Abstract
Purpose: Ghrelin has been shown to have antiepileptic function. However, the underlying mechanisms by which, ghrelin exerts its antiepileptic effects are still unclear. In the present study; we investigated antiepileptic mechanism of ghrelin through GABAB receptors using CGP35348 (selective GABAB receptor antagonist). Methods: Male Wistar rats' hippocampi were bilaterally microinjected with the single dose or 10-day ghrelin (0.3 nmol/µl/side). CGP35348, GABAB receptor antagonist, (12.5 µg/µl/side) or saline injected into the dorsal hippocampus 20 minutes before ghrelin administration. Thirty min after ghrelin microinjection, a single convulsive dose of pentylenetetrazole (PTZ) (50 mg/kg) was injected intraperitoneally (i.p). Afterwards, seizure duration and total seizure score (TSS) were assessed for 30 minutes in all animals. Results: Our results demonstrated that acute and chronic intrahippocampal (i.h.) injection of ghrelin could significantly (p<0.001) attenuate the severity of seizures. Ghrelin 0.3 nmol/µl/side decreased duration of seizure significantly both in acute (p<0.001) and chronic (p<0.01) injections. The ghrelin antiepileptic effect was completely antagonized by GABAB blockade. The suppression of both duration and TSS induced by ghrelin in hippocampus was significantly (p<0.001) blocked by CGP35348 in PTZ-induced seizures. Conclusion: In summary, our findings suggest that GABAB receptors may mediate the antiepileptic action of ghrelin in the hippocampus. Therefore, it is possible to speculate that ghrelin acts in the hippocampus to modulate seizures via GABA.
Keywords: Ghrelin, Hippocampus, Epilepsy, PTZ, CGP35348
Introduction
Epilepsy is one of the oldest neurological conditions known to humankind and as a major public health problem worldwide, it afflicts 0.5–1% of the population in industrialized countries.1,2 There are many therapeutic strategies for epilepsy such as pharmaceutical agents, administration of gonadal steroids, neurotrophic factors, dietary interventions and hormones. The effects of hormones either peripheral or endogenous on the nervous system have been well-established.3 Ghrelin is a brain-gut hormone, which is mainly produced by stomach.4,5 Other tissues that express ghrelin include different hypothalamic nuclei such as arcuate nucleus, ventromedial nucleus, dorsomedial nucleus, paraventricular nucleus; ependymal layer of third ventricle, pituitary, hippocampus, immune cells, lung, placenta, kidney, ovary and testis.6 Ghrelin receptor is located in hypothalamus nuclei, hypophysis, and different brain regions such as CA1, CA2, CA3 and dentate gyrus of hippocampal formation, substantia nigra, ventral tegmental region, raphe nucleus, nodose ganglion and cortex.6,7 Therefore, hippocampus could be a target for the central effects of ghrelin.8
Ghrelin is a multifaceted peptide hormone.5 Ghrelin stimulates GH secretion, increases food intake, and decreases fat utilization.9 Concerning ghrelin’s behavioral effects, it has been reported that intracerebroventricular administration in rats induced anxiety and improved memory retention.10 Recently, Obay etal 2007, demonstrated that dose-dependent ghrelin administration significantly delay the onset time of the first myoclonic jerk, generalized clonic seizure and tonic generalized extension, diminish the duration of tonic generalized extension and suppress the onset time of PTZ-induced seizures.11 Moreover, activation of the ghrelin receptor results in the attenuation of seizures in pilocarpine-induced limbic seizures in rats.12 Ghrelin protects against cell death of hippocampal neurons in pilocarpine-induced seizures in rats and promotes the formation of spine synapses in the stratum radiatum of the CA1 subfield of the hippocampal formation.7,13
Epilepsy is thought to be due to an imbalance between glutamate mediated excitatory and GABAergic inhibitory networks, changes in ionotropic receptor function and composition, altered calcium-mediated second messenger activity, or altered endogenous anticonvulsant and neuroprotective activities.14
GABA is the major inhibitory neurotransmitter in the central nervous system (CNS) and as such plays a key role in modulating neuronal activity.15 GABA acts on two types of receptors, namely GABAA, the ligand-operated ion channels, and GABAB, the G protein coupled metabotropic receptors. GABAA receptors are responsible for fast inhibitory postsynaptic potential (IPSP), whereas GABAB receptors cause slower IPSP.16 Postsynaptic GABAB receptors trigger the opening of K+ channels through the G subunits. This results in a hyperpolarization of the postsynaptic neuron. Moreover, GABAB activates Ca2+sensitive K+ channels and small conductance K+ channels in rat hippocampal neurons.15
Ghrelin may exert modulatory effects on neurotransmission.9 The possible involvements of GABA in the ghrelin-mediated effects have been previously shown. Orexigenic action and appetite stimulation of ghrelin, directly or indirectly is mediated through GABA.9,17-19
The present study was designed to investigate ghrelin possible antiepileptic mechanism through GABAB receptor using CGP 35348 (a selective GABAB receptor antagonist).
Materials and Methods
Chemicals and Drugs
Rat ghrelin, CGP3534 and PTZ were purchased from Tocris Bioscience (Bristol, UK). Ghrelin was dissolved in saline (1mg/100µl), and stocked at -20°C. Immediately before i.h. microinjection, ghrelin was diluted with 0.9% saline to give a final concentration of 0.3nmol/µl. The control group received equal amount of saline (1µl). CGP35348 was dissolved in saline (1mg/100µl) and i.h. injection of 12.5 μg/µl/side performed.
Animals and Treatment
The Regional Ethics Committee of Tabriz University of Medical Sciences approved all experimental procedures. Every effort was made to minimize the number of used animals and their suffering. Animals were obtained from the colony of Tabriz university of Medical Sciences. The experiments were performed in adult male Wistar rats (n=50) weighing 220-250 g at the beginning of experiments. They were housed in a temperature (22±2 °C) and humidity-controlled room. The animals were maintained under a 12:12-h light/ dark cycle, with lights off at 8:00 p.m. Food and water provided ad libitum except for the periods of behavioral testing. The behavioral testing was done during the light phase.
Surgery
Rats were implanted with bilateral canula aimed at the dorsal hippocampus. Before surgery, animals were anesthetized with i.p. injection of ketamine (60 mg/kg) and xylazine (12 mg/kg). The animals were mounted into a stereotaxic frame used to position the 22-gauge stainless steel guide canula in the dorsal hippocampus. Coordinates obtained from Paxinos and Watson brain atlas (mm from bregma: AP= -3.8; ML = ± 2.2; DV = -2.7).20 The guide canula was anchored to the skull using stainless steel screws and acrylic cement. The animals were allowed 7 days recovery after guide canula surgeries before the behavioral test.
Microinjection Procedure
All microinjections were done slowly (1 µl/2 min) using a 5µl Hamilton syringe connected by Pe-20 polyethylene tube. The stainless steel injection needle (30 G) was cut to protrude 0.5 mm beyond the tips of the guide cannulae and left in place for 1 min after injection to allow diffusion of the solution and to prevent back flow.
Saline or ghrelin 0.3 nmol/μl were injected bilaterally in dorsal hippocampus for 10 day. At the tenth day, ghrelin was injected 30 min before intraperitoneally (i.p.) injection of PTZ with a single convulsive dose of 50 mg/kg.
CGP 35348 was used to evaluate the role of GABAB receptors in antiepileptic effect of ghrelin. The conscious animals were gently restrained by hand, the injection needle was inserted through the guide cannulae, and saline or CGP 35348 and ghrelin (0.3 nmol/μl), were sequentially injected. A twenty min interval between i.h. injection of receptor antagonist or saline and ghrelin was considered. Thirty minutes after the last microinjection, a single convulsive dose of PTZ (50 mg/kg) was administered intraperitoneally. The doses and administration schedule of antagonist were established in accordance with some earlier studies. The dose of ghrelin was obtained according to the lowest effective dose to inhibit seizure.21 Microinjections were done between 9:00 and 12:00 a.m. to prevent variations determined by circadian rhythms.
Seizure Assessment
The rats were housed in Plexiglas cages (50 cm × 50 cm × 40 cm) after PTZ injection and their behavior was observed and videotaped for 30 min. The latency to seizure onset, duration, and severity of seizures were monitored as parameters of seizure in all animals. Then videotapes were reviewed, and detected seizures were scored based on Racine’s scale as following: (0) normal, nonepileptic activity; (1) mouth and facial movements, hyperactivity, grooming, sniffing, scratching, wet dog shakes; (2) head nodding, staring, tremor; (3) forelimb clonus, forelimb extension; (4) rearing, salivating, tonic clonic activity; (5) falling, status epilepticus.22 Rats were assigned a seizure score (SS) for each 5 min interval over the course of the 30 min session, after which a mean SS was calculated for the entire 30 min session for each rat and referred as total seizure score (TSS).23
Experimental Design
After 7 days of recovery rats were randomly divided into five groups (n = 10) as follows:
Group (saline): 1 µl/side saline i.h.
Group (acute ghrelin): a single dose of 0.3 nmol/µl/side ghrelin i.h.
Group (chronic ghrelin): 0.3 nmol/µl/side ghrelin i.h. for 10 days
Group (saline + ghrelin): 1µl/side saline 20 min before 0.3 nmol/µl/side ghrelin i.h.
Group (CGP35348 + ghrelin): 12.5 μg/µl/side CGP35348, 20 min before 0.3 nmol/µl/side ghrelin i.h.
Then, PTZ (50 mg/kg) was injected intraperitoneally 30 min after the administration of ghrelin or saline in all groups.
On completion of each experiment, the rats were sacrificed, their brains were removed, fixed in formalin, and injection sides were verified in coronal sections. Only animals with the correct injection sides were taken for a further analysis.
Statistical Analysis
Data are expressed, as means ± S.E.M. The statistical analysis of the data was carried out by one-way ANOVA-followed by Tukey's test. In all comparisons, P<0.05 was considered significant.
Results
The Effect of Acute and Chronic Intrahippocampal Microinjection of Ghrelin on the Latency to Seizure Onset, Duration of Seizures and the Total Seizure Score in Epileptic Rats
As shown in Figure 1, a one-way ANOVA indicated that latency to seizure onset after microinjection of single dose and 10-day ghrelin (0.3 nmol/µl/side), was not significant.
Figure 1 .
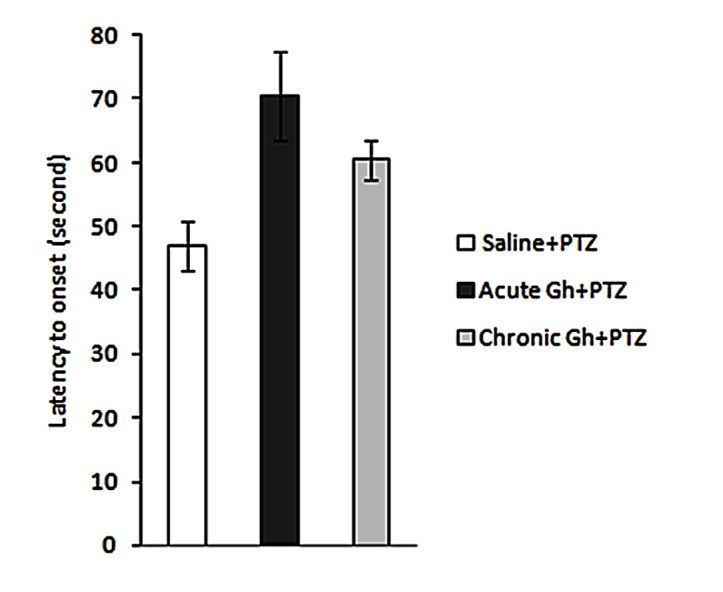
Effect of single dose and 10 days intrahippocampal microinjection of ghrelin (0.3 nmol/µl/side) on the latency to onset in PTZ-induced seizure. Results are expressed as mean ± SEM; n=10 animals per group.
The effect of acute and chronic microinjection of ghrelin on duration of seizures was determined. Single dose of ghrelin 0.3 nmol/µl/side decreased the duration of seizures and it was extremely significant (p<0.001) with respect to saline group. Animals treated with ghrelin 0.3 nmol/µl/side for 10 days had also significantly (p<0.01) reduced duration. But the difference in duration of seizure between acute and chronic ghrelin was not significant (Figure 2).
Figure 2 .
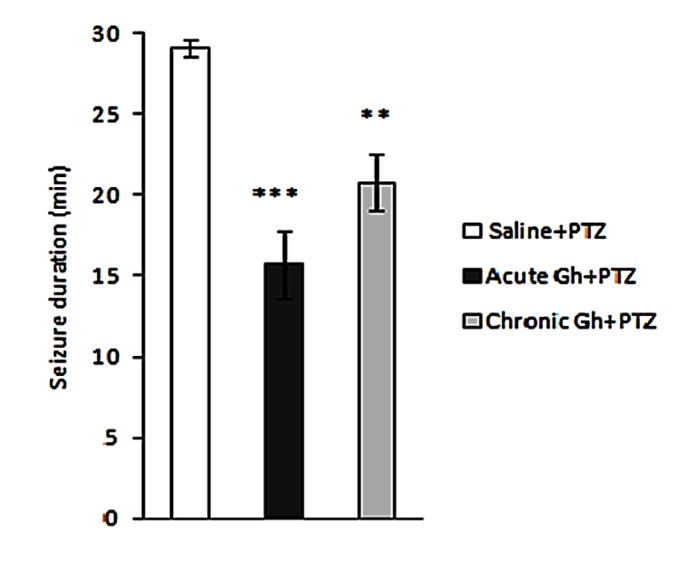
Effect of single dose and 10 days intrahippocampal microinjection of ghrelin (0.3 nmol/µl/side) on the duration in PTZ-induced seizure. Results are expressed as mean ± SEM; n=10 animals per group; ** p<0.01, *** p<0.001.
As illustrated in Figure 3, a repeated measure ANOVA revealed that ghrelin 0.3 nmol/µl/side could decrease total seizure score (scores during 30 minutes) significantly (p<0.001) both in the single dose and 10 - day groups, compared with saline group.
Figure 3 .
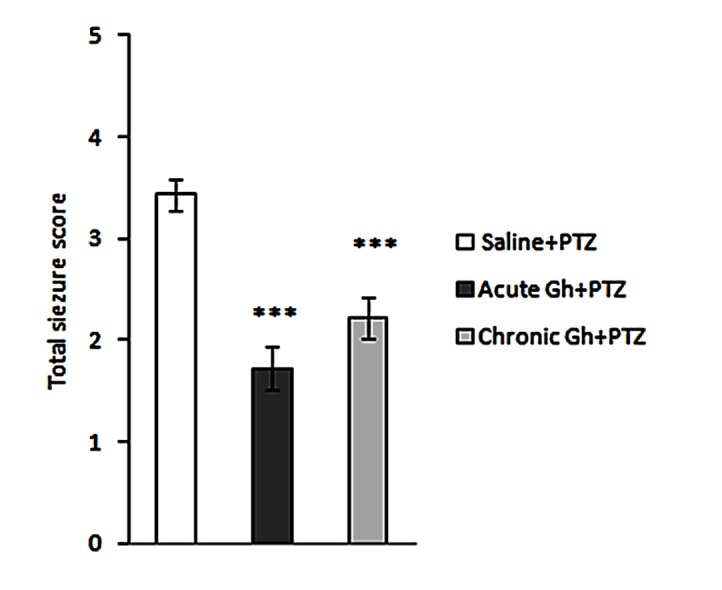
Effect of single dose and 10 days intrahippocampal microinjection of ghrelin (0.3 nmol/µl/side) on the total seizure score in PTZ-induced seizure. Results are expressed as mean ± SEM; n=10 animals per group, *** p<0.001.
The Effect of Intrahippocampal Microinjection of CGP35348 on the Latency to Seizure Onset, Duration of Seizures and Total Seizures Score in Epileptic Rats
Pre-treatment with CGP35348 (GABAB receptor antagonist), in dorsal hippocampus, 20 min prior to ghrelin administration reversed the antiepileptic effects of ghrelin. CGP35348 administration significantly prolonged duration of seizures (p<0.001) (Figure 4) and intensified total seizure score (p<0.001) (Figure 5). Intrahippocampal administration of CGP35347 alone did not induce convulsion (figure is not shown).
Figure 4 .
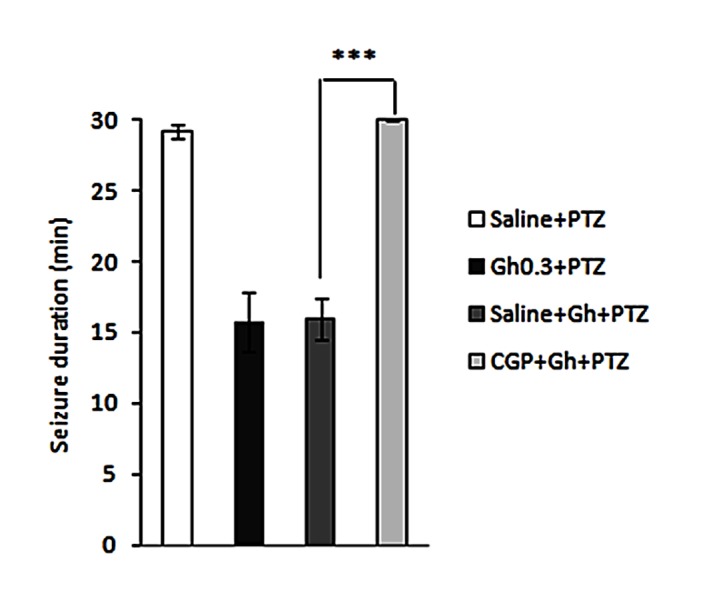
Effect of intrahippocampal injection of ghrelin preceded by CGP35348 (GABAB receptor antagonist) or saline on the duration of seizures during the 30-min post-PTZ behavior assessment. Results are expressed as mean ± SEM, n=10 animals per group; *** P<0.001.
Figure 5 .
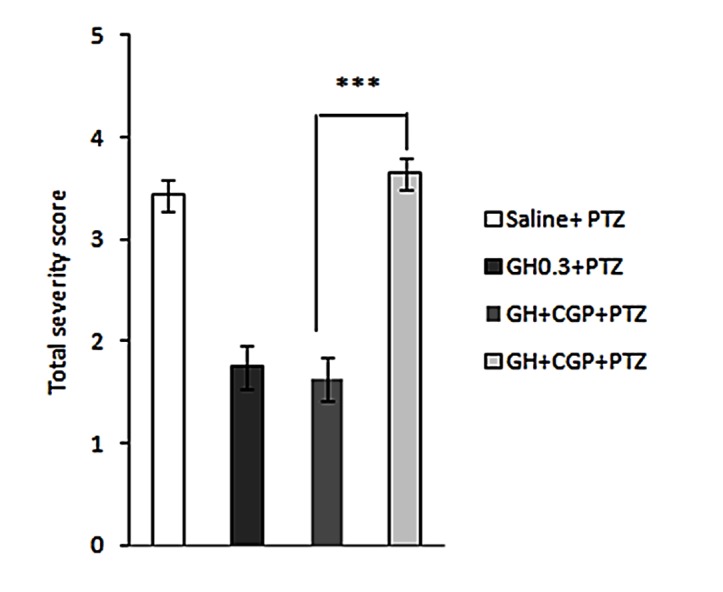
Effect of intrahippocampal injection of ghrelin preceded by CGP35348 (GABAB receptor antagonist) or saline on the total seizure score during the 30-min post-PTZ behavior assessment. Results are expressed as mean ± SEM, n=10 animals per group; *** P<0.001.
Discussion
In the present study, we assessed the antiepileptic effect of acute and chronic i.h. microinjection of ghrelin and one of the possible mechanisms of ghrelin action through GABAB receptors (CGP35348). To evaluate the contribution of specific receptor subtype to the anticonvulsant actions of ghrelin we used PTZ model of epilepsy. Our findings demonstrated that acute and chronic microinjection of ghrelin to dorsal hippocampus could significantly attenuate the severity and duration of seizures. In addition, our findings demonstrated that CGP35348, GABAB receptors antagonist, completely antagonized antiepileptic effects of ghrelin in the dorsal hippocampus.
Ghrelin is a peptide that expresses in a variety of tissues, and it plays a role in physiological and pathophysiological conditions.4 Growth hormone release and stimulation of feeding] are the most known physiological functions for ghrelin.6,10,24 Other findings indicate a role for ghrelin in mediating neuroprotective and behavioral responses. The neuroprotective action of ghrelin has been evidenced in different animal models of neuronal injury, such as cerebral ischemia/reperfusion neuronal loss, dopaminergic neurodegeneration and pilocarpine-induced hippocampal neuronal loss.13,25 Ghrelin can also promote dendritic spine synapse formation and neurogenesis in adult rat.7,26 Recently, it has been shown that ghrelin or its agonists have anti-epileptic action in rodents.11,12,27 Our results in acute and chronic 10-day intrahippocampal administration of ghrelin are in accordance with Obay etal 2007 and Aslan etal 2009 studies. Therefore, ghrelin could be a potential benefit treatment for relieving the intensity of epilepsy and the hippocampal neuron demise caused by seizures.
A number of naturally occurring brain substances, such as GABA, adenosine, and the neuropeptides galanin and neuropeptide Y, may function as endogenous anticonvulsants and, in addition, may interact with the process of epileptogenesis. GABA is one of the main inhibitory neurotransmitters in the brain.28 Focal augmentation of GABA in the limbic system is an obvious strategy for seizure control.14
GABAB receptors are G protein–linked receptors that hyperpolarize the neuron by increasing potassium conductance. GABAB receptors decrease calcium entry and have a slow inhibitory effect.29 The involvement of GABAB receptors in controlling seizures has been reported in various models of epilepsy.30 GABAB receptors are important in controlling partial or tonic – clonic seizures, despite their role in enhancing absence seizures.31 Seizure disorders are often associated with a decreased efficacy of GABA receptor-mediated inhibition that is mainly mediated by two receptor subtypes (termed A and B) located pre- and postsynaptically on both interneurons and principal cells.32 GABAB receptors are considered promising drug targets for the treatment of neurological and mental health disorders.33 CGP 35348 is a selective GABAB receptor antagonist and It is used as a tool in the studies of the role of GABAB receptors in brain.34
GABA mediates ghrelin action, in some areas of CNS. In mouse spinal cord slices, have been show that ghrelin significantly enhances inhibitory (GABAergic/glycinergic) neurotransmission.35 In arcuate nucleus ghrelin hyperpolarizes POMC neurons that, likely mediated by the GABAergic NPY/AGRP neurons.9 Circulating ghrelin enter the hippocampus, where specially has been shown to be a critical region for temporal lobe epilepsy, and bind to the hippocampal neurons.3,13 It is possible that ghrelin affect epilepsy parameters through GABAB receptors.
Our findings showed that intrahippocampal administration of the GABAB receptor antagonist, CGP35348; prior to intrahippocampally administration of ghrelin antagonize the antiepileptic effect of ghrelin in PTZ-induced seizures in rats. Therefore, it is possible to speculate that ghrelin acts in the hippocampus to modulate seizures via GABAB receptor dependent mechanism. The anti-epileptiform effects of ghrelin may be due to the stimulation of GABA release. It is beneficiary to measure GABA level after ghrelin administration to prove that ghrelin acts through GABA release to control limbic seizures in dorsal hippocampus.
Conclusion
In conclusion in vivo, acute and repeated i.h. applications of ghrelin exert anticonvulsant properties on seizures of PTZ-induced model. The anti-epileptiform action of ghrelin was diminished by GABAB selective antagonist, CGP 35348. Therefore, these findings may imply on GABAB receptors participate in the anti-epileptiform activity of ghrelin.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 2005;46(7):1133–9. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- 2.Luef G, Rauchenzauner M. Epilepsy and hormones: a critical review. Epilepsy Behav. 2009;15(1):73–7. doi: 10.1016/j.yebeh.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84(4):363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leontiou CA, Franchi G, Korbonits M. Ghrelin in neuroendocrine organs and tumours. Pituitary. 2007;10(3):213–25. doi: 10.1007/s11102-007-0023-0. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 6.Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin--a hormone with multiple functions. Front Neuroendocrinol. 2004;25(1):27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Diano S, Farr SA, Benoit SC, Mcnay EC, Da Silva I, Horvath B. et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9(3):381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 8.Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, De Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313(3):635–41. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- 9.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 10.Carlini VP, Monzon ME, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN. et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299(5):739–43. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- 11.Obay BD, Tasdemir E, Tumer C, Bilgin HM, Sermet A. Antiepileptic effects of ghrelin on pentylenetetrazole-induced seizures in rats. Peptides. 2007;28(6):1214–9. doi: 10.1016/j.peptides.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Portelli J, Aourz N, Ver Donck L, Michotte Y, Smolders I. Anticonvulsant effects of ghrelin receptor ligands against pilocarpine-induced limbic seizures. Acta Physiologica. 2009;197:3. [Google Scholar]
- 13.Xu J, Wang S, Lin Y, Cao L, Wang R, Chi Z. Ghrelin protects against cell death of hippocampal neurons in pilocarpine-induced seizures in rats. Neurosci Lett. 2009;453(1):58–61. doi: 10.1016/j.neulet.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 14.Boison D. Cell and gene therapies for refractory epilepsy. Curr Neuropharmacol. 2007;5(2):115–25. doi: 10.2174/157015907780866938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84(3):835–67. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 16.Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35(7):984–99. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- 17.Riediger T, Traebert M, Schmid HA, Scheel C, Lutz TA, Scharrer E. Site-specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci Lett. 2003;341(2):151–5. doi: 10.1016/s0304-3940(02)01381-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG. et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 19.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD. et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116(12):3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed. Sydney: Academic press; 2004. [Google Scholar]
- 21.Ghahramanian Golzar M, Ataei Z, Babri S, Ebrahimi H, Mirzaie F, Mohaddes G. Effect of acute and chronic intrahippocampal microinjection of ghrelin on pentylenetetrazole-induced seizures in rats. Pharm Sci. 2011;17(1):11–8. [Google Scholar]
- 22.Meurs A, Clinckers R, Ebinger G, Michotte Y, Smolders I. Seizure activity and changes in hippocampal extracellular glutamate, GABA, dopamine and serotonin. Epilepsy Res. 2008;78(1):50–9. doi: 10.1016/j.eplepsyres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Toscano CD, Ueda Y, Tomita YA, Vicini S, Bosetti F. Altered GABAergic neurotransmission is associated with increased kainate-induced seizure in prostaglandin-endoperoxide synthase-2 deficient mice. Brain Res Bull. 2008;75(5):598–609. doi: 10.1016/j.brainresbull.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K. et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 25.Guneli E, Onal A, Ates M, Bagriyanik HA, Resmi H, Orhan CE. et al. Effects of repeated administered ghrelin on chronic constriction injury of the sciatic nerve in rats. Neurosci Lett. 2010;479(3):226–30. doi: 10.1016/j.neulet.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 26.Johansson I, Destefanis S, Aberg ND, Aberg MA, Blomgren K, Zhu C. et al. Proliferative and protective effects of growth hormone secretagogues on adult rat hippocampal progenitor cells. Endocrinology. 2008;149(5):2191–9. doi: 10.1210/en.2007-0733. [DOI] [PubMed] [Google Scholar]
- 27.Aslan A, Yildirim M, Ayyildiz M, Guven A, Agar E. The role of nitric oxide in the inhibitory effect of ghrelin against penicillin-induced epileptiform activity in rat. Neuropeptides. 2009;43(4):295–302. doi: 10.1016/j.npep.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Czapinski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem. 2005;5(1):3–14. doi: 10.2174/1568026053386962. [DOI] [PubMed] [Google Scholar]
- 29.Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42 Suppl 3:8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- 30.Qu L, Boyce R, Leung LS. Seizures in the developing brain result in a long-lasting decrease in GABA(B) inhibitory postsynaptic currents in the rat hippocampus. Neurobiol Dis. 2010;37(3):704–10. doi: 10.1016/j.nbd.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Tsai ML, Shen B, Leung LS. Seizures induced by GABAB-receptor blockade in early-life induced long-term GABA(B) receptor hypofunction and kindling facilitation. Epilepsy Res. 2008;79(2-3):187–200. doi: 10.1016/j.eplepsyres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Motalli R, D'antuono M, Louvel J, Kurcewicz I, D'arcangelo G, Tancredi V. et al. Epileptiform synchronization and GABA(B) receptor antagonism in the juvenile rat hippocampus. J Pharmacol Exp Ther. 2002;303(3):1102–13. doi: 10.1124/jpet.102.040782. [DOI] [PubMed] [Google Scholar]
- 33.Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP. et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50(4):589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olpe HR, Karlsson G, Pozza MF, Brugger F, Steinmann M, Van Riezen H. et al. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990;187(1):27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- 35.Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7(1):37–49. doi: 10.2174/157015909787602779. [DOI] [PMC free article] [PubMed] [Google Scholar]


