Abstract
Purpose: The purpose of this study was the isolation and structure elucidation of chemical compounds from the rhizomes of Eremostachys laciniata (L) Bunge (EL), an Iranian traditional medicinal herb with a thick root and pale purple or white flowers as well as the clinical studies on the therapeutic efficacy and safety of topical application of the EL extract in the management of some inflammatory conditions, e.g., arthritis, rheumatoid arthritis and septic arthritis (Riter’s syndrome). Methods: The structures of the isolated compounds were elucidated unequivocally on the basis of one and two dimensional NMR, UV and HR-FABMS spectroscopic data analyses. A single-blinded randomized clinical trial was carried out with the extract of the rhizomes of E. laciniata (EL) to determine the efficacy and safety of the traditional uses of EL compared to that of piroxicam in treatment of inflammatory diseases, e.g., osteoarthritis, rheumatoid arthritis and Reiter’s syndrome. Results: Eleven iridoid glycosides, two phenylethanoids and two phytosterols were isolated and identified for the first time from the rhizomes of EL. After 14 days of treatment with the EL and piroxicam ointments, all groups showed significant improvements compared to the control groups. EL (5%) ointment induced better initial therapeutic response than piroxicam (5%) onitment. Conclusion: This clinical trial established that EL was suitable for topical applications as a safe and effective complementary therapy for inflammatory diseases.
Keywords: Eremostachys laciniata, Lamiaceae, Iridoid, Phenylethanoid, Phytosterol, Inflammatory diseases
Introduction
Eremostachys laciniata (L) Bunge (family: Lamiaceae alt, Labiatae; sub-family: Lamioideae) is a perennial medicinal herb with a thick root and pale purple or white flowers. It is one of the fifteen endemic Iranian species of the genus Eremostachys, and is also grown in other countries of the Middle-East Asia, Western Asia, and Caucasus.1,2 Traditionally, a decoction of the roots and flowers of E. laciniata (EL) has been used to treat allergies, headache and liver diseases.3-5 This plant is also used to alleviate inflammatory conditions. It is usually given as a remedy in the form of herbal teas, or tisanes of the roots and flowers. The merit of the traditional uses of EL has been supported by some previous phytochemical studies on the genus Eremostachys, providing the isolation and identification of several bioactive compounds. Previous phytochemical study on EL established the presence of mono- and sesqui-tepenes in its essential oils.6 The crude extract of this plant was reported to possess free-radical-scavenging property.7 As a part of our on-going studies on plants of Iranian flora,4,5,8-17 we now report on the isolation and structure elucidation of 11 iridoid glucosides, two phenylethanoids and two phytosterols from the rhizomes of EL as well as the clinical studies on the therapeutic efficacy and safety of topical application of the EL extract in the management of some inflammatory conditions, e.g., arthritis, rheumatoid arthritis and septic arthritis (Riter’s syndrome).
Materials and Methods
General experimental procedures
UV spectra were obtained in MeOH using a Shimadzu UV-160A spectrometer. NMR spectra were recorded in CD3OD on a Bruker DRX 500 MHz NMR spectrometer (500 MHz for 1H and 125 MHz for 13C) using the residual solvent peaks as an internal standard. HMBC spectra were optimized for a long range JH-C of 9 Hz and a NOSEY experiment was carried out with a mixing time of 0.8 s. MS analyses were performed on a Finnigan MAT95 spectrometer. HPLC separation was performed on a Shimadzu HPLC system coupled with a photo-diode-array detector (SPD-M20A). A Supelco Sep-Pak C18 10 g cartridge was used for pre-HPLC fractionation.
Plant material
The rhizomes of Eremostachys laciniata (L) Bunge, were collected during September-October 2005 from Ajabshir county in East Azarbaijan province in Iran (37o 36’ 46.7’’North latitude, 46o 11’ 15.6’’East longitude and altitude 1900 m over sea level). A voucher specimen (TUM-ADE 0204) for this collection has been retained in the herbarium of the School of Pharmacy, Tabriz University of Medical science, Iran.
Extraction and isolation
The dried and ground rhizomes of EL (100 g) were Soxhlet-extracted, successively, with n-hexane, dichloromethane (DCM) and MeOH (1.1 L each). All these extracts were separately concentrated using a rotary evaporator at a maximum temperature of 45 °C to yield 1.52 g, 0.92 g and 14.72 g of dried n-hexane, DCM and MeOH extracts, respectively. The MeOH extract (2 g) was subjected to Sep-Pack fractionation (Sep-Pak, C18 cartridge; 10 g) using a step gradient of MeOH-water mixture (10:90, 20:80, 40:60, 60:40, 80:20 and 100:0). The preparative reversed-phase HPLC analysis of the 10% methanolic Sep-Pak fraction afforded eight iridoid glycosides (compounds 1-8) and the 20% methanolic Sep-Pak fraction of afforded two iridoid glycosides (compounds 9 and 10). Similar HPLC purification of the 40% methanolic Sep-Pak fraction provided an iridoid glycoside and two phenyl ethanoids (compounds 11, 12 and 13, respectively). The n-hexane extract (1.28 g) was subjected to vacuum liquid chromatography (VLC) on silica gel 60H using a step gradient of n-hexane: ethyl acetate (100:0, 90:10, 80:20, 60:40, 40:60, 20:80, 100:0). Further purification of the 10% ethyl acetate fraction was carried out by preparative thin layer chromatography on silica gel GF254 using chloroform:acetone (92:8) resulted in the isolation of the compounds 14 and 15 (Rf = 0.51 and 0.54, respectively). The relative physical characteristics, retention times and weights of the compounds 1-15 are outlined in Table 1. The chemical structures of these compounds were elucidated by spectroscopic means.
Table 1. Appearance, molecular formula, retention time and weights of the compounds (1-15) isolated from Eremostachys laciniata.
| Compound name | Physical characteristic | Molecular formula |
Retention Time (min) /Rf value |
Weight (mg) |
| 9-epi-Phlomiol (1) | Pale yellow amorphous solid | C17H26O13 | 12.8 | 16.2 |
| 9-epi-pulchelloside II (2) | White amorphous solid | C17H26O12 | 17.9 | 8.3 |
| 6-ß-Hydroxy-7-epi-loganin (3) | Pale yellow amorphous solid | C17H26O11 | 19.1 | 10.0 |
| Lamalbide (4) | Pale yellow amorphous solid | C17H26O12 | 23.8 | 98.9 |
| Sesamoside (5) | Pale yellow amorphous solid | C17H24O12 | 32.2 | 78.9 |
| 6'-O-ß-D-Glucopyranosyl- sesamoside (6) |
White amorphous solid | C23H34O17 | 38.9 | 1.8 |
| Shanzhiside methyl ester (7) | Pale yellow amorphous solid | C17H26O11 | 47.1 | 13.9 |
| 5, 9-epi-Phlomiol (8) | White amorphous solid | C17H26O13 | 13.6 | 9.5 |
| Phloyoside II (9) | White amorphous solid | C17H25O12Cl | 29.6 | 5.1 |
| 5,9-epi-Penstemoside (10) | Pale yellow amorphous solid | C17H26O11 | 40.3 | 2.0 |
| 6,9-epi-8-O-Acetyl-shanzhiside metyl ester (11) |
Brown amorphous solid | C19H27O12 | 25.9 | 6.5 |
| Forsythoside B (12) | Brown amorphous solid | C34H44O19 | 41.1 | 15.0 |
| Verbascoside (13) | Brown amorphous solid | C29H36O15 | 48.6 | 7.9 |
| Stigmasterol (14) | White crystalline solid | C29H48O | 0.51* | 2.3 |
| β-Sistosterol (15) | White crystalline solid | C29H50O | 0.54* | 2.8 |
| * Rf value on silica gel GF254 using chloroform: acetone (92:8) as the mobile phase | ||||
Clinical study
Efficiency of the dried MeOH extract of EL in topical management of inflammatory diseases was studied in patients suffering from osteoarthritis, rheumatoid arthritis and Reiter’s syndrome. A single-blinded randomized clinical trial was designed to evaluate the therapeutic efficiency of EL alone and also in comparison with piroxicam. Ethics Committee of Tabriz University of Medical Sciences approved this study. Written informed consent was obtained from each participant before the commencement of the project. One hundred and thirty seven patients with an age range of 18-80 years of either sex (female 60% and male 40%) were randomly divided into two major groups. One group (67 patients) received EL and the other (70 patients) was treated with topical piroxicam. Both the EL extract and piroxicam were applied in the form of topical ointments with 5% of active ingredient. Each major group was divided into three minor groups according to the type of disease (osteoarthritis, rheumatoid arthritis and reiter’s syndrome). Medications which were administered along with our treatment protocol were cartigene, indomethacin and methotrexate, in the case of osteoarthritis, rheumatoid arthritis and reiter’s syndrome, respectively. Furthermore, the piroxicam and EL ointments were well matched for colour, smell, and consistency. Neither the nurse nor the patient could distinguish the differences between preparations. The ointments (piroxicam and EL) were gently massaged around the affected joint, mostly knee joints, for 2 to 3 minutes, two times a day, for a consecutive 14 days. Massaging for several minutes was used to facilitate penetration of the cream by increasing local blood supply and encouraging local movement. After application, the area was covered with a cotton cloth. The patients were personally examined by a physician every week during the course of the study. During these visits, the patients were asked about compliance, correctness of the ointment application and the level of pain they had, as well as the size of inflammated joint. Patients were instructed to record the level of their pain on a 10 cm visual analogue scale (VAS) labelled as “no pain” at one side and “worst pain ever” at the other side. The amount of inflammation for each joint was measured by nurses using a centimetre. As a control, inflammation of the joints and the associated pain for each group was recorded at the beginning of the study before application of the ointments. Additionally, any irritations or possible side effects in the patients were assessed to evaluate the safety of the EL in topical application. All nondrug modalities of treatment and all adjunctive drug treatments remained constant during the study for the parallel groups with the same types of diseases.
Statistical analysis
All data were statistically analysed by the analysis of variance or Tukey’s multiple comparison tests. A probability level of P<0.05 was taken to be statistically significant in the analysis.
Results and Discussion
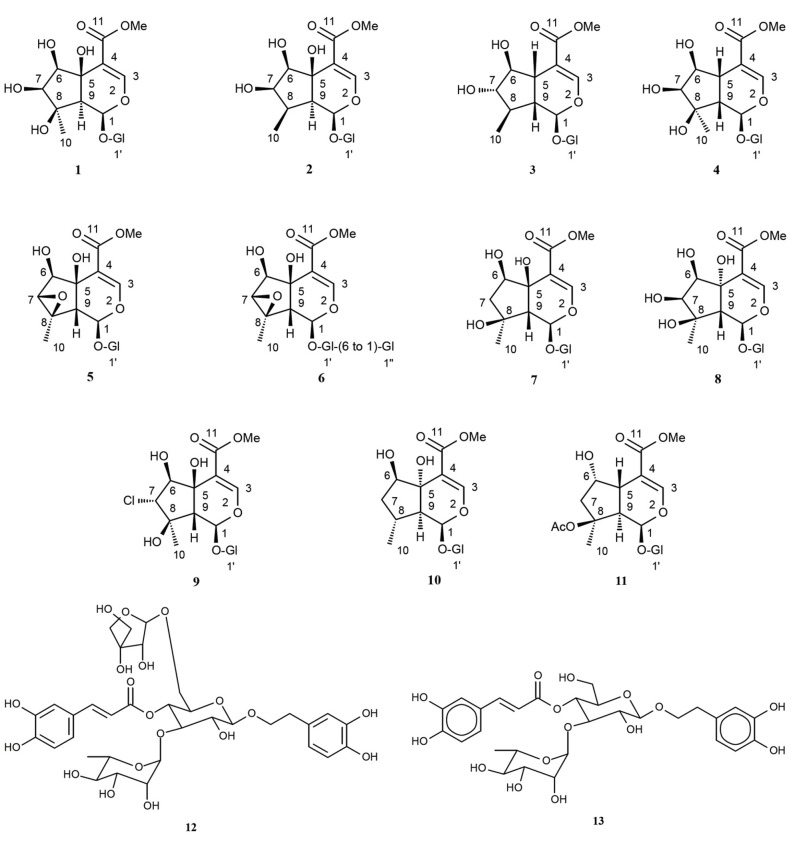
A combination of solid-phase extraction and preparative reversed-phase HPLC of the MeOH extract of the rhizomes of EL afforded a total of 13 compounds (1-13), 11 of which were iridoid glycosides (1-11), and two other were phenylethanoids (12 and 13) (Table 1; Figure 1). The VLC followed by prep-TLC of the n-hexane extract of this plant yielded two phytosterols (14 and 15) (Table 1). The chemical structures of these compounds were elucidated unambiguously on the basis of one and two dimensional NMR, UV and HR-FABMS spectroscopic data analyses, and also by comparing experimental data with literature data. The 1H and 13C NMR data of all iridoids (1-11) are presented in Tables 2-4. The identified compounds were: 9-epi-phlomiol (1),18 9-epi-pulchelloside ІІ (2),18,19 6-β-hydroxy-7-epi-loganin (3) 20-22, lamalbide (lamiridoside, 4),23sesamoside (5)18,24-26,6'-O-β-D-glucopyranosyl sesamoside (6),5,6, 27-30 shanzhiside methyl ester (7),31 5,9-epi-phlomiol (8),28,29 phloyoside ІІ (9),18 5,9-epi-penstemoside (10),4 6,9-epi-8-O-acetyl shanzhiside methyl ester (11),4,32 forsythoside B (12) and verbascoside (13),16 stigmasterol (14) and β-sitosterol (15).16
Figure 1 .
Structures of iridoids (1-11) and phenylethanoidsisolated (12 and 13) from Eremostachys laciniata.
Table 2. 13C NMR (125 MHz, CD3OD) data of iridoids 1-11.
| Chemical shift dc in ppm | |||||||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| 1 | 93.1 | 95.0 | 95.1 | 93.9 | 95.9 | 96.5 | 93.8 | 93.9 | 92.0 | 94.8 | 94.7 |
| 2 | - | - | - | - | - | - | - | - | - | - | - |
| 3 | 153.7 | 153.6 | 152.0 | 151.9 | 154.6 | 154.4 | 151.8 | 154.2 | 152.8 | 154.3 | 152.7 |
| 4 | 115.0 | 114.9 | 110.2 | 110.7 | 112.0 | 112.0 | 110.4 | 114.4 | 113.6 | 112.5 | 108.8 |
| 5 | 64.9 | 68.7 | 40.1 | 36.6 | 74.0 | 74.2 | 40.4 | 70.1 | 65.1 | 72.5 | 41.3 |
| 6 | 79.7 | 83.3 | 83.4 | 77.7 | 76.5 | 76.8 | 77.0 | 77.2 | 79.7 | 75.7 | 74.9 |
| 7 | 83.7 | 83.0 | 84.8 | 76.9 | 65.0 | 65.0 | 48.2 | 80.5 | 72.1 | 39.6 | 46.6 |
| 8 | 74.9 | 37.8 | 38.4 | 78.0 | 62.8 | 62.6 | 78.0 | 78.4 | 73.6 | 30.5 | 88.8 |
| 9 | 57.6 | 53.9 | 43.5 | 48.2 | 53.4 | 53.4 | 50.7 | 56.9 | 57.4 | 49.6 | 48.9 |
| 10 | 17.2 | 16.0 | 16.2 | 21.2 | 16.8 | 17.0 | 23.7 | 22.3 | 17.5 | 15.6 | 21.2 |
| 11 | 168.3 | 168.5 | 169.1 | 168.6 | 168.1 | 169.8 | 168.7 | 168.8 | 167.1 | 167.3 | 168.0 |
| 11-OMe | 51.9 | 51.8 | 50.9 | 51.1 | 51.4 | 51.3 | 50.9 | 51.7 | 51.0 | 50.7 | 50.8 |
| Me-COO | - | - | - | - | - | - | - | - | - | - | 21.2, 172.2 |
| 1' | 99.7 | 100.0 | 99.1 | 98.8 | 99.0 | 99.2 | 98.8 | 99.5 | 98.7 | 98.8 | 99.4 |
| 2' | 74.4 | 74.4 | 73.7 | 73.6 | 73.7 | 73.8 | 73.6 | 74.4 | 73.3 | 73.4 | 73.7 |
| 3' | 77.4 | 77.5 | 77.0 | 76.9 | 76.7 | 76.7 | 76.5 | 77.5 | 76.4 | 76.4 | 77.0 |
| 4' | 71.7 | 71.6 | 70.6 | 70.6 | 70.8 | 70.8 | 70.6 | 71.6 | 70.6 | 70.7 | 70.6 |
| 5' | 78.4 | 78.5 | 77.4 | 77.3 | 77.7 | 77.2 | 77.4 | 78.5 | 77.4 | 77.4 | 77.3 |
| 6' | 62.8 | 62.7 | 61.7 | 61.8 | 62.0 | 71.0 | 61.9 | 62.6 | 61.8 | 61.8 | 62.0 |
| 1’’ | - | - | - | - | - | 104.7 | - | - | - | - | - |
| 2’’ | - | - | - | - | - | 73.6 | - | - | - | - | - |
| 3’’ | - | - | - | - | - | 76.7 | - | - | - | - | - |
| 4’’ | - | - | - | - | - | 70.7 | - | - | - | - | - |
| 5’’ | - | - | - | - | - | 77.0 | - | - | - | - | - |
| 6’’ | - | - | - | - | - | 61.8 | - | - | - | - | - |
Table 4. 1H NMR (500 MHz, coupling constant J in Hz in parentheses, CD3OD) data of iridoids 7-11 .
| Chemical shift dH in ppm | |||||
| Position | 7 | 8 | 9 | 10 | 11 |
| 1 | 5.61 d (2.6) | 5.38 s | 5.89 s | 5.85 s | 5.95 s |
| 2 | - | - | - | - | - |
| 3 | 7.44 d (0.9) | 7.46 s | 7.54 s | 7.59 s | 7.48 d (1.3) |
| 4 | - | - | - | - | - |
| 5 | 3.04 dd (10.1, 2.9) | - | - | - | 3.11 dd (9.0, 1.4) |
| 6 | 4.08 m | 4.18 d (4.5) | 3.78 d (9.7) | 4.32 t (4.47) | 4.37 m |
| 7a | 1.86 dd (13.2, 6.0) | 3.66 d (4.5) | 4.08 d (9.7) | 1.52 m | 2.05 dd (14.9, 5.3) |
| 7b | 2.06 dd (13.2, 6.4) | - | - | 1.83 m | 2.24 d (15.0) |
| 8 | - | - | - | 2.54-2.70* | - |
| 9 | 2.65 dd (10.2, 2.5) | 2.51 s | 2.56 s | 2.54-2.70* | 3.03 dd (9.0, 2.1) |
| 10 | 1.30 s | 1.39 s | 1.16 s | 0.98 d (6.9) | 1.55 s |
| 11 | - | - | - | - | - |
| 11-OMe | 3.77 s | 3.73 s | 3.78 s | 3.76 s | 3.75 s |
| Me-COO | - | - | - | - | 2.05 s |
| 1’ | 3.67 (d (7.8) | 4.61 d (8.0) | 4.64 d (7.8) | 4.61 d (7.7) | 4.67d (7.8) |
| 2’ | 3.20 t (8.3) | 3.20 t (8.5) | 3.23 t (8.8) | 3.22 dd (8.9, 7.9) | 3.21 t (8.4) |
| 3’ | 3.36-3.44* | 3.35-3.38* | 3.36-3.47* | .3.36-3.46* | 3.36-3.45* |
| 4’ | 3.25-3.34* | 3.22-3.31* | 3.27-3.35* | 3.22-3.35* | 3.25-3.35* |
| 5’ | 3.33-3.40* | 3.31-3.36* | 3.32-3.39* | 3.33-3.39* | 3.33-3.40* |
| 6’a | 3.68 dd (12.0, 5.7) | 3.66-3.71* | 3.70 dd (11.9, 5.5) | 3.69 dd (12.0, 5.8) | 3.64-3.75* |
| 6’b | 3.94 dd (11.9, 1.6) | 3.90 d (12.0) | 3.94 dd (11.9, 1.6) | 3.95 dd (11.9, 1.8) | 3.90-3.96* |
To the best of our knowledge, this is the first report on the thorough phytochemical investigation on the rhizomes of EL growing in Iran as well as the presence of 6'-O-β-D-glucopyranosyl sesamoside (6) in the genus Eremostachys. This compound was first reported in Lamiophlomis rotata, another plant from the family Lamiaceae.30 Most of these iridoid glycosides were, however, previously reported from the aerial parts of this plant growing in Turkey,29 and phloyoside І, pulchelloside І and phlomiol exhibiting anti-bacterial activities also were reported before from this plant.17
Iridoid glycosides also occur in other species of the genus Eremostachys4,28,29 which is taxonomically close to the genus Phlomis. Interestingly, the Phlomis is also well known for producing variety of iridoid glycosides.4 Both the genera, Eremostachys and Phlomis, belong to the subtribe Lamieae of the family Lamiaceae4,33 and they are morphologically similar.Anatomical and cytological studies on the species of these genera also established this close affinity between these two genera. During the preliminary chemotaxonomic studies on the family Lamiaceae using flavonoids as the markers, some degrees of similarities between these genera were also identified.4 Iridoids have been considered as valuable chemotaxonomic markers,33 and in fact, they have been employed successfully to describe chemotaxonomic relationships among the taxa within various families, e.g. Acanthaceae, Bigoniaceae, Cornaceae, Oleaceae, and Rubiaceae. Within the family Lamiaceae, iridoid glycosides have recently been employed as chemotaxonomic markers for the species of the genus Lamium.25 Therefore, the co-occurrence of iridoid glycosides, in the closely related genera Eremostachys and Phlomis could be significant chemotaxonomically. It is also worth-mentioning that the iridoid profiles in the aerial parts and the rhizomes were found to be similar, and so is within the plant samples collected from two different geographical locations, one from Turkey28 and the other from Iran.
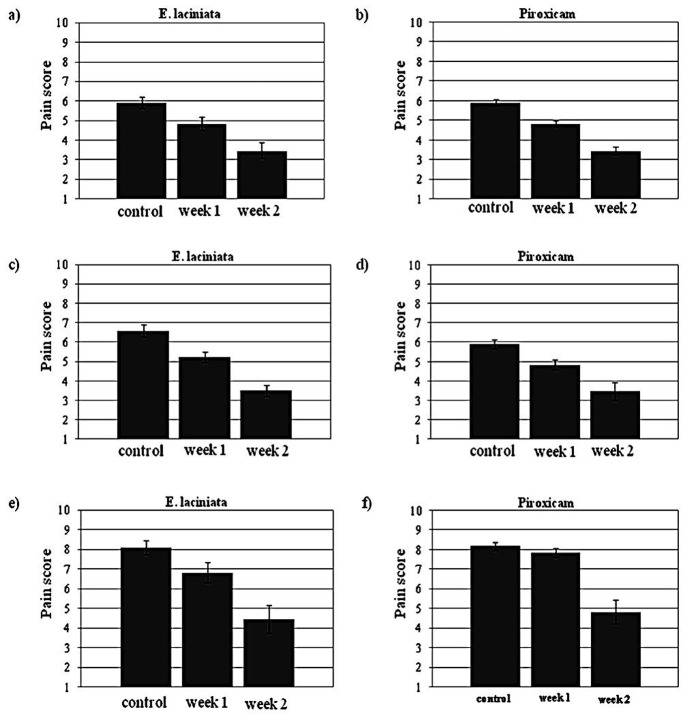
In order to evaluate the efficacy and safety of topical treatment of EL extract containing ointment (EL) in patients with inflammatory complications, a total of 137 patients were screened during 14 days of the study. Data from patients were analyzed, and the demographics are presented in Figures 2 and 3. Figure 2 illustrates the pain scores (VAS) before and after treatment in both groups treated with EL and piroxicam. In terms of VAS values for EL, the mean score in patients suffering from arthritis, rheumatoid arthritis and Reiter’s syndrome fell from 5.9, 6.6 and 8.1 to 3.5, 3.5 and 4.4 points after 14 days, respectively. In treatment with piroxicam, the values showed a decrease from 6.3, 7.0 and 8.1 to 3.7, 3.7 and 4.8 for the patients with arthritis, rheumatoid arthritis and Reiter’s syndrome correspondingly. After 14 days of treatment with the EL and piroxicam ointments, all groups showed significant improvement when compared with their control groups (p<0.001) consequently, both ointments were effective in relieving patients’ pains. Nevertheless, EL induced much better initial therapeutic response than piroxicam. In the cases of osteoarthritis and rheumatoid arthritis, notable differences between the two groups (piroxicam and EL) were observed in the first week of treatment (Figure 2), indicating enhanced onset of drug action for EL.
Figure 2.
Effects of EL and piroxicam on pain relief in patients; (a/b) arthritis, (c/d) rheumatoid arthritis and (e/f) Reiter’s syndrome.
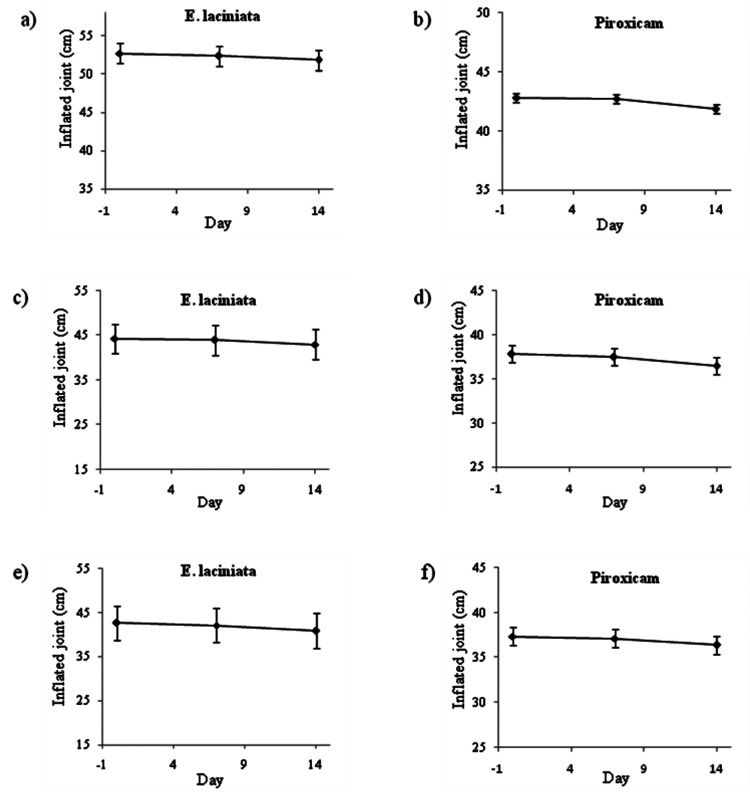
Figure 3 .
Effects of EA and piroxicam on the size of inflammated joints in patients; (a/b) arthritis, (c/d) rheumatoid arthritis and (e/f) Reiter’s syndrome.
Table 3. 1H NMR (500 MHz, coupling constant J in Hz in parentheses, CD3OD) data of iridoids 1-6.
| Chemical shift dH in ppm | ||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | 5.83 s | 5.67 d (2.0) | 5.44 d (3.9) | 5.65 s | 5.53 d (8.6) | 5.49 d (9.3) |
| 2 | - | - | - | - | - | - |
| 3 | 7.49 s | 7.48 s | 7.47 s | 7.45 s | 7.62 s | 7.60 s |
| 4 | - | - | - | - | - | - |
| 5 | - | - | 2.78 dd (8.6, 4.8) | 2.97 dd (10.7, 2.6) | - | - |
| 6 | 3.56 d (9.0) | 3.67 d (7.0) | 3.50 dd (8.7, 5.9) | 3.99 t (3.8) | 4.36 s | 4.45 d (1.4) |
| 7a | 3.82 d (9.0) | 3.43 dd (7.0, 6.5) | 3.72 d (5.2) | 3.59 d (4.2) | 3.51 s | 3.56 s br |
| 7b | - | - | - | - | - | - |
| 8 | - | 1.35 m | 1.73 m | - | - | 2.54 d (9.3) |
| 9 | 2.47 s | 1.96 dd (12.0, 1.5) | 2.07 dt (9.4, 3.8) | 2.84 d (10.9) | 2.56 d (8.6) | 1.57 s |
| 10 | 1.02 s | 1.14 d (6.5) | 1.19 d (6.6) | 1.24 s | 1.54 s | - |
| 11 | - | - | - | - | - | 3.78 s |
| 11-OMe | 3.74 s | 3.73 s | 3.77 s | 3.77 s | 3.78 | 4.77 d (7.8) |
| Me-COO | - | - | - | - | - | 3.20-3.30* |
| 1’ | 4.60 d (7.5) | 4.58 d (8.0) | 4.67 d (7.8) | 4.66 d (7.8) | 4.77 d (7.8) | 3.35-3.52* |
| 2’ | 3.19 dd (9.5, 8.0) | 3.19 dd (9.0, 8.0) | 3.21 t (8.2) | 3.21 t (8.3) | 3.27 dd (8.8, 3.1) | 3.26-3.36* |
| 3’ | 3.38 t (9.0) | 3.37 t (8.8) | 3.36-3.43* | 3.37-3.46* | 3.36-3.44* | 3.33-3.42* |
| 4’ | 3.28 t (9.3) | 3.27 t (9.3) | 3.24-3.36* | 3.25-3.34* | 3.26-3.36* | 3.56-3.78* |
| 5’ | 3.32-3.35* | 3.32-3.35 * | 3.30-3.40* | 3.34-3.42* | 3.33-3.40* | 4.23 d br (11.8, 1.5) |
| 6’a | 3.66 dd (12.0, 6.0) | 3.67 dd (12.0, 5.5) | 3.69 dd (12.4, 5.3) | 3.70 dd (12.1, 5.1) | 3.65 dd (11.8, 6.5) | 4.38 d (7.4) |
| 6’b | 3.90 dd (11.8, 2.3) | 3.90 dd (11,8, 1.8) | 3.93 d (12.1) | 3.96 d (13.3) | 3.96 d (11.7) | 3.20-3.30* |
| 1’’ | - | - | - | - | - | 3.35-3.52* |
| 2’’ | - | - | - | - | - | 3.26-3.36* |
| 3’’ | - | - | - | - | - | 3.33-3.42* |
| 4’’ | - | - | - | - | - | 3.56-3.78* |
| 5’’ | - | - | - | - | - | 3.91 dd (12.0, 1.4) |
| 6’’a | - | - | - | - | - | 7.60 s |
| 6’’b | - | - | - | - | - | - |
Scatter diagrams were used to determine and compare variations in the size of inflammated joints for patients in different groups, during the 14 days of the study (Figure 3). The profiles for the inflammated joints did not alter significantly within the EL and piroxicam treated groups. However, the results showed a significant difference in efficiency of the ointments between the EL and piroxicam in the arthritis-patients (p<0.005; Figure 3a and 3b). Moreover, two of the patients demonstrated dermatitis leading to withdrawal from the study and five patients reported itching after applying the ointment; but no more serious side effects from other patients were recorded.
To the best of our knowledge, this clinical trial is the first attempt to provide evidence-based scientific support for topical application of EL extract containing ointment for the management of inflammatory diseases. Clinical observations in relation to anti-inflammatory property of EL described in this paper are in good agreement with the findings from previous animal study on mice model.34
Conclusion
The present study has shown that the rhizomes of EL are rich in irirdoid glycosides. Since the analgesic, anti-inflammatory and anti-arthritic properties of iridoid glycosides have previously been demonstrated by several researchers in animal models,35 it is therefore reasonable to infer that the clinical effects of EL on managing inflammation in arthritic patients, as demonstrated in the present study, is probably owing to the presence iridoids in this plant. Some of the anti-inflammatory effects may also be partially due to the phenylethanoids (12, 13), as these compounds are also known to produce anti-inflammatory and antioxidant effects.36-38
Acknowledgement
We thank the EPSRC National Mass Spectrometry Service Centre (Grove Building, Swansea University, Singleton Park, Swansea, SA2 8PP, Wales, UK) for MS analyses. The Medicinal Plants Research Network of the Ministry of Health and Medical Education Iran is thanked for financial support for this study.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran: Farhang Moaser; 1996. [Google Scholar]
- 2.Germplasm Resources Information Network - (GRIN). USDA, ARS, National Genetic Resources Program. [Online Database] Beltsville, Maryland: National Germplasm Resources Laboratory; 2013 [cited 2013 06 August]; Available from: http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?15378. [Google Scholar]
- 3.Said O, Khalil K, Fulder S, Azaizeh H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J Ethnopharmacol . 2002;83(3):251–65. doi: 10.1016/s0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 4.Delazar A, Byres M, Gibbons S, Kumarasamy Y, Modarresi M, Nahar L. et al. Iridoid glycosides from Eremostachys glabra. J Nat Prod . 2004;67(9):1584–7. doi: 10.1021/np040044b. [DOI] [PubMed] [Google Scholar]
- 5.Delazar A, Modarresi M, Shoeb M, Nahar L, Reid RG, Kumarasamy Y. et al. Eremostachiin: a new furanolabdane diterpene glycoside from Eremostachys glabra. Nat Prod Res . 2006;20(2):167–72. doi: 10.1080/13518470500047082. [DOI] [PubMed] [Google Scholar]
- 6.Navaei MN, Mirza M. Chemical composition of the oil of Eremostachys laciniata (L.) Bunge from Iran. Flavour Frag J. 2006;21(4):645–6. [Google Scholar]
- 7.Erdemoglu N, Turan NN, Cakoco I, Sener B, Aydon A. Antioxidant activities of some Lamiaceae plant extracts. Phytother Res. 2006;20(1):9–13. doi: 10.1002/ptr.1816. [DOI] [PubMed] [Google Scholar]
- 8.Delazar A, Reid RG, Sarker SD. GC-MS analysis of essential oil of the oleoresin from Pistacia atlantica VAR mutica. Chem Nat Comp . 2004;40(1):24–7. [Google Scholar]
- 9.Delazar A, Celik S, Gokturk RS, Unal O, Nahar L, Sarker SD. Two acylated flavonoid glycosides from Stachys bombycina, and their free radical scavenging activity. Die Pharmazie. 2005;60(11):878–80. [PubMed] [Google Scholar]
- 10.Delazar A, Biglari F, Esnaashari S, Nazemiyeh H, Talebpour AH, Nahar L. et al. GC-MS analysis of the essential oils, and the isolation of phenylpropanoid derivatives from the aerial parts of Pimpinella aurea. Phytochemistry . 2006;67(19):2176–81. doi: 10.1016/j.phytochem.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Delazar A, Talischi B, Nazemiyeh Z, Rezazadeh H, Nahar L, Sarker SD. Chrozophorin: a new acylated flavone glucoside from Chrozophora tinctoria (Euphorbiaceae) Braz J Pharmacogn. 2006;16(3):286–90. [Google Scholar]
- 12.Delazar A, Gibbons S, Kosari AR, Nazemiyeh H, Modarresi M, Nahar L. et al. Flavonoid C-glycosides and cucurbitacin glycosides from Citrullus colocynthis. DARU . 2006;14(3):109–14. [Google Scholar]
- 13.Delazar A, Naseri M, Nazemiyeh H, Talebpour A-H, Imani Y, Nahar L. et al. Flavonol 3-methyl ether glucosides and a tryptophylglycine dipeptide from Artemisia fragrans (Asteraceae) Biochem Syst Ecol . 2007;35(1):52–6. [Google Scholar]
- 14.Delazar A, Naseri M, Nahar L, Moghadam SB, Esnaashari S, Nazemiyeh H. et al. GC-MS analysis and antioxidant activities of essential oils of two cultivated Artemisia species. Chem Nat Comp . 2007;43(1):112–4. [Google Scholar]
- 15.Nazemiyeh H, Maleki N, Mehmani F, Kumarasamy Y, Shoeb M, Garjani A. et al. Assessment of anti-inflammatory properties of ethyl acetate extract of Stachys schtschegleevii Sosn. DARU . 2007;15(4):174–82. [Google Scholar]
- 16.Nazemiyeh H, Delazar A, Ghahramani MA, Talebpour AH, Nahar L, Sarker SD. Phenolic glycosides from Phlomis lanceolata (Lamiaceae) Nat Prod Commun . 2008;3(1):53–6. doi: 10.1007/s11418-007-0194-z. [DOI] [PubMed] [Google Scholar]
- 17.Modaressi M, Delazar A, Nazemiyeh H, Fathi-Azad F, Smith E, Rahman MM. et al. Antibacterial iridoid glucosides from Eremostachys laciniata. Phytother Res . 2009;23(1):99–103. doi: 10.1002/ptr.2568. [DOI] [PubMed] [Google Scholar]
- 18.Gao YL, Lin RC, Wang GL, Zhao HR, Gao Y, Ciren B. Studies on the chemical constituents of Phlomis younghusbandii. Zhong Yao Cai . 2007;30(10):1239–42. [PubMed] [Google Scholar]
- 19.Franzyk H, Jensen SR, Olsen CE, Quiroga JM. A 9-hydroxyiridoid isolated from Junellia seriphioides (Verbenaceae) Org Lett . 2000;2(5):699–700. doi: 10.1021/ol0055521. [DOI] [PubMed] [Google Scholar]
- 20.Boros CA, Stermitz FR. Iridoids – An updated review, Part I. J Nat Prod. 1990;53(5):1055–147. [Google Scholar]
- 21.Boros CA, Stermitz FR. Iridoids – An updated review, Part II. J Nat Prod . 1991;54(5):1173–465. [Google Scholar]
- 22.Boros CA, Stermitz FR, McFarland N. Processing of the Iridoid Glycoside Antirrinoside from Maurandya antirrhiniflora (Scrophulariaceae) by Meris paradoxa (Geometridae) and Lepipolys Species (Noctuidae) J Chem Ecol . 1991;17(6):1123–33. doi: 10.1007/BF01402938. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Gu D, Tao B. Iridoid glycosides from Pedicula dicora Franch. Zhongguo Zhong Yao Za Zhi. 1999;24(1):40–1, 64. [PubMed] [Google Scholar]
- 24.Kang J, Jia Z. Chemical constituents of Pedicularis muscicola Maxim. Zhongguo Zhong Yao Za Zhi . 1997;22(3):167–8, 91. [PubMed] [Google Scholar]
- 25.Alipieva KI, Taskova RM, Evstatieva LN, Handjieva NV, Popov SS. Benzoxazinoids and iridoid glucosides from four Lamium species. Phytochemistry. 2003;64(8):1413–7. doi: 10.1016/j.phytochem.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Liu P, Teng J, Zhang YW, Takaishi Y, Duan HQ. Chemical constituents from rhizome of Phlomis umbrosa. Yao Xue Xue Bao. 2007;42(4):401–4. [PubMed] [Google Scholar]
- 27.Eigtved P, Jensen SR, Nielsen B. A novel iridoid glucoside isolated from Lamium album L. Acta Chem Scand B . 1974;28:85–91. [Google Scholar]
- 28.Calis I, Guvenc A, Armagan M, Koyuncu M, Gotfredsen CH, Jensen SR. Iridoid glucosides from Eremostachys molucelloides Bunge. Helv Chim Acta . 2007;90:1461–66. [Google Scholar]
- 29.Calis I, Guvenc A, Armagan M, Koyuncu M, Gotfredsen CH, Jensen SR. Secondary metabolites from Eremostachys laciniata. Nat Prod Commun . 2007;3:117–24. [Google Scholar]
- 30.Tan JJ, Tan CH, Jiang SH, Zhu DY. Iridoid glycosides from Lamiophlomis rotata. Helv Chim Acta . 2007;90(1):143–8. [Google Scholar]
- 31.Yu ZX, Wang GL, Bianba C, Lin RC. Studies on chemical constituents in root of Phlomis medicinalis I. Zhongguo Zhong Yao Za Zhi . 2006;31(8):656–8. [PubMed] [Google Scholar]
- 32.Jensen SR, Calis I, Gotfredsen CH, Sotofte I. Structural revision of some recently published iridoid glucosides. J Nat Prod . 2007;70(1):29–32. doi: 10.1021/np060452a. [DOI] [PubMed] [Google Scholar]
- 33.Azizian D, Cutler DF. Anatomical, cytological and phytochemical studies on Phlomis L and Eremostachys Bunge (Labiatae) Bot J Linn Soc . 1988;85:249–81. [Google Scholar]
- 34.Delazar A, Habibi Asl H, Mohammadi O, Afshar FH, Nahar L, Modarresi M. et al. Evaluation of analgesic activity of Eremostachys laciniata in mice. J Nat Remedies . 2009;9:1–7. [Google Scholar]
- 35.Dinda B, Debnath S, Harigaya Y. Naturally occurring secoiridoids and bioactivity of naturally occurring iridoids and secoiridoids. A review, part 2. Chem Pharm Bull (Tokyo) 2007;55(5):689–728. doi: 10.1248/cpb.55.689. [DOI] [PubMed] [Google Scholar]
- 36.Backhouse N, Delporte C, Apablaza C, Farias M, Goity L, Arraus S. et al. Antinociceptive activity of Buddleja globosa (matico) in several models of pain. J Ethnopharmacol. 2009;119:160–5. doi: 10.1016/j.jep.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Asnaashari S, Delazar A, Alipour SS, Nahar L, Williams AS, Pasdaran A. et al. Chemical composition, free-radical-scavenging and insecticidal activities of the aerial parts of Stachys byzantina. Arch Biol Sci . 2010;62:653–62. [Google Scholar]
- 38.Speranza L, Franceschelli S, Pesce M, Reale M, Menghini L, Vinciguerra I. et al. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother Res. 2010;24(9):1398–404. doi: 10.1002/ptr.3173. [DOI] [PubMed] [Google Scholar]