Abstract
Because mothers and fathers are more or less dissimilar at multiple HLA loci, mother considers her fetus as a semi-allograft. Mother's immune system may recognize paternal HLA as foreign antigen and may develop anti-paternal HLA antibodies and cytotoxic T lymphocyte. There are some mechanisms that modulate maternal immune responses during pregnancy, in order to make uterus an immune privileged site. This immunosuppression is believed to be mediated, at least partly, by HLA-G, non-classical class I human leukocyte antigen (HLA) molecule that is strongly expressed in cytotrophoblast and placenta. The major HLA-G function is its ability to inhibit T and B lymphocytes, NK cells and antigen-presenting cells (APC).Since HLA-G is expressed strongly at the maternofetal interface and has an essential role in immunosuppression, HLA-G polymorphism and altered expression of HLA-G seems to be associated with some complications of pregnancy, such as pre-eclampsia, recurrent misscariage and failure in IVF.This perspective discusses recent findings about HLA-G genetics, function, expression and polymorphism; and focus on HLA-G role in the etiology of recurrent miscarriage.
Keywords: HLA-G, Placenta, Recurrent miscarriage
Introduction
Recurrent Miscarriage (RM) is referring to ≥3 consecutive fetus losses in first-trimester or ≥2 in second-trimester. By means of this definition, RM involves 1-2% of all couples trying pregnancy.1 Although the frequency of 3 miscarriages accidentally is approximately 0.34%, the possibility of a spontaneous miscarriage is about 12–14%.2-3 This discrepancy indicates that, besides accidental causes, risk of RM is increased in some couples pathologically as a result of genetic disorders or anatomical, infectious and endocrine complications. In about 50% of RM cases, the etiology still remains unknown.4 Immunological factors play a significant role in RM etiology since the fetus and placenta are immunologically different from the mother.5
Fetus is considered as a semiallograft for maternal immune system, and ordinarily, the mother would be expected to produce antibodies and CTL (cytotoxic T lymphocytes) to foreign paternal HLA or other antigens expressed by fetal cells.6 Thus, particular mechanisms must modulate the maternal immune system in favor of success of pregnancy, so that help fetus resides inside the uterus for 9 months.7
several mechanisms protect the semiallogeneic fetus from maternal graft rejection responses.6 These strategies include lack of any physical connection between maternal and fetal tissues and fully separation of the blood circulations; lack of fetal antigens that could cause graft rejection; late appearance of transplantation antigens in the fetus; immunosuppression of leukocytes which present at the maternofetal interface; development of tolerance, build up the pregnant uterus as an immune privileged site by both the fetus and the mother.6 Failure in these mechanisms may cause complications in pregnancy like maternal rejection of the embryo/fetus.7
Immunosuppression of leukocytes which present at the maternofetal interface is one of the mechanisms that might modulate maternal immune responses during pregnancy.8 HLA-G, Non-classical class Ib human leukocyte antigen (HLA) molecules, is believed to be involved in this immunosuppression. As HLA-G is expressed on trophoblast cells in the placenta,9 it seems to be occupied in development of pregnant uterus as an immune privileged site.6 Accordingly, diminished or aberrant HLA-G expression may involve in the etiology of immunological malfunction, like pre-eclampsia, recurrent miscarriage and implantation failure in IVF.5,7
HLA antigens are most powerful cause of graft rejection. Although anti-paternal HLA antibodies are detectable in pregnant women's sera, they do no damage and they are more tolerogenic rather than immunogenic.7
The human major histocompatibility complex (MHC) genes are located on the short arm of chromosome 6 and subdivided into class Ia, which includes HLA-A, -B, and -C, and class II, which includes HLA- DR, -DQ and -DP. The non-classical HLA class Ib genes encoding HLA-E, -G -H and –F that are clustered on chromosome 6p21 at the telomeric end of the MHC region. The HLA-G gene is located close to HLA-A and seems to have a close homology with that.10
HLA class Ib antigens are similar to the HLA class Ia antigens in some characteristics, but also differ from them in several major features, including: 1) HLA class Ia genes are highly polymorphic, with so many alleles, but HLA class Ib genes are distinguished by low numbers of alleles, for example HLA-G is almost monomorphic and has five alleles.11 2) All HLA class Ia antigens are membrane bound, but one member of the class Ib group has soluble isoforms too, for example HLA-G have seven alternatively spliced transcripts that encoded four membrane bound and three soluble proteins. 3) The expression of class Ia antigens is ubiquitous while expression of class Ib antigens is organ-specific and conditional.
Structural features of HLA-G is similar to other class I genes. It has eight exons; exon 1 encodes a signal peptide, exons 2, 3, and 4, encode external part (respectively α1, α2 and α3 domains), exon 5 encodes transmembrane domain and exons 6 and 7 encode intracellular domain.12 α1 and α2 domains construct the peptide-binding cleft and α3 domain is binding site for leukocyte Ig-like receptor 1 and 2 (LIR-1 and LIR-2), which are inhibitory receptors. HLA-G has two unique characteristic; first, a stop codon in exon 6 cause a shortened cytoplasmic tail that results in the prolonged expression of HLA-G at the cell surface, and inefficient presentation of exogenous peptides.10 Second, due to mRNA alternative splicing, HLA-G encodes multiple isoforms including four membrane-bound isoforms, HLA-G1 to -G4, and three soluble isoforms, HLA-G5 to -G7.13,14
Trophoblast cells, which originate from the fetus, regulate their expression of HLA genes and the production of their proteins. If these proteins recognize by maternal immune cells as foreign antigens, they would stimulate maternal CTL against fetal antigens, so destroy fetal cells which express HLA. In contrast, the antigens expressed in trophoblast cells induce tolerance in maternal leukocytes. A successful pregnancy is due to Th2 cytokine profile that entitled ‘Th2 phenomenon’, while Th1 response may cause some complications of pregnancy, such as RM.15
Except HLA-C which may has weak expression on trophoblast cells, other HLA class Ia and II antigens are not expressed by these cells.16 Hence, the fetal cells are not in direct contact with the maternal immune system. Although NK-cell will destroy cells that do not express MHC, the strong expression HLA-G on cytotrophoblast cells, in cooperation with the expression of HLA-E in the placenta, will prevent NK-mediated cell lysis.7
Even though HLA-G mRNA has been detected in various tissues, the HLA-G protein expression is restrictive and strongly expressed on invasive trophoblast cells of the placenta at the maternofetal interface.7 IL-10 can up-regulate HLA-G expression.15 The mouse monoclonal antibody W6/32 is used most regularly to identify HLA-G antigens.6,17 sHLA-G can be detected in serum/plasma from both women and men.18 Monocytes are the main source of sHLA-G in the blood of men and non-pregnant women.7 Although sHLA-G can only be detected in some serum samples, it is detectable in all plasma samples from non-pregnant and pregnant women; hence plasma samples are preferred. HLA-G polymorphisms may effect the level, or even the presence, of sHLA-G in serum/plasma.18
HLA-E in another class Ib MHC that is expressed by trophoblasts.10 Its binding site has a great affinity for the HLA-G signal peptide, and this binding plays an important role in HLA-E expression on the trophoblast cell surface.
The major function of HLA-G is not antigen presentation; it may play a significant role in immunosuppression and tolerance (Figure1).9 Therefore, HLA-G is somehow essential to immune privilege in pregnancy.6 HLA-G as an inhibitory ligand for natural killer cells and cytotoxic T-lymphocytes can inhibit CTL responses and NK functions,19 both through direct interaction with the receptors LIR-1 and LIR-2 and with the killer Ig-like receptor 2 (KIR2),20 hence may protect trophoblasts via these receptors.21 Additionally, antigen-presenting cells (APC), which have been transfected with HLA-G, are able to inhibit the proliferation of CD4+ T cells that leads to suppression in T helpers.22 Besides, soluble HLA-G (sHLA-G) can induce apoptosis in CD8+ T-cell.23
HLA-E is also help the fetus to avoid maternal immune surveillance, possibly by interacting with the CD94/NKG2A, NK-cell inhibitory receptor.24 HLA-E is the most important ligand for the inhibition of NK cells.10
In contrast to the highly polymorphic HLA class Ia and II, the gene polymorphism of the HLA class Ib is very sparse as HLA-G proteins are almost monomorphic.7 Most polymorphisms in the HLA-G gene do not alter the amino acid sequence; and some polymorphisms that do alteration are not change secondary structures.11 However, some of these polymorphisms in regulatory regions of the HLA-G gene may influence transcription and mRNA abundance25 and subsequently HLA-G expression.18,26
Some investigations carry out in order to find out if expression of HLA-G at the maternofetal interface in women with RM is different in comparison with normal pregnancies. Results indicated that expression pattern of HLA-G is associated with a risk of RM.25 sHLA-G levels in the serum of women experiencing RM in comparison with serum sHLA-G concentration of women with successful pregnancies were significantly lower.18
Although all evidences support the imperative role for HLA-G in maternal immunosuppression during pregnancy, in vivo experiments must be done to confirm this evidences. Since significance of HLA-G expression in pregnancy is proved, more research is needed to elucidate the molecular mechanisms associated with HLA-G expression and function.
Figure 1 .
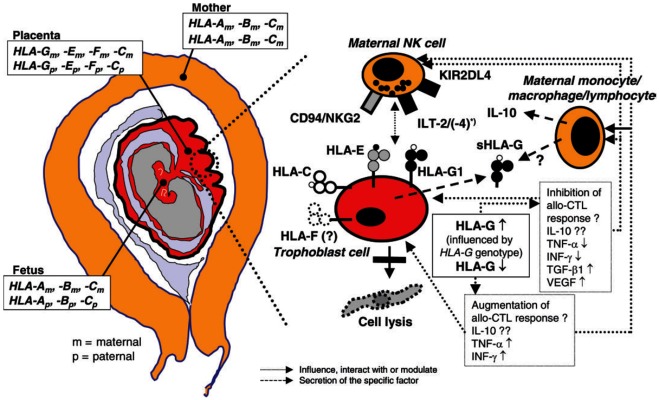
Expression HLA molecules during pregnancy and interactions between HLA class Ib molecules, natural killer (NK) receptors and cytokines at the feto–placental interface. The fetus inherits one HLA haplotype from the mother and one from the father and is thereby semiallogenic for the mother. However, the extreme polymorphic classical HLA class Ia and II antigens, HLA-A, -B and –DR, are not expressed by the trophoblast cells in the placenta. Instead, the nearly monomorphic non-classical HLA class Ib antigens, especially HLA-G, are expressed on the invasive cytotrophoblast cells. In this way, the trophoblast cells escape NK-cell-mediated lysis. Membrane-bound and soluble HLA-G (sHLA-G) can influence cytokine secretion and an allo-cytotoxic Tlymphocyte (CTL) response as described in detail in the text. *, ILT-4 is expressed on monocytes, macrophages and dendritic cells.7
According above, measuring of levels of HLA-G isoforms may be practical marker for the prediction of mother's capability to tolerate semiallograft fetus, success in IVF treatments and graft acceptance.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Coulam CB. Epidemiology of recurrent spontaneous abortion. Am J Reprod Immunol . 1991;26(1):23–7. doi: 10.1111/j.1600-0897.1991.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 2.Stirrat G. Recurrent miscarriage. Lancet . 1990;336:673–5. doi: 10.1016/0140-6736(90)92159-f. [DOI] [PubMed] [Google Scholar]
- 3.Edmonds DK, Lindsay KS, Miller JF, Williamson E, Wood PJ. Early embryonic mortality in women. Fertil Steril . 1982;38(4):447–53. [PubMed] [Google Scholar]
- 4.Lee RM, Silver RM. Recurrent pregnancy loss: summary and clinical recommendations. Semin Reprod Med . 2000;18(4):433–40. doi: 10.1055/s-2000-13733. [DOI] [PubMed] [Google Scholar]
- 5.Bhalla A, Stone PR, Liddell HS, Zanderigo A, Chamley LW. Comparison of the expression of human leukocyte antigen (HLA)-G and HLA-E in women with normal pregnancy and those with recurrent miscarriage. Reproduction . 2006;131(3):583–9. doi: 10.1530/rep.1.00892. [DOI] [PubMed] [Google Scholar]
- 6.Hunt JS, Petroff MG, Mcintire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J . 2005;19(7):681–93. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 7.Hviid TV. HLA-G in human reproduction: aspects of genetics, function and pregnancy complications. Hum Reprod Update . 2006;12(3):209–32. doi: 10.1093/humupd/dmi048. [DOI] [PubMed] [Google Scholar]
- 8.Loke YW, King A. Immunology of human placental implantation: clinical implications of our current understanding. Mol Med Today . 1997;3(4):153–9. doi: 10.1016/s1357-4310(97)01011-3. [DOI] [PubMed] [Google Scholar]
- 9.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H. et al. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol . 2003;171(3):1376–84. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 10.Heinrichs H, Orr HT. HLA non-A,B,C class I genes: their structure and expression. Immunol Res . 1990;9(4):265–74. doi: 10.1007/BF02935526. [DOI] [PubMed] [Google Scholar]
- 11.Ober C, Aldrich CL. HLA-G polymorphisms: neutral evolution or novel function? J Reprod Immunol . 1997;36(1-2):1–21. doi: 10.1016/s0165-0378(97)00062-4. [DOI] [PubMed] [Google Scholar]
- 12.Park B, Lee S, Kim E, Chang S, Jin M, Ahn K. The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity . 2001;15(2):213–24. doi: 10.1016/s1074-7613(01)00179-0. [DOI] [PubMed] [Google Scholar]
- 13.Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci U S A . 1992;89(9):3947–51. doi: 10.1073/pnas.89.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hviid TV, Moller C, Sorensen S, Morling N. Co-dominant expression of the HLA-G gene and various forms of alternatively spliced HLA-G mRNA in human first trimester trophoblast. Hum Immunol . 1998;59(2):87–98. doi: 10.1016/s0198-8859(97)00259-0. [DOI] [PubMed] [Google Scholar]
- 15.Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol . 2004;134(2):93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 16.Hunt JS, Andrews GK, Wood GW. Normal trophoblasts resist induction of class I HLA. J Immunol . 1987;138(8):2481–7. [PubMed] [Google Scholar]
- 17.Ober C, Aldrich C, Rosinsky B, Robertson A, Walker MA, Willadsen S. et al. HLA-G1 protein expression is not essential for fetal survival. Placenta . 1998;19(2-3):127–32. doi: 10.1016/s0143-4004(98)90000-5. [DOI] [PubMed] [Google Scholar]
- 18.Hviid TV, Rizzo R, Christiansen OB, Melchiorri L, Lindhard A, Baricordi OR. HLA-G and IL-10 in serum in relation to HLA-G genotype and polymorphisms. Immunogenetics . 2004;56(3):135–41. doi: 10.1007/s00251-004-0673-2. [DOI] [PubMed] [Google Scholar]
- 19.Le Gal FA, Riteau B, Sedlik C, Khalil-Daher I, Menier C, Dausset J. et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol . 1999;11(8):1351–6. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- 20.Ponte M, Cantoni C, Biassoni R, Tradori-Cappai A, Bentivoglio G, Vitale C. et al. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc Natl Acad Sci U S A . 1999;96(10):5674–9. doi: 10.1073/pnas.96.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allan DS, Colonna M, Lanier LL, Churakova TD, Abrams JS, Ellis SA. et al. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J Exp Med . 1999;189(7):1149–56. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci U S A . 2004;101(18):7064–9. doi: 10.1073/pnas.0401922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contini P, Ghio M, Poggi A, Filaci G, Indiveri F, Ferrone S. et al. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol . 2003;33(1):125–34. doi: 10.1002/immu.200390015. [DOI] [PubMed] [Google Scholar]
- 24.Braud VM, Allan DS, O'callaghan CA, Soderstrom K, D'andrea A, Ogg GS. et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature . 1998;391(6669):795–9. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 25.Ober C, Aldrich CL, Chervoneva I, Billstrand C, Rahimov F, Gray HL. et al. Variation in the HLA-G promoter region influences miscarriage rates. Am J Hum Genet . 2003;72(6):1425–35. doi: 10.1086/375501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hviid TV, Hylenius S, Rorbye C, Nielsen LG. HLA-G allelic variants are associated with differences in the HLA-G mRNA isoform profile and HLA-G mRNA levels. Immunogenetics . 2003;55(2):63–79. doi: 10.1007/s00251-003-0547-z. [DOI] [PubMed] [Google Scholar]


