Abstract
Purpose: There are several reports about effects of Salvia spp. on CNS. The present experiment is undertaken to study effect of S. limbata, S. hypoleuca and S. macrosiphon on withdrawal syndrome in mice. Methods: Antinociceptive activities of aerial parts of Salvia spp. is investigated using hot plate method. In addition, the effect of its aerial parts on morphine dependence is investigated in mice. After induction of morphine dependency, different concentrations of plant extract are injected. To assess morphine withdrawal, naloxone (5 mg kg-1, i.p.) are injected into mice on the 5th day. Withdrawal syndrome is assessed by placing each mouse in a glass box 30 cm in height and recording the incidence of escape jumps for 60 minutes. Results: A decrease in incidence of escape jumps is observed in morphine dependence mice. S. limbata and S. hypoleuca extracts produced a statistically significant inhibition of pain induced by hot plate latency at (500, 1000 and 1500 mg kg-1) i.p. A significant increase in pain threshold is observed after 30 and 60 minutes (p < 0.001). The activity was comparable to that of morphine (30 mg kg-1, i.p., p > 0.05). The antinociceptive activity increased up to 60 minutes. Conclusion: S. limbataand S. hypolecuca extracts produced statistically significant inhibition of pain and development of morphine dependence in mice.
Keywords: Salvia, Morphine dependence, Hot plate, Antinociceptive activity, Withdrawal syndrome
Introduction
It is well clear that repeated use of opioid drugs brings physical dependence and tolerance. A variety of agents and systems such as noradrenergic system1 adenosine receptor agonists,2 amino acid excitatory antagonists,3 protein kinase C inhibitors,4 glucocorticosteroids,5 benzodiazepines6 and arachidonic acid7 can modulate the morphine withdrawal syndrome. Pain is still one of the main health problems of the world’s populations. Many bioactive substances are involved in the modulation of pain sensation.8 Some physicians relied upon herbal medicines and natural remedies to treat diseases.9 Salviais an important genus consisting of about 900 species in the Lamiaceae family.10There are some reports that Salvia spp. has effects on the CNS.11S. labiatae, is generally known for its multiple pharmacological effects including analgesic and anti-inflammatory activities.12 S. leriifolia has effect on morphine dependence13 and hypoglycemic effects in morphine dependency.14 Antinociceptive and anti-inflammatory activities have also been reported for theses pecies.12 Jumping is the best indication of the abstinence in mice. This marker easily counted and jumping rate increases when dependence rises or dose of antagonist boosted. Investigation on plant, S. limbata, S. hypoleucaand S. macrosiphonrelatively revealed its beneficial effects to decrease dependence signs produced by morphine and increased pain threshold after 60 min, in comparison to the control. The present experiment was undertaken to study the effect of S. limbata, S. hypoleuca and S. macrosiphon on the development of morphine dependence in mice.
Materials and Methods
Animals
Male albino mice 25-30 g were used. They had free access to a standard commercial diet and water and maintained at 25 ± 1 °C with a 12/12h light/dark cycle.
Plant Material
S. limbata, S. hypoleuca and S. macrosiphon were collected from Tehran. Aerial parts (flowered browse) were dried at room temperature (RT) and coarsely ground before extraction. The powdered samples were extracted at RT by percolation with methanol and methanol/water (80:20). The resulting extract was concentrated over a rotary vacuum evaporator, until a solid extract sample was obtained which was freeze-dried. Extracts were prepared in phosphate buffer (pH 7.4) and tween 80 (4:1) for pharmacological studies.
Morphine Dependence
Morphine was injected i.p. into mice at doses of 50, 75,100 and 125 mg kg-1 three times daily (8:00 a.m., 12:00 and 16:00 p.m., respectively) for 4 days. On fifth day, a single dose of morphine (50 mg kg-1) was injected 2 h before naloxone treatment.
Morphine Withdrawal
Withdrawal signs were precipitated by injection of naloxone (5 g kg-1, i.p.) 2 h after the final administration of morphine. After the naloxone challenge, mice were immediately placed in a glass cylinder (30 cm in height, 20 cm in diameter). The number of jumping episodes was counted for 60 min after naloxone injection.
Extract Treatment
After induction of dependence by morphine, mice were divided into 10 groups. Normal saline was injected to control group. Plant extracts (100,200, 500, 1000, 1500 mg kg-1) were injected to other groups, 1.5 h after the last dose of morphine.
Antinociceptive Study
The hot-plate test was used. The temperature of the metal surface was maintained at 55± 0.2°C. Latency to a discomfort reaction was determined before and after drug administration. The cut-off time was 55 second. Morphine was injected i.p. into mice, as a single dose of 30 mg kg-1. Solvent was injected into the negative control group (10 mL kg-1). Extracts were given at the doses of 500, 1000, 1500 mg kg-1 i.p. to the animals. Antinociceptive activity was assessed by measuring the hot plate latency as described by Leimbach and Eddy.15 Results showed in Figure 1.
Figure 1 .
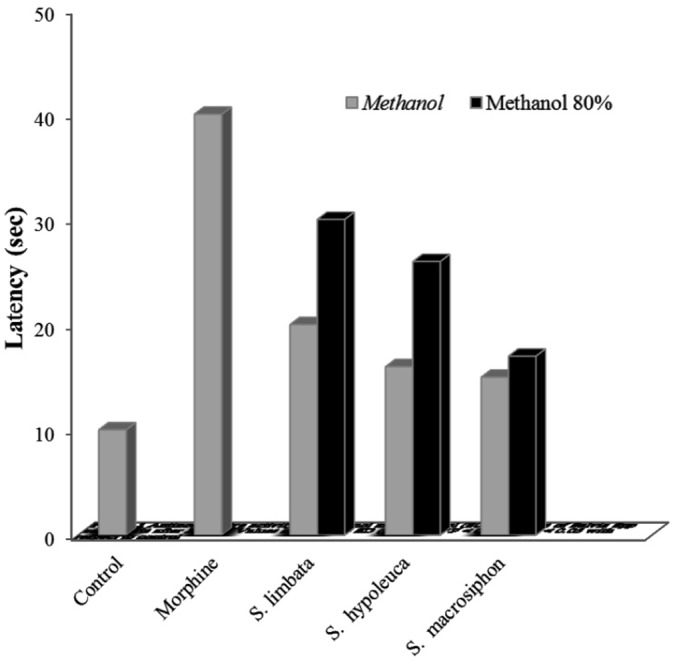
Antinociceptive activity of methanol and methanol (80%) extracts of Salvia Spp aerial parts after 30 min. Values are Mean ± SD (n = 7), ***P < 0.001, **P < 0.05 with respect to control.
Statistical Analysis
Statistical analysis was performed using the SPSS (Ver. 10) software for Windows. Data were analyzed by one-way analysis of variance (ANOVA) and presented as Mean ± SD. p < 0.05 was considered to be significant.
Results and Discussion
Effect of Extract on Morphine Withdrawal Signs
Animal receiving acute treatment with morphine displayed dependency. Extracts decreased jumping count following naloxone administration. The extract reduced the jumping episodes dose-dependently. The maximum effect was observed at the dose of 1.5 g.kg-1 (Table 1).
Table 1. Effects of salvia spp. on morphine withdrawal jumps in mice.
| Control | 100 mg/kg | 200 mg/kg | 500 mg/kg | 1000 mg/kg | 1500 mg/kg | |
| S. limbata | 95 ± 0.83 | 91 ± 0.82* | 83±0.58** | 72±0.74 *** | 62± 0.46*** | 55 ± 0.55*** |
| S. hypoleuca | 91 ± 0.81 | 80 ± 0.75** | 77 ±0.65*** | 67 ±0.69*** | 44 ± 0.45*** | 24 ± 0.56*** |
| S. macrosiphon | 93 ± 0.80 | 86 ± 0.88** | 73 ±0.68*** | 68 ±0.74*** | 41 ± 0.45*** | 21± 0.67*** |
| Each value represents jumping count during 60 min (Mean ± SD) (*, p<0.05; **, p<0.01; ***, p<0.001). | ||||||
Antinociceptive Activity of Salvia Spp Aerial Parts
The activity was weak, but was enough for treatment and blocking the pain. This activity was comparable to that of morphine (30 mg kg-101i.p, p> 0.05). The antinociceptive activity of extract was increased up to 60th min. Results indicated that all extracts reduced the withdrawal signs dose-dependently. Adenosine A1 receptor agonists suppressed withdrawal syndrome of morphine and its antagonists increased jumping episodes and blocked the effect of adenosine analogue.2 Extract increased the ATP level in the brain.16 Hence, it is possible that the extract can reduce morphine dependence by adenosine mechanism. Further study is needed to confirm this mechanism. Benzodiazepines, via GABAA receptors exert an inhibitory effect on morphine dependence.6 There is also a possibility that salvia acts via this pathway to affect morphine dependency, although the involvement of other mechanisms may also be considered. Active ingredient of S. miltorrhizan (called danshen) lead to inhibition of adenylate cyclase activity in the rat brain.17 It also inhibits phosphatidyl inositol system in acute myocardial ischemia.18
Conclusion
This study confirmed that methanolic extracts of S. limbata, S. hypoleuca and S. macrosiphon suppressed morphine withdrawal syndrome. But it is difficult to speculate on the exact mechanism of action at this time. The present results also indicate good antinociceptive activity of S. limbata, S. hypoleuca and S. macrosiphonvia CNS system.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Baraban SC, Stornetta RL, Guyenet PG. Effects of morphine and morphine withdrawal on adrenergic neurons of the rat rostral ventrolateral medulla. Brain Res . 1995;676(2):245–57. doi: 10.1016/0006-8993(95)00097-a. [DOI] [PubMed] [Google Scholar]
- 2.Michalska E, Malec D. Agonist and antagonists of adenosine receptors and morphine withdrawal syndrome in rats. Pol J Pharmacol . 1993;45(1):1–9. [PubMed] [Google Scholar]
- 3.Belozertseva I, Zvartau E, Bespalov A. Behavioral effect of MK-801in morphine dependent and non-dependent mice. Life Sci . 1996;58(4):55–61. doi: 10.1016/0024-3205(95)02297-x. [DOI] [PubMed] [Google Scholar]
- 4.Tokuyama S, Feng Y, Wakabayashi H, Ho IK. Possible involvement of protein kinases in physical dependence on opioids: studies using protein kinase inhibitors, H-7 and H-8. Eur J Pharmacol . 1995;284(1-2):101–7. doi: 10.1016/0014-2999(95)00370-z. [DOI] [PubMed] [Google Scholar]
- 5.Capasso A, Pinto A, Sorrentino L, Cirino G. Dexamethasone inhibition of acute opioid physical dependence in vitro is reverted by anti-lipocortin-1 and mimicked by anti-type II extracellular PLA2 antibodies. Life Sci . 1997;61(10):PL 127–134. doi: 10.1016/s0024-3205(97)00607-3. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Tsuda M, Narita M, Funada M, Mizoguchi H, Misawa M. Diazepam pretreatment suppresses morphine withdrawal signs in the mouse. Life Sci . 1996;58(4):349–57. doi: 10.1016/0024-3205(95)02294-5. [DOI] [PubMed] [Google Scholar]
- 7.Capasso A, Sorrentino L. Arachidonic acid and its metabolites are involved in the expression of morphine dependence in guinea-pig isolated ileum. Eur J Pharmacol . 1997;330(2-3):199–204. doi: 10.1016/s0014-2999(97)00177-5. [DOI] [PubMed] [Google Scholar]
- 8.Yang D, Yang S, Zhang Y, Liu Y, Meng X, Liang Z. Metabolic profiles of three related Salvia species. Fitoterapia . 2009;80(5):274–8. doi: 10.1016/j.fitote.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Winston D. The use of botanicals in eclectic pediatrics. J Am Herbalists Guide . 2004;3:59–64. [Google Scholar]
- 10.Rechinger KH. Salvia. In: Rechinger KH, Hedge IC, editors. Flora Iranica. Akademische Druck and Verlagsanstalt, Graz, Austria: 1982; p. 439.
- 11.Akbar S, Tariq M, Nisa M. A Study on CNS depressant activity of Salvia haematodes Wall. Int J Crude Drug Res . 1984;22(1):41–4. [Google Scholar]
- 12.Hosseinzadeh H, Yavari M. Anti-inflammatory effects of Salvia leriifolia Benth. leaf extract in mice and rats. Pharm Pharmacol Let. 1999;9:60–1. [Google Scholar]
- 13.Hosseinzadeh H, Lary P. Effect of Salvia leriifolia leaf extract on morphine dependence in mice. Phytother Res . 2000;14(5):384–7. doi: 10.1002/1099-1573(200008)14:5<384::aid-ptr641>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinzadeh H, Haddadkhoda parast MH, Shokohizadeh H. Antihyperglycemic effect of Salvia leriifolia Benth. leaf and seed extract in mice. Iran J Med Sci . 1998;23:74–80. [Google Scholar]
- 15.Eddy NB, Leimback D. Diethyl buteryl and diethienyl butyl amines. J Pharmacol Exp Ther. 1953;107(3):385–93. [PubMed] [Google Scholar]
- 16.Wang L, Milne B, Jhamandas K. Involvement of excitatory amino acid pathways in the expression of precipitated opioid withdrawal in the rostral ventrolateral medulla: an in vivo voltammetric study. Brain Res . 1995;697(1-2):130–42. doi: 10.1016/0006-8993(95)00803-x. [DOI] [PubMed] [Google Scholar]
- 17.Kohda H, Takeda O, Tanaka S, Yamasaki K, Yamashita A, Kurokawa T. et al. Isolation of inhibitors of adenylate cyclase from dan-shen, the root of Salvia miltiorrhiza. Chem Pharm Bull (Tokyo) 1989;37(5):1287–90. doi: 10.1248/cpb.37.1287. [DOI] [PubMed] [Google Scholar]
- 18.Tao YY. Effect of Salvia miltiorrhiza compositae on phosphoinositides metabolism in acute myocardial ischemia. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1993;13:354–5. [PubMed] [Google Scholar]


