Abstract
Purpose: To evaluate phytochemical composition, antibacterial and antioxidant properties of methanolic extracts of different parts viz., leaves, follicles and latex of Indian devil tree (Alstonia scholaris Linn.) R. Br. Methods: Antibacterial activities of the methanol extracts against Gram +ve (Bacillus subtilis and Staphylococcus aureus) and Gram -ve (Escherichia coli, Pseudomonas aeruginosa) bacteria were determined by well diffusion techniques. Aantioxidant profiles of methanol extracts were determined by 1,1-diphenyl-2-picryl-hydrazil (DPPH) free radical scavenging, superoxide anion radial scavenging and ferric thiocyanate reducing assays. Results: Phytochemical composition revealed abundance of flavonoids (97.3 mg QE/g DW), proanthocynidins (99.3 mg CE/g DW) and phenolics (49.7 mgGAE/g DW) in the leaf extract. Extracts of follicles and latex had comparatively very content of phenolics, flavonoids and proanthocyanidins. However, in follicle extract level of proanthocyanidins was significantly higher (46.8 mg CE/gDW). Latex extract among others exhibited most potent antibacterial activity. All the extracts displayed strong DPPH free radical and superoxide anion scavenging activities, only leaf extract displayed powerful reducing and ferrous ion chelating activities. Conclusion: Study revealed significant antioxidant activities of A. scholaris leaf, follicles and latex extracts and potential antibacterial activity of latex extract.
Keywords: Alstonia scholaris, Antibacterial, Antioxidant, Follicles, Latex, Phenolics
Introduction
Medicinal plants play important roles in our daily life to treat many diseases and ailments. Research in medicinal plants reflects the recognition of the validity of many herbal products.1 Plants has been a major source of natural products and medicines since Ayurveda one of the world’s oldest systems of medicine practiced in India.2,3 In developing countries like India several plants based medicine, also known as herbal medicine are used. Alstonia scholaris (L.) R. Br. belong to family Apocynaceae commonly called as Indian devil tree has been used as folklore medicines,1 possesses different pharmacological activities4,5 and potentially used as antimalaria drug Ayush-64, NRDC, India.6 Different parts of A. scholaris viz; leaves, follicles and latex a milky white secretion from folicles3 are used in the treatment of various types of disorders in different medicinal system.8 In alternative medicinal systems it is effective against different ailments such as asthma, malaria, fever, dysentery, diarrhoea, epilepsy, skin diseases and snakebite.5 Latex is useful in application in ulcers, sores, tumors and rheumatoid pain.2 Fruits are useful in syphilis and epilepsy and also used a tonic, antiperiodic and anthelmintic.8
Previous studies on A. scholaris have revealed the presence of different phytoconstituents1,3 as well as the antibacterial and antioxidant properties.2,6,8,9 While the antimicrobial properties of A. scholaris are well known2,8,10-12 only little is known about the phytochemical compositions of the leaf, fruit and latex and their antibacterial and antioxidant properties. Therefore the present study was undertaken to investigate the phytochemical compositions, and antioxidant and antibacterial properties of leaves, follicles and latex parts of A. scholaris.
Materials and Methods
Plant material and extraction
Fresh leaves were collected from A. scholaris trees (8-12 feet) grown wild in the campus of Amity University and nearby areas in Noida (U.P.) India. Follicles were collected during the month February to March when the tree is laden of follicles. Follicles were cut with sharp blade and compressed to collect milky white latex in a beaker. Latex was stored at 0-4ºC in refrigerator until used.
A known amount of leaves and follicles (100 g each) were kept in an oven at 40 °C for drying. Dried leaves and follicles were powdered by using mortar and pestle. The powder (10 g) of leaves and follicles were separately extracted with methanol (25-50 ml) for 24 h in separate Erlenmeyer flasks. Extraction process was repeated three times and each time the extract obtained were filtered through 0.45-μm filter paper and collected in a beaker. The extract thus obtained was dried over reflection water bath. Dried extracts were stored at 4 °C.
Antibacterial assay
Antibacterial activities of methanolic extracts of leaves, follicles and latex were assessed against Gram +ve (Bacillus subtilis and Staphylococcus aureus) and Gram -ve (Escherichia coli, Pseudomonas aeruginosa) bacteria by agar well diffusion method.11 The culture plates were prepared by first sterilizing the nutrient agar (36 gm in 1000 ml) in an autoclave at 121 °C at 15 lb for 15 minutes and then by pouring 20 ml of media into sterilized Petri dishes. 1 ml inoculums suspension was spread uniformly over the agar in Petri dishes using sterile glass rod. Wells were made by sterile cork borer (6 mm) in each plate. Extracts 100 μl (at concentration of 50, 100 mg/ml) dissolved in dimethyl sulfoxide (DMSO) was added aseptically into the well. Simultaneously, a control with DMSO was also run. Plates were incubated at 37 °C for 24 hrs. After incubation, microbial growth was observed in the Petri dishes. The antibacterial activity was expressed as the mean of diameter of the inhibition.
Determination of phenolics content
Total content of phenolics, flavonoids and proanthocynidins were determined according to previously published report.13 0.5 ml of methanol extract of each sample (concentration 1 mg/ml) were taken in separate test tubes and 0.5 ml of the Folin-Ciocalteu reagent was added. Contents were mixed gently. After 2 min, 0.5 ml of sodium carbonate (100 mg/ml) was added gently to the content and allowed to stand for 2 h. The optical density of the solution turned blue was measured at 765 nm. Total phenolic contents were expressed as mg gallic acid equivalent (GAE)/g dry weight of the sample.
Flavonoids content was measured by the method of Jia et al.14 250 μl of extract from stock (1 mg/ml) was taken in a test tube containing 1.25 ml of distilled water. To this, added 75 μl of 5% sodium nitrate solution and left for 5 min. Then added 150 μl of 10% ammonium chloride followed and after 6 min 500 μl of 1 M sodium hydroxide was added. The content was diluted with 275 μl of distilled water. Absorbance of the solution was measured at 510 nm. Total flavonoids content was expressed as milligrams of quercetin equivalent (QE)/g dry weight of sample.
Proanthocyanidin content was measured according to the previously published method.15 0.5 ml of extract was mixed with 3 ml of 4% vanillin methanol and 1.5 ml of hydrochloric acid in a test tube. The content was mixed well and left at room temperature for 15 min. The absorbance of the solution was measured at 500 nm. Total proanthocyanidins content was expressed as milligrams of catechin equivalent (QE)/g dry weight of sample.
DPPH assay
The antioxidant activity of the each sample extract was assessed by the ability of the extract to scavenge 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radicals.13 The extracts were taken in separate test tubes and allowed to react with DPPH. DPPH free radical scavenging activity was monitored by measurement of decline in absorbance at 517 nm. Butylated hydroxyanisole (BHA) was used as the standard compound.
Superoxide anion scavenging assay
Superoxide anion scavenging activity was determined using riboflavin–light NBT system.16 The assay system consists of 0.5 ml phosphate buffer (50 mM pH 7.6), 0.3 ml riboflavin (50 μM), 0.25 ml PMS (20 mM), 0.1 ml NBT (0.5 mM) and 1 ml methanol extract of each sample. The reaction was initiated by illuminating fluorescence lamp. After 20 min of incubation, the absorbance was recorded at 560 nm. Superoxide anion scavenging activity was calculated using the formula of scavenging activity (%) = [(A560 of control – A560 of sample)/A560 of control] × 100. Quercetin and BHA were used as standards.
Determination of reducing power
Reducing power of each sample extract was determined in accordance with previous report.17 In a test tube, 2 ml of methanol extract was mixed with 2 ml of 0.2M (pH 6.6) phosphate buffer and 2 ml of potassium ferricyanide (10 mg/ml). The mixture was incubated at 50ºC for 20 min followed by addition of 2 ml of trichloroacetic acid (100 mg/ml). 2 ml aliquot from this mixture was transferred to fresh test tube and diluted with 2 ml of distilled water. To the diluted solution, added 0.4 ml of 0.1% ferric chloride and kept for 10 min The absorbance was measured at 700 nm.
Determination of ferrous ions chelating activity
Ferrous ion Fe2+ chelating ability of the methanol extract of each sample was measured determined by the ferrous iron-ferrozine complex method.18 Various dilution (10, 8, 6, 4, and 2 mg/ml) of the of the extracts were prepared in methanol. An aliquot (0.8 ml) of diluted extract was taken in a test tube and mixed with 50 μl (2mM) of FeCl2, 200 μl (5 mM) ferrozine and incubated at 25 ± 2 °C for 10 min. Absorbance was measured at 562 nm against methanol as blank. Ferrous ion chelating activity was calculated by the formula: Chelating ferrous ion (%) = [(A562 of control–A562 of sample/A517 of control] × 100.
Results
Phytochemical constituents
Total phenolics content including flavonoids and proanthocyanidins of leaves, follicles and latex extracts are presented in Table 1. The results revealed marked variation in overall content of phenolics in leaf, follicle and latex extracts. The leaf extract had highest content of overall phenolics followed by follicle and latex extracts. In the leaf extract, flavonoids and proanthocyanidins were present in abundance with values observed 97 mgQE/g DW and 99 mgCE/g DW, respectively, whereas the phenolics were only 50 mgGAE/gDW. In the follicle extract, level of flavonoids and phenolics were comparatively lower than leaf extract, however proanthocynidins (47 mgCE/g DW) was found significant. Latext extract had lowest content of phenolics (17 mgGAE/g DW), flavonoids (11 mgQE/g DW) and proanthocynidins (18mgCE/g DW).
Table 1. Phenolics content of methanolic extracts of leaves, follicles and latex of A. scholaris.
| Phytoconstituents | Plant parts | ||
| Leaves | Follicles | Latex | |
| Phenolics (mg GAE/g) |
49.66±1.52 | 18.68±1.53 | 17.0±2.0 |
| Flavonoids (mg QE/g) |
97.33±1.52 | 15.86±01.64 | 11±1.0 |
| Proanthocyanidins (mg CE/g) |
99.33±1.52 | 46.86±1.75 | 18.33±.57 |
Antibacterial properties
The antibacterial profiles of the three extracts viz., leaves, follicles and latex are presented in Table 2. Results revealed that all the extracts tested showed fairly well bactericidal activity against a set of Gram +ve than Gram –ve bacteria. However, the latex extract exhibited more potent antibacterial activity than the leaf and follicle extracts. Extract of latex (100 mg/ml) did best against B. subtilis with zone of inhibition 21 mm. However, extracts tested were found least effective against P. aeruginosa with measured zone of inhibition of 8 mm. In comparison to the standard antibiotics used, antibacterial potential of the extracts of the samples were found moderately effective.
Table 2. Antibacterial activity of methanolic extracts of leaves, follicles and latex of A. scholaris.
| Extracts / Standard | Concentration (mg/ml) | Zone of inhibition (mm) | |||
| S. aureus | B. subtilis | P. aeruginosa | E.coli | ||
| Leaves | 50 | 12 | 15 | 8 | 11 |
| 100 | 15 | 18 | 12 | 14 | |
| Follicles | 50 | 11 | 15 | 9 | 10 |
| 100 | 13 | 17 | 11 | 13 | |
| Latex | 50 | 13 | 17 | 10 | 12 |
| 100 | 18 | 21 | 13 | 16 | |
| Ampicillin | 10 | 23 | 21 | 17 | 16 |
DPPH free radical and superoxide anion scavenging activities
Antioxidant profiles of methanol extracts of leaves, follicles and latex in terms of their ability to scavenge DPPH free radicals are depicted in Figure 1. Among the extracts tested, leaf extract displayed most potent antioxidant activity (70-80%) followed by follicle (50-80%) and latex (40-58%). However, leaf and follicle extracts exhibited similar antioxidant (75-80%) activities at concentrations, 20 and 50 mg/ml. The riboflavin–light NBT system revealed almost similar superoxide anion radical scavenging profiles (80-90%) for all the three extracts (Figure 2). Interestingly, lower concentration of the extracts (10-20 mg/ml) displayed higher superoxide anion radical-scavenging
Figure 1.
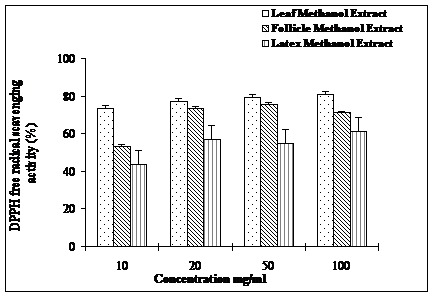
DDPH free radical scavenging activity of methanolic extracts leaves, follicles and latex of A. scholaris.
Figure 2.
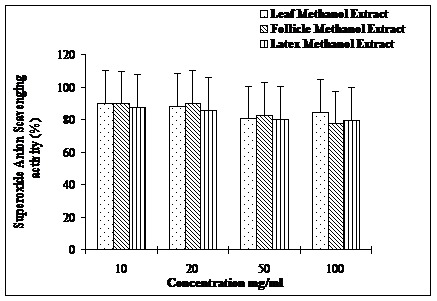
Superoxide anion scavenging activities of methanolic extracts of leaves, follicles and latex of A. scholaris.
Ferrous ion chelating and reducing activities
As shown in the Figure 3, ability of the leaf extract to chelate ferrous ion was the highest followed by follicles and latex extracts. Ferrous ion chelating activity increased linearly with increase in the concentration (1-8 mg/ml) of extracts. Higher ferrous ion chelating activities 80, 68 and 57 %, respectively of leaves, follicles and latex were observed at a concentration 8 mg/ml. A similar trend of the reducing activities was observed for the three extracts with leaves extract displaying highest reducing activity (Figure 4).
Figure 3.
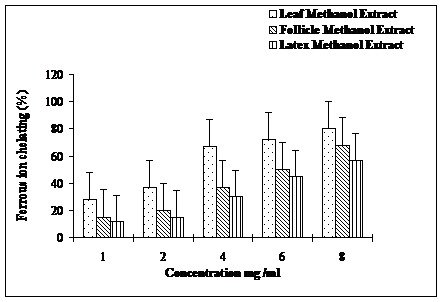
Ferrous ion chelating abilities of methanolic extracts of leaves, follicles and latex of A. scholaris.
Figure 4.
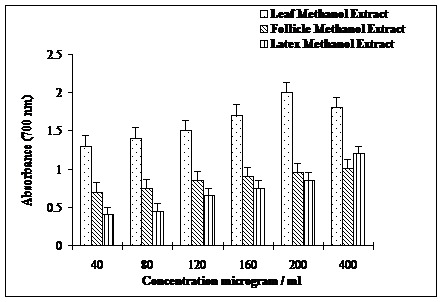
Reducing power of methanolic extracts of leaves, follicles and latex of A. scholaris.
Discussion
Previously, several reports have been published on phytochemical composition and antimicrobial properties of different parts viz., leaves, flowers, fruits, roots, latex and bark of A. scholaris from different regions. Still reports on antioxidant properties of A. scholaris are limited. Since awareness towards natural products in healthcare is rapidly increasing, interest in medicinal plants has been multiply increased.1 Plants produce many important compounds such as phenolics and flavonoids which possesses antioxidant and antimicrobial properties.6 Phenolics and flavonoids provide protection against free radicals and regulate various oxidative reactions occurring naturally. Also, they are used to protect food quality mainly by the prevention of oxidative deterioration of constituents of lipids.9
Here we have reported phytochemical composition of leaves, follicles and latex of A. scholaris along with their antibacterial and antioxidant properties. Study revealed that A. scholaris leaves accumulated high content of phenolics including flavonoids and proanthocynidins whereas follicles and latex had comparatively lesser phenolics content. Higher levels of phenolics accumulated in the green tissues (leaves) may be due to higher rates at which photosynthesis proceeds in these parts. Total phenolic contents (49.7mg/ml) of A. scholaris leaf extract reported here matches with phenolics content (35-36 mg/ml) of A. scholaris from Karnataka region, however, flvonoids and proanthocynidins content were significantly higher.8,9 Kumar et al.19 have also reported significantly higher (80mg/ml) phenolics content in leaf methanolic extract of A. scholaris than the levels observed in the present study A. scholaris bark methanolic extract also contained significantly higher (46 mg/ml) phenolics content.20 Here we have not investigated the bark extract for phenolics. Variations in the phytochemical compositions of the different plant parts of A. scholaris are typical of many other plant species reported previously.21-24 Phytochemical contents are also reported to be influenced by several other factors such as geographical, genetic, environmental, degree of maturity at the time of harvest. Higher levels of phenolics accumulated in the green tissues (leaves) may be due to higher rates at which photosynthesis proceeds in these parts.
Phenolics content of the plants/parts are often correlated with their strong antioxidant activities.25,26 In the present study, methanol extracts of A. scholaris leaves, follicles and latex extracts prepared with methanol exhibited strong antioxidant activities in terms of scavenging DPPH free radicals and superoxide anions. Leaf extract also had significant ferrous ion chelating and reducing abilities than follicle and latex extracts. The hydrogen donating potential is known to be one of the various mechanisms for measuring antioxidant activity. In DPPH assay, the radical scavenging ability of the extract was determined by the DPPH which itself is a stable nitrogen-centered free radical. The color of ethanolic DPPH solution changes from purple to yellow due to the formation of diphenyl picryl hydrazine, a stable diamagnetic molecule upon reduction by either the process of hydrogen radical or electron-donation.27,28 Superoxide anions are weak oxidants that produce very strong and harmful hydroxyl radicals and singlet oxygen causative agents of the oxidative stress.29 Polyphenols are well-known to chelate pro-oxidant metal ions (ferrous ions) and prevent free radical formation from this pro-oxidants.30. Here, the leaf extract showed 78 % DPPH free radical scavenging activity, much higher than that reported previously.31 Whereas, superoxide anion scavenging activity (89% ) was only slightly higher than (73%) that reported in leaf methanol extract of A. scholaris.9 The methanolic extract of bark also displayed strong antioxidant (90%) activity.20Also, higher antioxidant activities of A. scholaris plant parts reported here also coincide with the previous study reported significant amount of phyto constituents as well as antioxidant activities of A. scholaris.8 However, no report is available on antioxidant activity of A. scholaris follicles and latex methanolic extracts except that by James et al.32
Similarly, the antibacterial activities of the methanol extracts of latex and follicles are studied for the first time. Latex extract among others showed stronger antibacterial activity against bacteria, particularly B. subtilis. Several other studies have also reported more or less similar antibacterial potential of the leaf meathonic extracts.2,11 Stronger antibacterial effects of the extracts against Gram +ve bacteria might be due to absence of phospholipid membrane in Gram +ve bacteria.12 Antibacterial activity of A. scholaris plant parts reported here are comparatively lesser than the standard antibiotic used. In conclusion, methanolic extracts of A. scholaris plant parts leaf, follicles and latex exhibited significant antioxidant and antibacterial activity.
Acknowledgements
Corresponding author of this article is grateful to Dr. Ashok Kumar Chauhan, Founder President and Atul Chauhan, Chancellor, Amity University, Uttar Pradesh, Noida, India for providing necessary facilities and support. Also, I duly acknowledge research grant from Council of Scientific and Industrial Research (CSIR), New Delhi.
Conflict of Interest
No conflicts of interest among the authors
References
- 1.Saxena N, Shrivastava PN, Saxena RC. Preliminary physcio-phytochemical study of stem bark of Alstonia scholaris (L) RBR-A medicinal plant. Int J Pharma Sci Res. 2012;3(4):1071–5. [Google Scholar]
- 2.Misra CS, Pratyush K, Dev LMS, James J, Vettil AKT, Thankamani V. A comparative study on phytochemical screening and antibacterial activity of roots of Alstonia scholaris with roots, leaves and stem bark. Int J Res Phytochem Pharmacol. 2011;1(2):77–82. [Google Scholar]
- 3.Kaushik P, Kaushik D, Sharma N, Rana AC. Alstonia scholaris: It’s phytochemistry and pharmacology. Chron Young Sci. 2011;2(2):71–8. [Google Scholar]
- 4.Pratyush K, Misra CS, James J, Dev LMS, Veettil AKT, Thankamani V. Ethnobotanical and pharmacological study of Alstonia (Apocynaceae) - A Review. J Pharm Sci Res. 2011;3(8):1394–403. [Google Scholar]
- 5.Dey A. Alstonia scholaris R.Br.(Apocynaceae): Phytochemistry and pharmacology: A concise review. J Appl Pharm Sci. 2011;1(6):51–7. [Google Scholar]
- 6.Arulmozhi S, Mazumder PM, Narayanan LS, Thakurdesai PA. In vitro antioxidant and free radical scavenging activity of fractions from Alstonia scholaris Linn.R.Br. Int J Pharm Tech Res. 2010;2(1):18–25. [Google Scholar]
- 7.Meena AK, Nitika G, Jaspreet N, Meena RP, Rao MM. Review on ethanobotany, phytochemical and pharmacological profile of Alstonia scholaris. Int Res J Pharm. 2011;2(1):49–54. [Google Scholar]
- 8.Pankti K, Payal G, Manodeep C, Jagadish K. A phytopharmocological review of Alstonia scholaris: A panoramic herbal medicine. Int J Res Ayu Pharm . 2012;3(3):367–71. [Google Scholar]
- 9.Ramachandra YL, Ashajyothi C, Padmalatha RS. Antioxidant activity of Alstonia scholaris extracts containing flavonoids and phenolic compounds. Int J Pharm Pharma Sci. 2012;4(3):424–6. [Google Scholar]
- 10.Khan MR, Omoloso AD, Kihara M. Antibacterial activity of Alstonia scholaris and Leea tetramera. Fitoterapia. 2013;74(8):736–40. doi: 10.1016/s0367-326x(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 11.Khyade MS, Vaikos NP. Phytochemical and antibacterial properties of leaves of Alstonia scholaris R.Br. Afr J Biotechnol. 2009;8(22):6434–6. [Google Scholar]
- 12.Patel JP, Gami B, Patel K, Solanki R. Antibacterial activity of methanolic and acetone extract of some medicinal plants used in indian folklore. Int J Phytoremediation. 2011;3(2):261–9. [Google Scholar]
- 13.Liu X, Zhao M, Wang J, Yang B, Jiang Y. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J Food Compos Anal. 2008;21(3):219–28. [Google Scholar]
- 14.Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–9. [Google Scholar]
- 15.Manikandan S, Devi RS. Antioxidant property of alpha-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacol Res. 2005;52(6):467–74. doi: 10.1016/j.phrs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra S, Mishra KP, Maurya R, Srimal RC, Yadav VS, Pandey R. et al. Anticellular and immunosuppressive properties of ethanolic extract of Acorus calamus rhizome. Int Immunopharmacol. 2003;3(1):53–61. doi: 10.1016/s1567-5769(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 17.Oyaizu M. Antioxidative activities of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography. J Jpn Soc Food Sci Technol. 1998;35(11):771–5. [Google Scholar]
- 18.Meyer AS, Isaksen A. Application of enzymes as food antioxidants. Trends Food Sci Technol. 1995;6(9):300–4. [Google Scholar]
- 19.Kumar A, Kaur R, Arora S. Free radical scavenging potential of some Indian medicinal plants. J Med Plant Res. 2010;4(19):2034–42. [Google Scholar]
- 20.Kulkarni PM, Juvekar AP. Effect of Alstonia scholaris R.Br. on stress and cognition in mice. Indian J Exp Biol. 2008;47(1):47–52. [PubMed] [Google Scholar]
- 21.Kannan P, Ganjewala D. Antibacterial Activity of Nyctanthes Arbor-tristis (Lour.) flowers, leaves, fruits and seeds. Res J Phytol. 2007;1(2):61–7. [Google Scholar]
- 22.Song W, Wang HJ, Bucheli P, Zhang PF, Wei DZ, Lu YH. Phytochemical profiles of different mulberry (Morus sp.) species from China. J Agric Food Chem. 2009;57(19):9133–40. doi: 10.1021/jf9022228. [DOI] [PubMed] [Google Scholar]
- 23.Asha D, Ganjewala D. Antioxidant activities of methanolic extracts of Acorus calamus (L.) rhizome and leaves. J Herb Spic Med Plant. 2011;17(1):1–11. [Google Scholar]
- 24.Bhakta D, Ganjewala D. Effects of leaf position on total phenolics, flavonoids and proanthocynidines and their antioxidant activities in Lantana camara (L.) J Sci Res. 2009;1(2):363–9. [Google Scholar]
- 25.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20(7):933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 26.Sim KS, Nurestri AM, Norhanom AW. Phenolic content and antioxidant activity of Pereskia grandifolia Haw.(Cactaceae) extracts. Pharmacogn Mag. 2010;6(23):248–54. doi: 10.4103/0973-1296.66945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF. et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113 Suppl 9B:71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 28.Oktay M, Gulcin I, Kufrevioglu OI. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extract. LWT-Food Sci Technol. 2003;36(2):263–71. [Google Scholar]
- 29.Tepe B, Sokmen A. Screening of the antioxidative properties and total phenolic contents of three endemic Tanacetum subspecies from Turkish flora. Bioresour Technol. 2007;98(16):3076–9. doi: 10.1016/j.biortech.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Wang HZ, Cheng YG, Fan CS. Review of studies on chemical constituents and pharmacology of genus Acorus in China. Acta Bot Yunnanica. 1998;20:96–100. [Google Scholar]
- 31.Kumar V, Gogoi BJ, Meghvansi MK, Singh L, Srivastava RB, Deka DC. Determining the antioxidant activity of certain medicinal plants of Sonitpur, (Assam), India using DPPH assay. J Phytol. 2009;1(1):49–56. [Google Scholar]
- 32.James J, Veettil AKT, Pratyush K, Misra CS, Sahadevan LDM, Thankamani V. In vitro antioxidant activity of flowers and fruits of Alstonia scholaris. Int J Phytomed. 2011;3(4):475–9. [Google Scholar]


