Abstract
Purpose: The paper describes some thin layer chromatographic procedures that allow simple and rapid separation and identification of penicillins and cephalosporins from complex mixtures. Methods: Using silicagel GF254 as stationary phase and selecting different mobile phases we succeeded in the separation of the studied beta-lactamins. Our aim was not only to develop a simple, rapid and efficient method for their separation but also the optimization of the analytical conditions. Results: No system will separate all the beta-lactams, but they could be identified when supplementary information is used from color reactions and/or by using additional chromatographic systems. Conclusion: The right combination of solvent system and detection method allows the identification of the studied penicillins and cephalosporins and can be successfully used in the preliminary analysis beta-lactam antibiotics.
Keywords: Thin layer chromatography, Beta-lactam antibiotics, Penicillins, Cephalosporins, Separation
Introduction
Among all the antibiotics, the group of beta-lactams ranks first regarding both the number of compounds on the market and also in terms of their use in the treatment of infectious disease. The most frequently used beta-lactams are the group of penicillins and cephalosporins respectively.1
The most popular analytical methods for the determination of beta-lactams are the chromatographic ones, including high performance liquid-chromatography (HPLC) and thin layer chromatography (TLC). HPLC offers high sensitivity and separation efficiency, establishing itself as the first choice method for the analysis of beta-lactams; however it is expensive and requires sophisticated equipment. TLC is a less expensive and less complicated chromatographic procedure, which can be successfully used, in the preliminary screening of pharmaceutical substances. In modern analysis, TLC is usually used as a separation method, which establishes the presence or absence of beta-lactam antibiotics above a defined level of concentration.2,3
TLC is used in 7th edition of the European Pharmacopoeia (Ph.Eur. 7) for separations of a particular beta-lactam antibiotic from its specific impurities, but the methods described refers to only one antibiotic and to its structure-related impurities and are less suitable for identification purposes from complex mixtures.4
A number of publications regarding the identification and separation of beta-lactams by TLC have appeared in literature, but few of them discuss the simultaneous separation from complex mixtures of structurally related derivatives.5-8
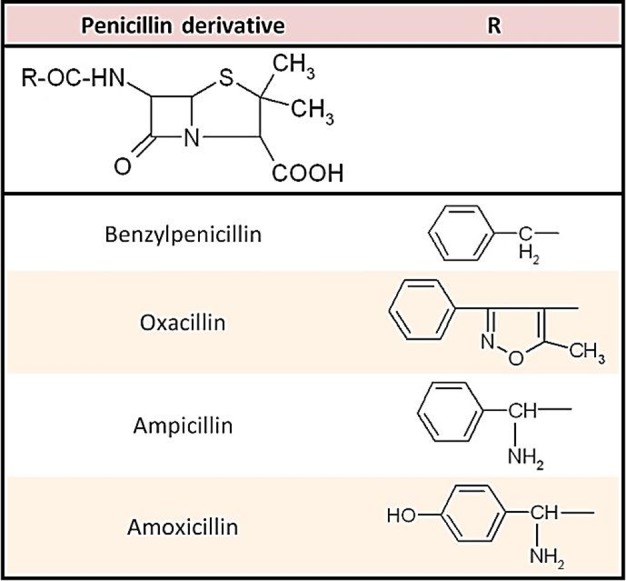
Structurally, penicillins are based on a heterocyclic skeleton, penam, formed by condensation of a beta-lactam ring with a thiazolidine one. In this study we analyzed four of the most frequently used penicillin derivatives with different structural characteristics: benzylpenicillin (PEN) – the first natural penicillin introduced in therapy; ampicillin (AMP) and amoxicillin (AMO) – two semisynthetic aminopenicillins; oxacillin (OXA) – a semisynthetic izoxazolilpenicillin. Penicillins differ from one another in the substituent R attached to the 6-aminopenicillanic acid residues. The chemical structures of the studied penicillins are presented in Figure 1.
Figure 1 .
The skeleton of the cephalosporins consits in a heterocycle, cefem, formed by condensation of a beta-lactam ring with a dihydrothiazine one. In this study we analyzed six frequently used cephalosporin derivatives: cefalexin (CEL), cefadroxil (CFD), cefaclor (CFC) – first generation oral cephalosporins, cefuroxim (CFR) – second generation parenteral cephalosporin, ceftazidim (CZI), ceftriaxon (CTR) – third generation parenteral cephalosporins. Substitution of the various R and R’groups results in cephalosporins with different pharmacological and pharmacokinetic properties. The chemical structures of the studied cephalosporins are presented in Figure 2.
Figure 2 .
Our aim was the separation of beta-lactams from complex mixtures and also the „optimization” of the generic chromatographic process, in order to enhance the quality of the separation.
Materials and Methods
Instrumentation
The TLC system consisted of a Camag Nanomat III automatic sampler, a Camag Linomat IV semiautomatic sampler (Camag, Switzerland), a 2-ml Hamilton microsyringe (Hamilton, USA), a Camag Normal Development Chamber and a Camag fluorescence inspection lamp (Camag, Switzerland). As stationary phase we used 10x20 and 20x20 cm pre-coated silicagel GF254 HPTLC glass plates (Merck, Germany).
Reagents
Penicillins: amoxicillin trihydrate, ampicillin trihydrate, benzylpenicillin sodium, oxacillin sodium (Antibiotice Iaşi, Romania). Cephalosporins: cefalexin monohydrate, cefadroxil monohydrate, cefaclor monohydrate (Sandoz, Romania), cefuroxim sodium (Medochemie, Cyprus), ceftazidim pentahydrate, ceftriaxon sodium (Antibiotice Iaşi, Romania). All the studied beta-lactams were of pharmaceutical grade.
Reagents: acetone, acetic acid, benzene, butanol, ethanol, ethyl acetate, formaldehyde, methanol, sulphuric acid (Reactivul Bucureşti, Romania). All reagents were of analytical grade.
Samples
PEN and OXA, were used as sodium salts, consequently samples were prepared in water at a concentration of 0.2%. AMP and AMO, used as trihydrates, exhibit poor solubility in water; consequently samples of 0.2% were prepared in a 2% sodium bicarbonate solution. Cephalosporin samples were prepared by dissolving the substances in methanol and then diluting with water (1:1). Amounts of 0.5 ml were applied on the chromatoplates using a Hamilton syringe.
Method
The chromatographic chambers were saturated with the mobile phase for 30 minutes. The plates were developed over a distance of 15 cm in filter-paper-lined chromatographic chambers, dried in a stream of hot air, and examined under UV radiation at wavelengths of 254 and 366 nm. The spots were then visualized by placing the plates in a chromatographic chamber saturated with iodine vapors. Some specific in situ color reactions were used in order to increase specificity of the method. All experiments were carried out at room temperature. Photographs of the chromatoplates were taken with a Nikon D-3100 camera, equipped with a UV filter.
Chromatographic detection procedure
Three detection procedures were used; first with iodine vapors and then using in situ plate color reactions with iodine and ninhydrine, after an alkaline hydrolysis.
A few iodine crystals were placed on the base of tightly sealed chromatographic chamber, stored in a fume cupboard. After a few hours during which violet iodine vaporizes and distributes itself homogenously throughout the interior of the chamber, the chromatographic plates were introduced in the chamber. After 30 minutes the plates were sprayed with a 1% starch solution.
Chromatograms were first sprayed with a 1N sodium hydroxide solution, in order to hydrolyze the beta-lactam ring, and after 15 minutes with a solution containing 0.2 g potassium iodine, 0.4 g iodine dissolved in 20 ml ethanol and 5 ml 10% hydrochloric acid.
Chromatoplates were first sprayed with a 1N sodium hydroxide solution, in order to hydrolyze the beta-lactam ring, and after 15 minutes with a 0.1% ninhydrine solution in ethanol, and heated in an oven at 120 °Cfor 10 minutes.
Results and Discussion
The purpose of the method (simultaneous separation of a multicomponent mixture), and the information about the samples (structure, polarity, solubility, stability) were important as initial hints for the choice of the chromatographic system, using the rule of the Stahl’s triangle.2,10,11
The most widely used stationary phase for the analysis of beta-lactam is silicagel, but if we consult the literature reversed-phase or cellulose plates have also been used. Silicagel surface bears Si-OH groups capable of hydrogen bonding with polar substances. Mobile phases for the separation of both penicillins and cephalosporins are polar, usually containing variable quantities of water.5-7,12
An acid (acetic acid) was added to the mobile phase in order to avoid decomposition of the beta-lactam ring on silicagel.
Around twenty solvents were tested and six mobile phases were selected (Table 1 ).
Table 1. The selected mobile phases.
| No | Mobile phases (V/V) |
| I | butanol – water – ethanol – acetic acid 50:20:15:15 |
| II | butanol – water –acetic acid 60:20:20 |
| III | ethyl acetate – water – acetic acid 60:20:20 |
| IV | ethyl acetate – methanol – acetic acid 45:50:5 |
| V | acetone – acetic acid 95:5 |
| VI | acetone – benzene – water – acetic acid 65:14:14:7 |
All beta-lactams can be detected in UV light at 254 nm (green fluorescence) and 366 nm (blue fluorescence). Applying reagents such as ninhydrin or exposing the chromatoplate to iodine vapor can diminish the detection limit.
Figure 3 and 4 show photographs of the chromatograms obtained with mobile phase III in UV light at 254 and 366 nm respectively.
Figure 3.
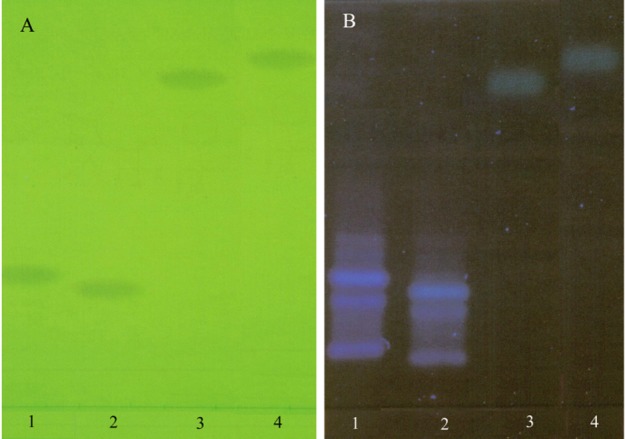
Chromatogram obtained at the separation of penicillins using mobile phase III (ethyl acetate – water – acetic acid 60:20:20), detection in UV light at 254 nm(A) and 366 nm(B) (1 - AMP, 2 - AMO, 3 – PEN, 4 - OXA)
Figure 4.
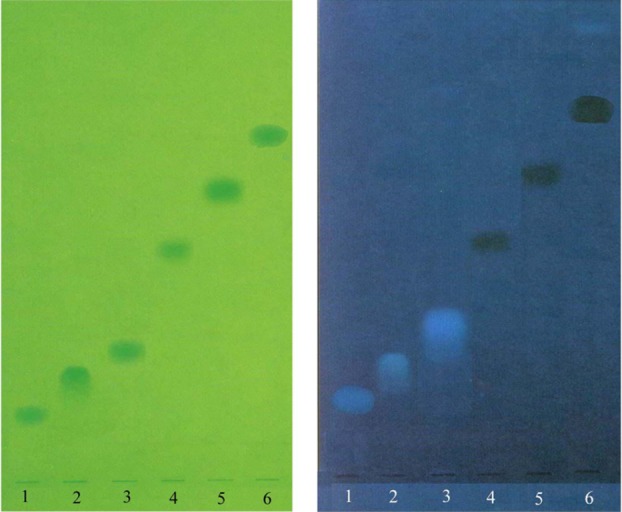
Chromatogram obtained at the separation of cephalosporins using mobile phase III (ethyl acetate – water – acetic acid 60:20:20), detection in UV light at 254 nm (A) and 366 nm (B) (1 - CFD, 2 - CEL, 3 – CFC, 4 – CFR, 5 – CZI, 6 - CTR)
The Rf values, colors and fluorescence of the spots are mentioned in Table 2 and 3 respectively.
Table 2. Rf values of the studied beta-lactams in the six-development system.
| Beta-lactam derivative | Rf values | |||||
| I | II | III | IV | V | VI | |
| AMP | 0.43 | 0.45 | 0.48 | 0.36 | 0.40 | 0.45 |
| AMO | 0.38 | 0.41 | 0.42 | 0.34 | 0.38 | 0.39 |
| PEN | 0.79 | 0.82 | 0.87 | 0.66 | 0.75 | 0.80 |
| OXA | 0.84 | 0.85 | 0.92 | 0.71 | 0.77 | 0.84 |
| CFD | 0.15 | 0.17 | 0.19 | 0.14 | 0.15 | 0.18 |
| CEL | 0.20 | 0.20 | 0.22 | 0.18 | 0.21 | 0.24 |
| CFC | 0.23 | 0.23 | 0.26 | 0.18 | 0.19 | 0.21 |
| CFR | 0.42 | 0.45 | 0.48 | 0.36 | 0.40 | 0.46 |
| CZI | 0.58 | 0.60 | 0.61 | 0.46 | 0.51 | 0.57 |
| CTR | 0.67 | 0.69 | 0.72 | 0.55 | 0.60 | 0.64 |
Table 3. Colours and fluorescence of beta-lactams developed by the six TLC systems.
| Beta-lactam derivative | - | Detection | in (with) | - |
| - | UV 254 | UV 366 nm | Iodine vapors | Ninhydrine reaction |
| AMP | brown | fluorescent blue | yellow-brown | reddish |
| AMO | brown | fluorescent blue | yellow-brown | reddish |
| PEN | brown | fluorescent green | yellow | pale red |
| OXA | brown | fluorescent green | yellow | pale red |
| CFD | brown | fluorescent blue | yellow-orange | pale red |
| CEL | brown | fluorescent blue | yellow-orange | pale red |
| CFC | brown | fluorescent blue | yellow-orange | pale red |
| CFR | brown | fluorescent dark blue | yellow-orange | negative |
| CZI | brown | fluorescent dark blue | yellow-orange | negative |
| CTR | brown | pale dark blue | yellow-orange | negative |
Difficulties appear at the separation of the two aminopenicillins (AMP, AMO) and of the three first generation oral cephalosporins (CEL, CFD, CFC) respectively; substances with similar structural and physico-chemical characteristics.
It is often advantageous in TLC to be able to obtain preliminary impression of a substance separation by exposing the plate to a rapidly carried out, economically priced universal reaction before passing on to final characterization using group-specific specific reactions.2,3,12 Detection by iodine is usually based on physical concentration of iodine molecule in the lipophilic chromatogram zones, without the occurrence of any chemical reaction. Iodine is more strongly enriched in the substance zones than in the neighboring polar substance-free silicagel layer.2 The result was the appearance of brown chromatogram zones on a yellow background. The chromatogram zones can be stabilized by spraying them with 1 % starch solution; when the well-known blue clathrates are formed (starch-iodine inclusion compounds), which remain stable for months. The presence of different beta-lactams is demonstrated by the appearance of pale spots on a blue-purple background.
When detection takes place by spraying the chromatoplate with an iodine-potassium iodide solution, the iodine contained in the reagent reacts chemically with the penicilloic acid, as the beta-lactam ring is initially opened by alkaline hydrolysis. The subsequent treatment with starch solution employed after the iodine treatment for the stabilization and enhancement of the “iodine” chromatogram zones cannot be employed here since the layers - even after evaporation of the excess iodine - still contain so much iodine that the whole background is colored blue.
Aminopenicillins (AMP, AMO) proved to be more sensitive to ninhydrin reaction after an alkaline hydrolysis than PEN or OXA, which were scarcely visible. Cephalosporins couldn’t be detected clearly with ninhydrine, as ninhydrine reacts actually with a degradation product of penicillins in alkaline environment, D-penicillamine. In general, amino - acid related compounds could be visualized using the ninhydrin reagent.
Penicillins can be also detected by spraying the plates with sulphuric acid – formaldehyde reagent (37% formaldehyde solution in conc. sulphuric acid 1:10), dark yellow spots were detected for the two aminopenicillins (AMP, AMO), PEN gave a brown spot while OXA a yellow one. All the cephalosporins gave pale yellow spots with this reagent. Heating was not necessary.
Dragendorff reagent (solution of potassium bismuth iodide) was also applied for detection, but beta-lactams proved to be less sensitive to this commonly used chromatographic reagent, as all the penicillins gave a very pale orange spot.
Although the stability of beta-lactam antibiotics in solid state is generally satisfying, beta-lactams dissolved in water or other solvents gradually convert to different degradation products through hydrolysis. After dissolution of each antibiotic the sample solutions were analyzed using mobile phase III, several times over duration of a week. The extent of the hydrolysis of beta-lactams is highly dependant on the time and temperature. Some degradation products detectable by TLC were found in the case of PEN after 48 hours storage. Possible degradation was noticeable on the chromatoplate for OXA, CEL, CFD and CFC after samples were stored in a refrigerator for a week, as they produced more than one spot on the TLC plate. Therefore the concentration value of beta-lactams determined in a certain sample solution is valid just for the application time on the chromatoplate, but does not provide valid information about the amount of beta-lactam at the time of the sampling step. It is highly advisable for sample solutions to be stored under refrigeration, especially if the sample cannot be analyzed shortly after dissolution of the antibiotic. This is especially important if the TLC method is applied for direct determination from biological samples.
With the unstable nature of these antibiotics in mind, degradation of the drugs may occur during the run and shadow spots and tailing may result from interactions between the sample and the developing solvent; however in our case these disadvantages were insignificant with the selected chromatographic systems.
Conclusion
All the studied beta-lactam derivatives may be separated using silicagel as stationary phase with an appropriate mobile phase. The use of a mobile phase, or a combination of two mobile phases, or a combination of a mobile phase and a color reaction enables the identification of the studied substances from complex mixtures. No system will separate all the beta-lactams, but they could be identified when supplementary information is obtained from color reactions and/or by using additional TLC systems. This identification technique is easy to perform and can be applied in preliminary analytical screening.
With some restriction regarding stability of beta-lactam antibiotics, the solvents and detection system mentioned above allow a rapid and convenient separation and detection of a large number of penicillins and cephalosporins and can find useful applications in the purity and stability control of beta-lactams in solution and dosage forms.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Cristea A. Tratat de Farmacologie. Bucureşti: Editura Medicală; 2011. [Google Scholar]
- 2.Fried B, Sherma J. Thin-layer Chromatography. 4th ed. New York: Marcel Dekker Inc; 1999.
- 3.Wall P. Thin-layer chromatography – A modern practical approach, RSC Chromatography Monographs. London: Royal Society of Chemistry; 2005.
- 4.European Pharmacopoeia. 7th ed. Strasbourg: Council of Europe; 2010.
- 5.Hendrickx S, Roets E, Hoogmartens J, Vanderhaeghe H. Identification of penicillins by thin-layer chromatography. J Chromatogr A . 1984;291:211–8. [Google Scholar]
- 6.Quintens I, Eykens J, Roets E, Hoogmartens J. Identification of cephalosporins by thin layer chromatography and color reactions. J Plan Chromatogr . 1993;6:181–6. [Google Scholar]
- 7.Nabi SA, Laiq E, Islam A. Selective separation and determination of cephalosporins by TLC on stannic oxide layers. Acta Chromatogr . 2004;14:92–101. [Google Scholar]
- 8.Choma IM. TLC Separation of cephalosporins: searching for better selectivity. J Liq Chromatogr Relat Technol . 2007;30(15):2231–40. [Google Scholar]
- 9.Block JH, Beale JM. Wilson and Gisvold’s textbook of Organic Medicinal and Pharmaceutical Chemistry. 11th ed. Philadelphia: Lippincott Williams and Wilkins; 2004.
- 10.Sadek PC. Ilustrated Pocket Dictionary of Chromatography. New Jersey: Wiley and Sons Inc; 2004.
- 11.Cazes J. Encyclopedia of Chromatography. 3rd ed. New York: Marcel Dekker Inc; 2009.
- 12.Pachaly P. DC-Atlas – Dünnschicht Chromatographie in der Apotheke. Stuttgart: Wissenschaftliche mbH; 2010.



