100 |
| Aspartame | – | – | – | – | 20 | 30 | 40 | 60 | 70 | – | 80 | – | 80 | 80 |
| Sucrose | 20 | – | – | – | – | – | – | – | – | 20 | – | 20 | 20 | 20 |
| Acesulfame k | – | – | – | – | 35 | – | – | – | – | – | – | 50 | – | – |
| Mint | 5 | 15 | 20 | 25 | 20 | – | – | – | – | – | – | – | – | – |
| Cherry | – | – | – | – | – | – | 20 | 30 | – | – | – | – | – | – |
| Tutti-frutti | – | – | – | – | – | – | – | – | 20 | 30 | – | – | – | – |
| Raspberry | – | – | – | – | – | 20 | 20 | – | – | – | 25 | – | – | – |
| Orange | – | – | – | – | – | – | – | – | – | – | – | 20 | 25 | 40 |
Evaluating the Mixture of Powders and Granules
The main flowability properties of granules and powders (before compression) were characterized by the angle of repose, compressibility index (Carr's index), and Hausner's ratio.
Angle of Repose (Ө)
The frictional forces in a loose powder or granules may be measured by repose angle. It is defined as the maximum possible angle between the surface of a powder pile or granules and the horizontal plane. The granules were allowed to flow through a funnel fixed to a stand at a definite height. The angle of repose (Ө) was then calculated by measuring the height (h) and radius (r) of the formed granules heap and putting the values into the formula :
Tan Ө = (h/r).12
Compressibility Index
The flowability of powder may be evaluated by comparing the bulk density (ρb) and tapped density (ρt) of powder and the rate at which it packs down. The percentage of compressibility index was calculated as .13
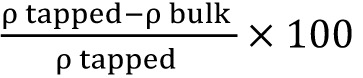
Hausner's Ratio
Hausner’s ratio is an important character to determine the flow property of powder and granules. This can be calculation by the following formula: ρt/ρb.14
Particle Size Distribution
In order to evaluate particle size distribution, powders and granules are sieved. Powders or granules were then disposed on a series of sieves sized 20, 25, 30, 35, 40, 70, and 100 and placed on the device. The remaining powders or granules on each sieve were weighed and the mean particle size (d) was calculated as where xi was the average size of both upper and lower sieves and di was the percent of value i in the range of that bulk (Figure 1).15
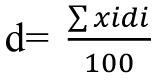
Figure 1 .
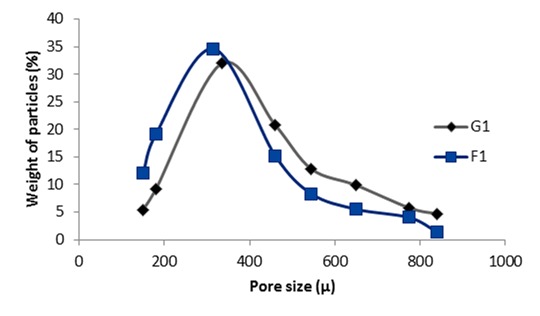
Particle size distribution of F1 and G1 300 mg tablets formulations
Preparation of Effervescent Tablets by Direct Compression Method
After mixing the powder with appropriate characteristics, the tablets were made. Ranitidine was first triturated with sweeteners and then mixed with the effervescent base. The powder was subsequently pressed in a single punch machine (Kilian & Co, Germany) with a rod number 14. The prepared tablets were dried in an oven at 60°C for 1 hour. They were finally packaged.
Preparation of Effervescent Tablets by Fusion Method
The selected acid and alkali were placed on a heater at 54°C to release the crystallization water of citric acid. The formed granules were then dried in an oven at 60°C. Afterwards, the mixture of ranitidine and the sweeteners was added. The powders were pressed in a single punch machine (Kilian & Co, Germany) with a rod number 14. The tablets were again dried in an oven at 60°C for 1 hour and finally packaged.
Physicochemical Evaluation of the Effervescent Tablets
The following physicochemical tests were conducted to evaluate the tablets.
Weight Variation
Twenty tablets were randomly selected and weighed individually and the weights of tablets were compared with the calculated mean weight. In this method, not more than two tablets should have a deviation greater than pharmacopoeia limits ± 5% of the weight.16
Friability Test
Friability of the tablets was determined using friabilator (Erweka, TAP, Germany). It subjected the tablets to the combined abrasion and shock in a plastic chamber revolving at 25 rpm for 4 minutes and dropping a tablet at height of 6 inches in each revolution. The tablets were reweighed. Tablets were de-dusted using a soft muslin cloth and reweighed. The percentage of the tablets friability was calculated as . The desirable friability was determined as lower than 1%.16
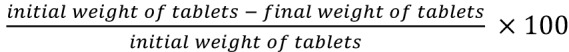
Thickness
A vernier caliper (For-Bro Engineers, India) was used to determine the thickness of randomly 10 selected tablets.17
Hardness Test
The force required to break down a tablet in a compression is defined as the hardness or crushing strength of a tablet. In this study, ten tablets were randomly selected and individually placed in a hardness tester (Erweka, 24-TB, Germany) and then the hardness of tablets reported in N.18
CO2 Content
Three tablets were placed in 100 ml of sulphuric acid solution 1N in 3 separate beakers. In order to determine the amount of released CO2 (mg), the difference in weight before and after dissolving the tablets was calculated.19
Evaluating the Solution pH
Using a pH meter (Metrohm, 632, Switzerland), the pH of the solution was measured by dissolving 3 tablets in 3 beakers containing 200 ml of water.20
Effervescence Time
Three tablets were put in 3 beakers of water and the effervescence time was measured using a stopwatch. Effervescence time was defined as the moment when a clear solution was obtained.18
Assay
Twenty tablets were weighed and grounded into a fine powder. An amount of powder equivalent to 200 mg of ranitidine HCl was weighed accurately and mixed with 70 ml of pure water in a 100 ml volumetric flask. The mixture was shaken for about 20 minutes. Purified water was then added to fill the flask. After mixing well, the solution was filtered using a Whatman No. 42 filter paper. The first 10 ml of the filtrate was discarded. A suitable aliquot was subsequently subjected to analysis by titrimetry. The filtrate (equivalent to 2 mg/ml) was diluted appropriately to obtain a 100 µg/ml solution which is then analyzed by spectrophotometry.21
Content Uniformity
After selecting 10 tablets randomly, the content of each tablet was determined separately.15
Water Content
Ten tablets were dried for 4 hours in a desiccator containing silica gel. The percentage of water content was calculated as .18
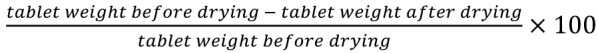
Equilibrium Moisture Content
Three tablets were placed in 3 desiccators containing saturated salt solutions of sodium nitrite (RH, 60%), sodium chloride (RH, 71%), and potassium nitrate (RH, 90%). The percentage of equilibrium moisture content was determined on the first and seventh days by the following method. First, about 50 ml of methanol was poured in Autotitrator (Mettler, TOLEDO-DL53, Switzerland) while a dry magnet was present with methanol. It was titrated by the endpoint with Karl Fischer reagent. In a dry mortar, the pellets were grounded to fine powder of which 100 mg was accurately weighed and transferred to the titration vessel quickly. It was stirred by the end point.20 The equilibrium moisture content was then calculated as V×F×100 in which F was a factor of Karl Fischer reagent and V, the volume of Karl Fischer reagent consumed for sample titration in ml.
Results
Examining the standard curves of ranitidine HCl in purified water led to the curve equation, y=0.044x+0.086 and the regression R2 = 0.998.
Finally, some of the formulations were obtained by measuring effervescent components and eighteen formulations listed in Table 1. The formulations were selected with the best solubility, effervescence time and pH. The formulation with an effervescence time of over 180 seconds or a sediment formation were deleted. The P1-P5 formulations were fixed in amount of citric acid and sodium bicarbonate but variable in amount of tartaric acid. The P7-P11 formulations varied in the amount of citric acid and according to the previous results, tartaric acid was 85.9 mg but sodium bicarbonate was fixed. Thus, citric acid was not less than its original value because of its pH rises. The P14-P18 formulations varied in the amount of sodium bicarbonate but citric acid was fixed. Therefore‚ the amount of sodium bicarbonate should be 146.1- 292.2 mg. After altering the ratio of effervescent components, the materials had a lot of effect on solubility and pH. The P12-P16 formulation were selected as the appropriate base formulations in tableting process.
To improve the unpleasant taste of ranitidine HCl, various sweeteners were used and then the sweeteners added to the formulation of F6 (according to Table 3) and the mixture of sweeteners utilized (S14 formulation). Different flavors were then added to the formulations and surveyed by Latin square method. Mint and orange flavors were finally selected as the best flavors (Table 2).
Table 3. Compositions of 300 mg ranitidine HCl effervescent tablets.
| Ingredients (mg) | Formulations | |||||
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Ranitidine | 336 | 336 | 336 | 336 | 336 | 336 |
| Citric acid | 128.8 | 171.8 | 128.8 | 171.8 | 128.8 | 171.8 |
| Na bicarbonate | 146.1 | 146.1 | 219.2 | 219.2 | 292.2 | 292.2 |
| Mannitol | 150 | 150 | 150 | 150 | 150 | 150 |
| Sorbitol | 100 | 100 | 100 | 100 | 100 | 100 |
| Aspartame | 80 | 80 | 80 | 80 | 80 | 80 |
| Sucrose | 20 | 20 | 20 | 20 | 20 | 20 |
| PVP | 8 | 8 | 8 | 8 | 8 | 8 |
| PEG 6000 | 15 | 15 | 15 | 15 | 15 | 15 |
| Mint | 20 | 20 | 20 | 20 | 20 | 20 |
Based on the previous stages, 6 formulations for 300 mg tablets were selected as the best (Table 3).
Evaluation of Powders Blend and Granules
The results for evaluation of powder blend and granular formulations are provided in Table 4, and their results were compared with standard tables.15
Table 4. Evaluation of physical characteristics of powders and granules blend in 300 mg tablets.
| Physical characteristics | Formulations | |||||||||||
| F1 | G1 | F2 | G2 | F3 | G3 | F4 | G4 | F5 | G5 | F6 | G6 | |
| Angle of repose (Ө) | 27.7 | 26.3 | 29.7 | 27.3 | 29.3 | 25.5 | 30.1 | 25.3 | 28.2 | 27.6 | 27.3 | 26.1 |
| Compressibility index | 7.35 | 3.72 | 5.26 | 5.21 | 3.74 | 1.74 | 3.2 | 2.41 | 8.79 | 4.92 | 5.40 | 4.32 |
| Hausner's ratio | 1.08 | 1.04 | 1.06 | 1.06 | 1.04 | 1.02 | 1.03 | 1.02 | 1.1 | 1.05 | 1.06 | 1.04 |
| Mean particle size | 302.9 | 385.3 | 304.6 | 385.4 | 303.6 | 375.9 | 304.5 | 380.7 | 318.6 | 383.9 | 308.5 | 371.6 |
Physicochemical Evaluation
Tablets were prepared by direct compression and fusion methods. They was exposed to all of the physicochemical tests. The weight of formulated effervescent tablets met the pharmacopoeia criteria. Physicochemical tests were conducted on complete tablets including assay, hardness, friability, thickness, weight variation, CO2 content, water content and equilibrium moisture content (Tables 5, 6). All tablets had similar conditions in the weight variation test in pharmacopoeia limits i.e ± 5% .21 The drug content of the whole formulations were put down in the range of 85-115%.1
Table 5. Physicochemical evaluation of 300 mg effervescent ranitidine HCl tablets by direct compression method (Mean ± SD).
| Physicochemical evaluation | Formulations | |||||
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Weight variation (%) | 1.95±0.04 | 2.32±0.04 | 1.76±0.05 | 1.12±0.08 | 1.08±0.05 | 1.30±0.04 |
| Friability test (%) | 0.78±0.10 | 0.89±0.18 | 0.78±0.18 | 0.84±0.22 | 0.57±0.13 | 0.43±0.13 |
| Thickness (mm) | 5.08±0.03 | 5.73±0.04 | 5.18±0.05 | 5.23±0.08 | 5.50±0.01 | 5.75±0.06 |
| Hardness (N) | 58±8.50 | 42±6.46 | 59±4.12 | 47.5±3.30 | 65±4.28 | 66±6.43 |
| pH | 5.36±0.03 | 5.02±0.02 | 5.85±0.02 | 5.38±0.01 | 6.1±0.04 | 5.94±0.02 |
| Effervescence time (sec) | 98±1.53 | 82±3 | 82±2.50 | 67±3.61 | 90±4.04 | 83±3.79 |
| CO2 content (mg) | 239±1 | 240±0.58 | 244±1.53 | 247±1.16 | 248±0.58 | 250±1.15 |
| Assay (mg) | 340±0.02 | 330.3±0.05 | 341.4±0.04 | 336.7±0.02 | 336±0.01 | 335±0.04 |
| Content uniformity (%) | 99.4±3.55 | 99.2±4.15 | 100±4.92 | 100.1±2.96 | 100.1±3.94 | 99.5±5.02 |
| Water content (%w/w) | 0.14±0.006 | 0.18±0.012 | 0.17±0.007 | 0.14±0.007 | 0.20±0.003 | 0.16±0.009 |
Table 6. Physicochemical evaluation of 300 mg effervescent ranitidine HCl tablets by fusion method (Mean ± SD).
| Physicochemical evaluation | Formulations | |||||
| G1 | G2 | G3 | G4 | G5 | G6 | |
| Weight variation (%) | 1.89±0.003 | 1.53±0.018 | 1.09±0.004 | 1.11±0.012 | 1.03±0.004 | 1.05±0.004 |
| Friability test (%) | 0.55±0.11 | 0.63±0.34 | 0.49±0.12 | 0.59±0.23 | 0.39±0.12 | 0.33±0.19 |
| Thickness (mm) | 5.12±0.02 | 5.15±0.03 | 5.25±0.03 | 5.34±0.05 | 5.40±0.02 | 5.45±0.05 |
| Hardness (N) | 64±5.34 | 54±4.35 | 65±6.57 | 63±6.73 | 68±5.27 | 73±3.15 |
| pH | 5.31±0.03 | 4.93±0.02 | 5.75±0.02 | 5.32±0.006 | 6.12±0.04 | 5.95±0.01 |
| Effervescence time (sec) | 91±3.05 | 72±1.53 | 70±2.52 | 69±2.51 | 86±2.52 | 72±2.64 |
| CO2 content (mg) | 236±1.16 | 238±0.58 | 241±1 | 244±1 | 245±1.16 | 249±0.58 |
| Assay (mg) | 336±0.03 | 340.6±0.06 | 337.5±0.03 | 336.7±0.02 | 336.5±0.06 | 338.9±0.04 |
| Content uniformity (%) | 99.6±4.30 | 99.6±3.63 | 99.7±4.41 | 99.9±3.87 | 99.6±4 | 99.9±4.40 |
| Water content (%w/w) | 0.020±0.003 | 0.04±0.001 | 0.05±0.002 | 0.04±0.001 | 0.05±0.001 | 0.04±0.002 |
Friability of the all formulations was found to be lower than 1%. The hardness of the tablets was determined using a hardness tester. The values were within the range of 40-80 (N). The thickness of the tablets varied between 3 and 6 mm. The tablets produced by the fusion method were thicker. The effervescence test was carried out in 200 ml of water. Effervescence times of all formulations were 67-98 seconds. The G1 and F1 formulations of 300 mg tablets had the longest effervescence time (91 and 98 seconds, respectively). Effervescent compounds basically absorb a lot of moisture. Water content of all formulations was lower than 0.5%.
Among 300 mg tablets, the F6 and G6 formulations had the lowest friability. In both methods, the F6 and F2 formulations had the highest and lowest hardness, respectively.
The pH of formulations should be within the range of 5.5 and 6.2, otherwise they may not be acceptable due to lack of stability and sediment production.
The results of equilibrium moisture content (%) of effervescent powders and granules formulations (F5 and F6) are provided in Table 7.
Table 7. Equilibrium moisture content (%) in effervescent powder and granular mixing of the F4 and F5 in temperature 18 °C.
| Formulations | Microclimates | Powder effervescent mixing | Variation (%w/w) | Effervescent granule | Variation (%w/w) | ||
| 1st Day | 7th Day | 1st Day | 7th Day | ||||
| F5 | RH 90% | 11.54(0.02)* | 15.63(0.03) | 26 | 13.32(0.01) | 19.39(0.01) | 31 |
| RH 71% | 4.98 (0.02) | 6.25(0.01) | 20 | 5.12 (0.02) | 7.18(0.01) | 28 | |
| RH 60% | 2.22 (0.02) | 2.73(0.03) | 18 | 3.11(0.01) | 3.65 (0.03) | 14 | |
| F6 | RH 90% | 12.93(0.01) | 18.67(0.01) | 44 | 14.77(0.02) | 21.32(0.01) | 44 |
| RH 71% | 6.42 (0.02) | 8.83(0.02) | 38 | 7.18(0.02) | 9.34 (0.01) | 30 | |
| RH 60% | 3.85 (0.01) | 5.29(0.02) | 37 | 5.60(0.02) | 6.78(0.02) | 21 | |
| *Mean (standard deviation), n=3 | |||||||
| The saturated salt solutions: sodium nitrite (RH, 60%), sodium chloride (RH, 71%), and potassium nitrate (RH, 90%). | |||||||
Discussion
Most of the oral pharmaceutical dosage forms such as conventional tablets and capsules are formulated to be swallowed or chewed. Old people and children frequently have difficulties in swallowing these dosage forms. Such problems are more serious for those confined to bed patients. Despite the attractiveness of effervescent pharmaceutical forms, 300 mg ranitidine HCl is not available in this form. Since it is better tolerated by patients and results in a faster recovery, as the previous studies show,22 we decided to formulate and research the 300 mg effervescent ranitidine HCl tablets.
The standard curve of ranitidine HCl in purified water was plotted using the UV spectrophotometer with λmax of 315.3 nm. This was in agreement with the results obtained from the other studies.23
Since the effervescent reaction in effervescent products requires acid and alkali resources, so they were used in all formulations. Then, pH of the solution, the solubility and the effervescence time were tested.24 Formulations containing tartaric acid (P1-P12) were eliminated due to the formation of clearly observed sediment and a lower pH. The P17 and P18 formulations with a higher amount of sodium bicarbonate were eliminated due to the observed sediment and the highest pH. Ratios of effervescent components in the formulations of P12-P16 led to a better solubility, a pH less than 6 and an appropriate effervescent reaction.
As mentioned earlier, the very bitter taste and the sulphur-like smell of ranitidine HCl are major problems for the patients. Hence, the next step was to add flavor and sweeteners to improve the taste of final product and to increase patient acceptance. A previous study also reported similar findings.25
Consequently, several sweeteners were used in this stage and none of them could not mask the unpleasant taste, so a mixture of sweeteners was used. Different flavors were used and 40 volunteers chose the best formulation in 3 stages and the formulation with a score about 4 was selected as the best (Table 2). The S14 formulations were the best.
Each of the physical properties listed in Table 4 were compared with USP tables. In both methods, most of the formulations had a suitable flowability. As the results showed, angle of repose was reduced in fusion method. For example, angles of repose of F6, G6 (the same formulations, but different manufacturing methods) were reported as 27.3, 26.1, respectively. Hausner´s ratio and compressibility index are reduced in fusion method. Fusion method increases flowability and decreases angle of repose due to increasing the particle size and its spherical shape.26 Compressibility of the granules was higher due to internal porosity of granules.
The mean diameter of particles in the fusion method is larger than the average diameter of the particles in the direct compression due to the adhesion of smaller particles and formation of larger particles. The particle size of all formulations was in the range of 150-800 microns. Effervescent granules had the particle size larger than of the effervescent powders blend.
The majority of formulations had the weight variation and friability of pharmacopoeia limits.26 The F5, F6,G3, G5, and G6 formulations were suitable friability. The F1,F3, F5, F6, G1-G6 formulations had the desired hardness. Due to a lower hardness of direct compression method, the friability of tablets was increased compared to the fusion method. Another study found similar results.27
CO2 content of fusion method is lower than that of the direct compression method. These differences are found in manufacturing process of the granules. In the studied formulations, CO2 contents of G5 and G6 were 245 and 249 mg, respectively. Other study reported that in each grams of formulas containing citric acid and sodium bicarbonate CO2 content, was 292 mg which is comparable with these results.20 In formulation G1, lower level of CO2 was obtained.
The pH of formulations should be within the range of 5.7 and 6.2. Therefore, the F3, F5 and F6 formulations of 300 mg tablets were selected.
The effervescence times of the all formulations were less than 3 minutes and all were in the range mentioned in BP.7 All of the formulations showed effervescence within 67 to 98 seconds.
Finally, a drug content was established in a range of 330.25-338.89 mg for 300 mg tablets which was within the normal range. Drug content of all formulations was in the range mentioned in USP.15
Water content was lower in formulations of fusion method, since they had lost some water during granulation process.
Measurements of relative humidity in some formulations revealed more moisture absorption in the fusion method, compared with direct compression method. Moreover, formulations with higher amounts of sodium bicarbonate absorbed more moisture. Therefore, the F5 and F6 formulations absorbed the highest amount of moisture.
Conclusion
Effervescent ranitidine HCl tablets were prepared by fusion and direct compression methods to replace the conventional tablets of ranitidine HCl in treatment of gastric and duodenal ulcers. The results obtained at each stage of formulation were utilized and the best formulations selected.
After performing the required studies, citric acid, sodium bicarbonate and sweeteners (including mannitol, sucrose and aspartame) were selected. Pre and post-compression tests were conducted on the prepared tablets. Aspartame, mint and orange flavors were more effective in masking the bitter taste of ranitidine.
Finally, the F5 and F6 formulations of 300 mg tablets were selected as the best formulation because of their physicochemical characteristics. It is significant that fusion method resulted in better tablets compared to direct compression method.
Acknowledgments
This study was supported by Isfahan University of Medical Sciences as a thesis research project numbered 390181.
Conflict of Interest
Authors have no conflict of interests.
References
- 1.Rajalakshmi G, Vamsi CH, Balachandar R, Damodharan N. Formulation and evaluation of diclofenac potassium effervescent tablets. Int J Pharm Biomed Res. 2011;2(4):237–43. [Google Scholar]
- 2.Prabhakar C, Krishna KB. A review on effervesent tablets. Int J Pharm Technol. 2011;3:704–12. [Google Scholar]
- 3.Palanisamy P, Abhishekh R, Yoganand Kumar D. Formulation and evaluation of effervescent tablets of aceclofenac. Int Res J Pharm. 2011;2(12):185–90. [Google Scholar]
- 4.Srinath KR, Chowdary CP, Palanisamy P, Krishna AV, Aparna S. et al. Formulation and evaluation of effervescent tablets of paracetamol. Int J Pharm Res Dev. 2011;3(3):76–104. [Google Scholar]
- 5.Lee RE. Effervescent tablets. 2010; Available from: http://www.amerilabtech.com/wp-content/uploads/ EffervescentTabletsKeyFacts.pdf.
- 6.Wadhwani AR, Prabhu NB, Nadkarni MA, Amin PD. Consumer friendly mucolytic formulations. Indian J Pharm Sci. 2004;7:506–7. [Google Scholar]
- 7.Bandeline FJ. Granulation. In: Liberman HA, Lachman L, Schwartz JB, editors. Pharmaceutical Dosage Forms: Tablets. New York: Marcel Dekker Inc; 1989. P. 287-92.
- 8.Bhusan SY, Sambhaji SP, Anant RP, Kakasaheb RM. New drug delivery system for elderly. Indian Drug. 2000;37:312–8. [Google Scholar]
- 9.Brunton L, Lazo J, Parker K. Goodman and Gilman’s the pharmacological basis of therapeutics. New York: McGraw-Hill; 2005. [Google Scholar]
- 10.Kitagami K, Yamao J. NSAID induced gastroduodenal lesions in patients with rheumatoid arthritis. Jpn Gastroent. 1990;87:2025–6. [Google Scholar]
- 11.Clarke-O’Neill S, Pettersson L, Fader M, Dean G, Brooks R, Cottenden A. A multicentre comparative evaluation: Washable pants with an integral pad for light incontinence. J Clin Nurs. 2002;11(1):79–89. doi: 10.1046/j.1365-2702.2002.00559.x. [DOI] [PubMed] [Google Scholar]
- 12.Gunn C, Carter SJ. Cooper and Gunn’s Tutorial Pharmacy. New Delhi: CBS Publishers; 1986. [Google Scholar]
- 13.Nagar P, Singh K, Chauhan I, Verma M, Yasir M. Orally disintegrating tablets: Formulation, preparation techniques and evaluation. J Appl Pharm Sci. 2011;1(4):35–45. [Google Scholar]
- 14.Patil MG, Kakade SM, Pathade SG. Formulation and evaluation of orally disintegrating tablet containing tramadol HCL by mass extrusion technique. J Appl Pharm Sci. 2011;1(6):178–81. [Google Scholar]
- 15.United States Pharmacopeia and National Formulary. 29th ed. Rockville, MD, USA: United States Pharmacopeial Convention; 2006.
- 16.Lachman L, Lieberman HA, Kanig JL. The Theory and Practice of Industrial Pharmacy. 3rd ed. Mumbai: Vargheese Publishing House; 1991. [Google Scholar]
- 17.Tadros MI. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Eur J Pharm Biopharm. 2010;74(2):332–9. doi: 10.1016/j.ejpb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Masareddy R, Yellanki SK, Patil BR, Manvi V. Development and evaluation of floating matrix tablets of riboflavin. Int J PharmTech Res. 2010;2(2):1439–45. [Google Scholar]
- 19.Prajapati ST, Patel LD, Patel DM. Gastric floating matrix tablets: design and optimization using combination of polymers. Acta Pharm. 2008;58(2):221–9. doi: 10.2478/v10007-008-0006-3. [DOI] [PubMed] [Google Scholar]
- 20.Yanze FM, Duru C, Jacob M. A process to produce effervescent tablets: fluidized bed dryer melt granulation. Drug Dev Ind Pharm. 2000;26(11):1167–76. doi: 10.1081/ddc-100100988. [DOI] [PubMed] [Google Scholar]
- 21.Basavaiah K, Nagegowda P, Ramakrishna V. Determination of drug content of pharmaceuticals containing ranitidine by titrimetry and spectrophotometry in non-aqueous medium. Sci Asia. 2005;31:207–14. [Google Scholar]
- 22.Gosai AR, Patil SB, Sawant KK. Formulation and evaluation of oro dispersible tablets of ondansetron hydrochloride by direct compression using superdisintegrants. Int J Pharm Sci Nanotechnol. 2008;26(1):106–11. [Google Scholar]
- 23.Jaiswal D, Bahattacharya A, Yadav IK, Singh HP, Chandra D, Jain DA. Formulation and evaluation of oil entrapped floating alginate beads of ranitidine hydrochloride. Int J Pharm Pharm Sci. 2009;1(3):128–40. [Google Scholar]
- 24.Moghimipour E, Akhgari A, Ghassemian Z. Formulation of glucosamine effervescent granules. Sci Med J. 2010 ;9(1):21–34. [Google Scholar]
- 25.Sharma V, Chopra H. Formulation and evaluation of taste masked mouth dissolving tablets of levocetirizine hydrochloride. Iran J Pharm Res. 2012;11(2):457–63. [PMC free article] [PubMed] [Google Scholar]
- 26.Twitchell A. Mixing. In: Aulton ME, editor. Pharmaceutics: The Science of Dosage Form Design. 3rd ed. New York: Churchill Livingstone; 2007. P. 181-96.
- 27.Bhardwaj V, Bansal M, Sharma PK. Formulation and evaluation of fast dissolving tablets of amlodipine besylate using different super disintegrants and camphor as sublimating agent. Am-Euras J Sci Res. 2010;5(4):264–9. [Google Scholar]


