Abstract
Purpose:Ghrelin has been shown to have antiepileptic function. However, the underlying mechanisms by which, ghrelin exerts its antiepileptic effects are still unclear. In the present study, we investigated whether neuropeptide Y (NPY) mediates ghrelin anticonvulsant effect in the brain through its Y1, Y2 or Y5 receptors. Methods:Male Wistar rats were bilaterally microinjected with ghrelin 0.3 nmol/μl/side and NPY antagonists; GR231118 (Y1 receptor antagonist), BIIE0246 (Y2 receptor antagonist), CGP71683 (Y5 receptor antagonist) or solvents (Saline, DMSO) into the dorsal hippocampus 20 minutes before ghrelin administration. Thirty minutes after ghrelin microinjection, a single convulsive dose of pentylenetetrazole (PTZ) (50 mg/kg) was injected intraperitoneally (ip). Afterwards, duration of seizure and total seizure score (TSS) were assessed for 30 minutes in all animals. Results:Intrahippocampal injection of 0.3 nmol/μl/side ghrelin decreased duration of seizure and TSS induced by PTZ. The suppression of both duration (p<0.001) and TSS (p<0.001) induced by ghrelin in hippocampus were significantly blocked by GR231118 (10 μg/μl/side), BIIE0246 (400 pmol/μl/side) and CGP 71683A (5 nmol/μl/side). Conclusion:Our findings suggest that NPY Y1, Y2 and Y5 receptors in the hippocampus may somehow mediate the anticonvulsive action of ghrelin. Therefore, it is possible to speculate that ghrelin acts in the hippocampus to modulate seizures via NPY.
Keywords: Ghrelin, Seizure, NPY, GR231118, BIIE0246, CGP71683
Introduction
Ghrelin is a brain-gut peptide, which is mainly produced by stomach.1,2 However, expression of the peptide has also been demonstrated in peripheral organs such as testis, ovary, placenta, kidney, pituitary, small intestine, pancreas, lymphocytes and brain.3 Central tissues that express ghrelin include hippocampus, ependymal layer of third ventricle, pituitary and different hypothalamic nuclei such as arcuate, ventromedial, dorsomedial and paraventricular nuclei.4 Two major forms of ghrelin are found in tissues and plasma: n-octanoyl-modified and des-acyl ghrelin and both cross the blood-brain barrier. Ghrelin receptor, growth hormone secretagogue receptor (GHSR), has been detected in many brain regions such as hypothalamus-pituitary unit, CA1, CA2, CA3 and dentate gyrus of hippocampal formation.7-9
Ghrelin has many physiological functions but growth hormone release and stimulation of feeding are the most known functions for ghrelin.4,10,11 Recently, it has been shown that there is a relationship between seizure and ghrelin. On the one hand, PTZ-induced seizure decreased acylated ghrelin of plasma.12 On the other hand, intraperitoneal and intrahippocampal administration of ghrelin attenuated the intensity of PTZ-induced seizures in rats. 13,14 Electrophysiological evidence also showed that the intracerebroventricular injection of ghrelin has an inhibitory effect against epileptiform activity in the penicillin model of epilepsy. 15 Therefore, previous studies show that ghrelin has an attenuating effect on the severity of seizures, but the mechanism by which ghrelin shows its effect is unclear.
Neuropeptide Y, a potent inhibitory neurotransmitter expressed in the central neurons, is capable of inhibiting epileptiform discharge and its expression, and release is significantly upregulated in hippocampal neurons following an epileptic seizure.16-18 Six NPY receptor subtypes have been reported (Y1–Y6) all of which belong to the G-protein coupled receptor superfamily. In the central nervous system, and specifically in hippocampus (an epileptogenic brain region), expression of Y1, Y2 and Y5 are most prominent.18
The possible involvement of NPY in the several ghrelin-mediated effects has been shown in different functions. Ghrelin affects feeding behavior), energy balance, growth hormone secretion and gastrointestinal motility through regulating NPY system.19-24
To further substantiate the role of NPY as mediator of ghrelin’s antiepileptic actions, intrahippocampal administration of ghrelin was done and the role of Y1, Y2 and Y5 receptors investigated in PTZ-induced seizures in rats.
Materials and Methods
Chemicals and Drugs
Rat ghrelin, GR231118 (Y1 receptor antagonist), BIIE0246 (Y2 receptor antagonist), CGP71683 (Y5 receptor antagonist), and PTZ were purchased from Tocris Bioscience, (Bristol, UK) and DMSO Sigma, (Germany). Ghrelin was dissolved in saline (1mg/100µl), and stocked at -20 °C. Immediately before intrahippocampal microinjection, ghrelin was diluted with 0.9% saline to give a final concentration of 0.3 nmol/ µl. The control group received equal amounts of saline (1µl). Both GR231118 and BIIE0246 were dissolved in saline and CGP71683 was dissolved in DMSO.
Animals and Treatments
The Regional Ethics Committee of Tabriz University of Medical Sciences approved all experimental procedures. Every effort was made to minimize the number of used animals and their suffering. Animals were obtained from the colony of Tabriz university of Medical Sciences. The experiments were performed in adult male Wistar rats weighting 220-250 g at the beginning of experiments. They were housed in a temperature (22±2 °C) and humidity-controlled room. The animals were maintained under a 12:12 h light/ dark cycle, with lights off at 8:00 p.m. Food and water provided ad libitum except for the periods of behavioral testing. The behavioral testing was done during the light phase.
Surgery
For surgical procedures, rats were anesthetized with i.p. injection of ketamine (60 mg/kg) and xylazine (12 mg/kg).25 Animals were positioned in a stereotaxic apparatus. Then trepanation of the skull cap was performed according to coordinates obtained from Paxinos and Watson brain atlas (mm from bregma: AP= -3.8; ML= ± 2.2; DV= -2.7).26 According to these coordinates, two 22-gauge guide cannulae were implanted bilaterally into the dorsal hippocampus. The guide cannulae were anchored to the skull using stainless steel screws and acrylic cement. After cannulae implantation, animals were individually housed and allowed 7 days recovery before the behavioral test.
Intrahippocampal Microinjection Procedure
All microinjections were done slowly (1µl/2 min) using a 5µl Hamilton syringe connected by Pe-20 polyethylene tube. The stainless steel injection needle (30 G) were cut to protrude 0.5 mm beyond the tips of the guide cannulae. The conscious animals were gently restrained by hand, the injection needle was inserted through the guide cannulae, and vehicles (1 μl saline or DMSO) or NPY receptor antagonists (GR231118 10 µg/µl; BIIE0246 400 pmol/µl; CGP71683 5 nmol/µl) and ghrelin (0.3 nmol/μl), were sequentially injected. A twenty min interval between i.h. injection of receptor antagonist or vehicles and ghrelin was considered. The injection needle was left in place for 1 min after injection to allow diffusion of the solution and to prevent back flow. Thirty minutes after the last microinjection, a single convulsive dose of PTZ (50 mg/kg) was administered intraperitoneally. The doses of antagonists and administration schedule were chosen based on previous studies demonstrating block of the relevant receptor subtype at the selected dose.27-29 Effective dose of ghrelin to attenuate seizure intensity obtained from our previous study.14 Microinjections were done between 9:00 and 12:00 a.m. to prevent variations determined by circadian rhythms.
Seizure Assessment
The rats were housed in Plexiglas cages (50 cm × 50 cm × 40 cm) after PTZ injection and their behavior was observed and videotaped for 30 min. The duration and severity of seizures were monitored in all animals. Then videotapes were reviewed, and detected seizures were scored based on Racine’s scale as following: (0) normal, non epileptic activity; (1) mouth and facial movements, hyperactivity, grooming, sniffing, scratching, wet dog shakes; (2) head nodding, staring, tremor; (3) forelimb clonus, forelimb extension; (4) rearing, salivating, tonic clonic activity; (5) falling, status epilepticus.30 Rats were assigned the score of the most severe seizure observed as seizure score (SS) for each 5 min interval over the course of the 30 min session.31 Then a mean SS was calculated for the entire 30 min session for each rat and referred as total seizure score (TSS).32
Experimental Design
After 7 days of recovery seventy rats were randomly divided into six groups (n=10) as follows:
Group (saline): 1µl/side saline i.h.
Group (ghrelin): 0.3 nmol/µl/side ghrelin i.h.
Group (Saline + ghrelin): 1µl/side saline, 20 min before 0.3 nmol/µl/side ghrelin i.h.
Group (DMSO + ghrelin): 1µl/side DMSO, 20 min before 0.3 nmol/µl/side ghrelin i.h.
Group (GR231118+ ghrelin): 10 µg/µl/side GR231118, 20 min before 0.3 nmol/µl/side ghrelin i.h.
Group (BIIE0246 + ghrelin): 400 pmol/µl/side BIIE 0246, 20 min before 0.3 nmol/µl/side ghrelin i.h.
Group (CGP71683 + ghrelin): 5nmol/µl/side CGP71683, 20 min before 0.3 nmol/µl/side ghrelin i.h.
In all experimental groups, PTZ (50 mg/kg) was injected intraperitoneally 30 min after the administration of ghrelin.
On completion of each experiment, the rats were sacrificed, their brains were removed, fixed in formalin, and injection sides were verified in coronal sections. Only animals with the correct injection sides were taken for a further analysis.
Statistical Analysis
Data are expressed, as means ± SEM. The statistical analysis of the data was carried out by one-way ANOVA-followed by Tukey's test. In all comparisons, P < 0.05 was considered significant.
Results
Effect of Intrahippocampal Microinjection of Y1 Antagonist on Seizure
Figure 1 shows the effects of GR231118 (Y1 receptor antagonist) (10 µg/µl, i.h.), on the anticonvulsive activity of ghrelin in PTZ-induced seizure. Administration of GR231118, 20 min before the effective dose of ghrelin (0.3 nmol/µl), significantly (P < 0.001) prolonged duration of the seizures (Figure 1A) and increased total seizure score (P < 0.001) (Figure 1B.) in rats.
Figure 1.
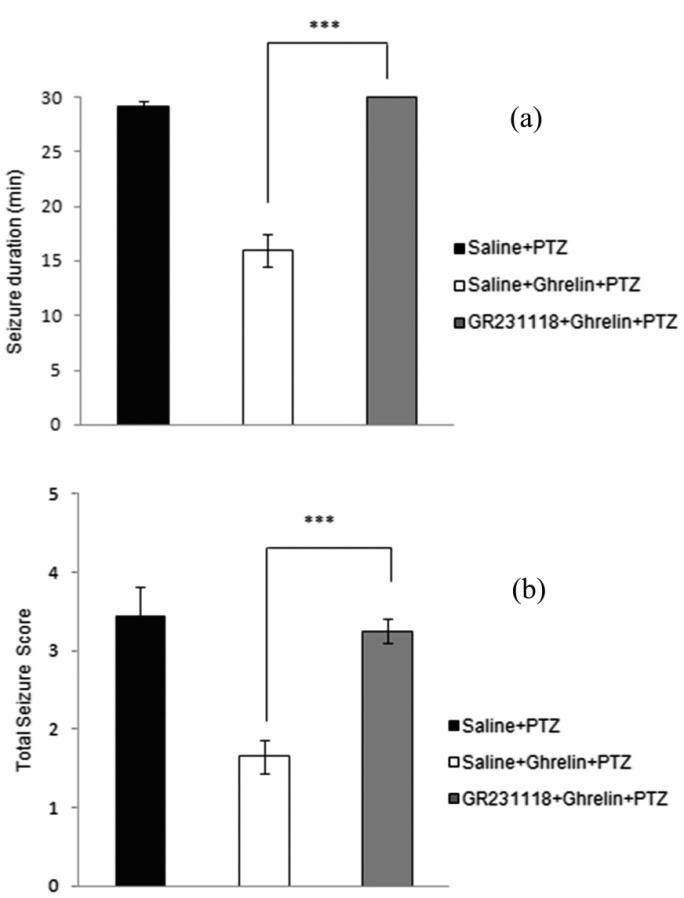
Effect of intrahippocampal injection of ghrelin preceded by GR231118 (Y1-receptor subtype antagonist) or saline on the duration of seizures (a) and total seizure score (b) during the 30-min post-PTZ behavior assessment. Data were analyzed by one-way ANOVA followed by Tukey’s test. Results are expressed as mean ± SEM, n=10 animals per group; *** P < 0.001
Effect of Intrahippocampal Microinjection of Y2 Antagonist on Seizure
As shown in Figure 2, pre-treatment with BIIE0246 (Y2 receptor antagonist) in dorsal hippocampus, 20 min prior to ghrelin administration (0.3 nmol/µl i.h.) reversed the anticonvulsant effects of ghrelin. BIIE0246 (400 pmol/µl, i.h.) administration significantly prolonged duration of seizure (p < 0.001) (Figure 2A) and intensified total seizure score (p < 0.001) (Figure 2B).
Figure 2 .
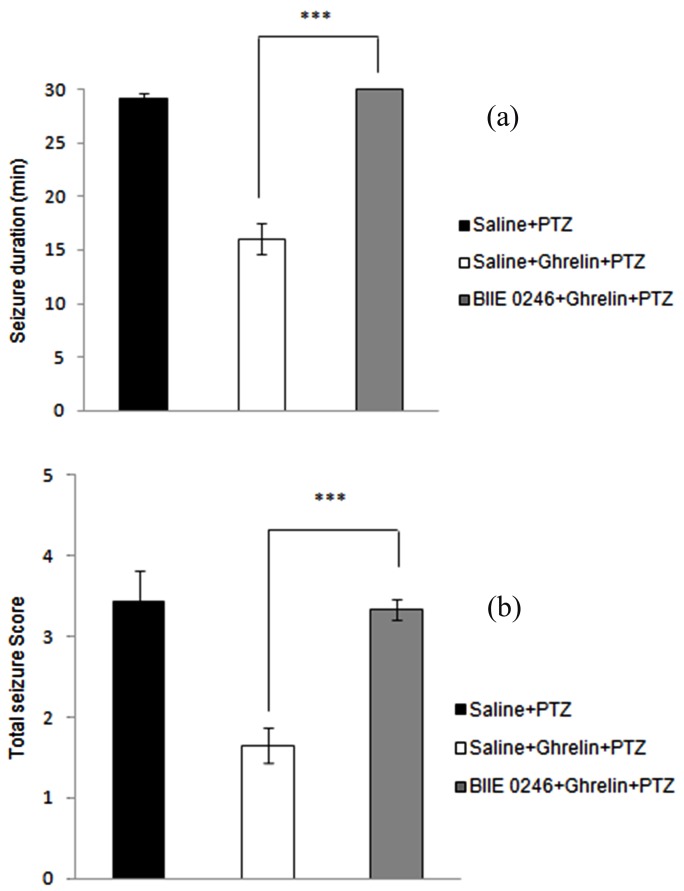
Effect of intrahippocampal injection of ghrelin preceded by BIIE 0246 (selective Y2-receptor subtype antagonist) or saline on the duration of seizures (a) and total seizure score (b) during the 30-min post-PTZ behavior assessment. Data were analyzed by one-way ANOVA followed by Tukey’s test. Results are expressed as mean ± SEM, n=10 animals per group; *** P < 0.001
Effect of Intrahippocampal Microinjection of Y5 Antagonist on Seizure
The effect of ghrelin in dorsal hippocampus of rats was reduced by GR231118 (Y5 receptor antagonist) at a dose of 1 µg/µl. Data analysis showed that duration of seizure was increased significantly (p < 0.001) after GR231118 administration as shown in Figure 3A. In addition, injection of CGP71683 prior to ghrelin administration significantly intensified total seizure scores (p < 0.001) (Figure 3B.) in PTZ-induced seizure in rats.
Figure 3 .
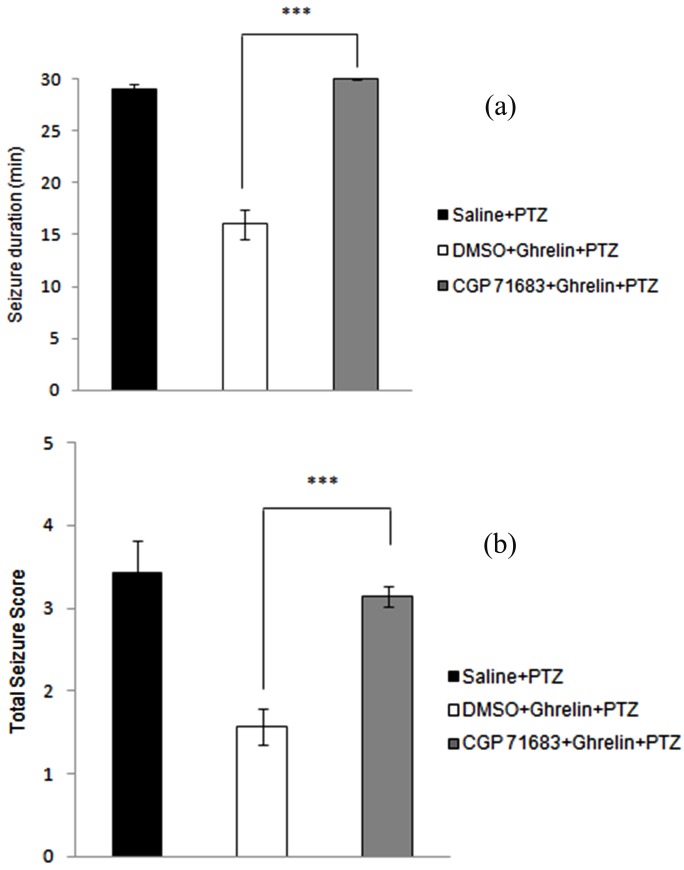
Effect of intrahippocampal injection of ghrelin preceded by CGP 71683 (Y5-receptor subtype antagonist) or DMSO on the duration of seizures (a) and total seizure score (b) during the 30-min post-PTZ behavior assessment. Data were analyzed by one-way ANOVA followed by Tukey’s test. Results are expressed as mean ± SEM, n=10 animals per group; *** P < 0.001
None of the antagonists induced seizure when administered intrahippocampally alone (in the absence of PTZ) and there were no significant differences between saline or vehicle and ghrelin treated groups with ghrelin alone group in seizure duration and TSS.
Discussion
In the present study, in vivo PTZ model of epilepsy was used to determine one possible mechanism of action for anticonvulsant effect of ghrelin. We assessed the response to ghrelin and different antagonists of NPY receptors in area CA1 of hippocampus in rats. Our findings demonstrated that the NPY type 1, 2, 5 receptors are primarily involved in the anticonvulsive action of ghrelin. Our evidences have been obtained using GR231118 (potent Y1 receptor antagonist), BIIE0246 (a specificY2 receptor antagonist) or CGP71683 (a potent and highly selective non-peptide antagonist).27,28,33
Ghrelin is a 28 amino acid peptide with growth hormone-releasing and appetite-inducing activities.1,2 Ghrelin is involved in many more processes than was initially postulated, and its endocrine, paracrine and autocrine effects play a role in its physiological and pathophysiological functions.1 Recently, it has been shown that ghrelin has an antiepileptic effect and simultaneous treatment of animals with the ghrelin receptor antagonist significantly attenuates the neuroprotective effect of ghrelin against KA-induced excitotoxicity.13,15,34,35
Circulating ghrelin enter the hippocampus, where specially has been shown to be a critical region for temporal lobe epilepsy, and binds to the hippocampal neurons.7,36 Ghrelin may exert modulator effects on neurotransmission.21 It enhances NPY and GABA-ergic activity in the brain.21 A number of studies show the possible involvement of NPY in the ghrelin-mediated effects.20,21,37
Neuropeptide Y is a 36 amino acid peptide which has been suggested to act as an endogenous anticonvulsant.18,38,39 NPY is a powerful endogenous modulator of limbic seizure activity.18 It has potent inhibitory effects on excitatory synaptic transmission from stratum radiatum to CA1 pyramidal cells and in both area CA1 and CA3 of hippocampus.40,41 Mice lacking NPY had an enhanced susceptibility to PTZ-induced seizures suggesting that the peptide is an important modulator of excitability in the CNS.42
Six different NPY receptor subtypes have been reported (Y1–Y6). In the central nervous system, and specifically in hippocampus (an epileptogenic brain region), expression of Y1, Y2 and Y5 are most prominent.18 All three subtype receptors have been shown to influence epileptic activity.43
Our results showed that pretreatment with GR231118, BIIE 0246 or CGP 71683 reverse anticonvulsant effect of intrahippocampal ghrelin. All these three NPY receptor antagonists increased the duration and TSS of PTZ-induced generalized seizures that had been attenuated by intrahippocampal administration of ghrelin.
NPY could theoretically be acting to suppress generalized seizures via postsynaptic Y1 or Y5 receptors enhancing GABAergic inhibition within the nucleus reticularis thalami or cortex, or presynaptically via Y2 receptors inhibiting GABA release from nRT axon terminals projecting onto VB neurons (and therefore reducing hyperpolarization-mediated T-channel de-inactivation).44 Y1, Y2 and Y5 receptors have all been shown to influence epileptic activity and their receptor agonists reduced seizure-like activity in hippocampal cultures.43 Silva also suggested that selective Y1, Y2 or Y5 receptor activation significantly inhibits glutamate (principal brain excitatory neurotransmitter) release in rat dentate gyrus of the epileptic hippocampus induced by kainite.45
The functional involvement of Y1 receptors in seizures has been demonstrated by several researchers with either anticonvulsant or proconvulsant effects.42,44,46 It has been reported that the Y1 receptor subtype predominantly mediates the antiepileptic activity of NPY in the frontal cortex.46 Conversely, Y1 receptors may mediate a facilitator role on seizure susceptibility and suggest that NPY Y1 receptors have a permissive role in seizures.18,42,47
In accordance with these studies, our results suggest that Y1 receptors mediate an attenuating action of ghrelin on seizures induced by PTZ.44,46 Thus, we suggest that these receptors may play a role in ghrelin effects on PTZ-induced seizure. The controversial results about Y1 receptors role may depend on several experimental difficulties such as the selection of the brain region, epilepsy model, the type and dosage of the used convulsant and the applied Y1 receptor antagonists.
Y2-like receptor is highly expressed in the hippocampal formation. Y5 receptors are also expressed in high levels in the hippocampus.48 Several studies showed that antiepileptic actions of NPY require activation of hippocampal Y2 or Y5 receptor subtypes.17,39,43,49,50 There are strong evidences from in vitro and in vivo studies that the effect of NPY to suppress hippocampal seizures and absence seizures was mediated by the Y2 receptors.39,41,44 Some studies have also suggested that in rat CA1 neurons, Y5 agonists reduce excitatory postsynaptic currents and the Y5 antagonist CGP71683A as well the Y2 antagonist BIIE0246 both block the inhibitory effect of NPY on glutamate release.51,52 Taken together, these data suggest that both Y2 and Y5 receptors regulate hippocampal seizures.53 Our results confirmed these findings and it seems that Y2 and Y5 receptors may play a critical role in modulating ghrelin induced hippocampal anticonvulsant effect.
In conclusion, intrahippocampal microinjection of ghrelin reduced the TSS and shortened the duration of epileptic activity in PTZ-induced seizures of rats and central administration of the NPY1, 2, 5 receptor antagonists, prior to ghrelin antagonize the ghrelin anticonvulsant effects. Therefore, it is possible to speculate that ghrelin acts in the central nervous system to modulate seizures via NPY receptor dependent mechanisms. It will be beneficial to measure NPY levels after ghrelin administration to find out whether it acts through NPY release to control seizures in the hippocampus.
Acknowledgments
The Neuroscience Research Centre of Tabriz University of Medical Sciences and Neuroscience Research Centre of Shahid Beheshti University of Medical Sciences supported this study. The article is based on the MSc thesis of Mrs. Mina Ghahramanain Golzar entitled "Evaluation of the mechanism of antiepileptic effect of intrahippocampal injection of ghrelin through neuropeptide Y1,2,5 receptors in pentylenetetrazole-induced seizure".
Conflict of Interest
The authors report no conflicts of interest in this work.
References
- 1.Leontiou CA, Franchi G, Korbonits M. Ghrelin in neuroendocrine organs and tumors. Pituitary. 2007;10(3):213–25. doi: 10.1007/s11102-007-0023-0. [DOI] [PubMed] [Google Scholar]
- 2.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 3.Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7(1):37–49. doi: 10.2174/157015909787602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin a hormone with multiple functions. Front Neuroendocrinol. 2004;25(1):27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279(3):909–13. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302(2):822–7. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 7.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B. et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci . 2006;9(3):381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 8.Bennett PA, Thomas GB, Howard AD, Feighner SD, van der Ploeg , Smith RG. et al. Hypothalamic growth hormone secretagogue-receptor (ghs-r) expression is regulated by growth hormone in the rat. Endocrinology . 1997;138(11):4552–7. doi: 10.1210/endo.138.11.5476. [DOI] [PubMed] [Google Scholar]
- 9.Van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 10.Carlini VP, Monzón ME, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN. et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun . 2002;299(5):739–43. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- 11.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 12.Ataie Z, Golzar MG, Babri Sh, Ebrahimi H, Mohaddes G. Does ghrelin level change after epileptic seizure in rats? Seizure. 2011;20(4):347–9. doi: 10.1016/j.seizure.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Obay BD, Tasdemir E, Tumer C, Bilgin HM, Sermet A. Antiepileptic effects of ghrelin on pentylenetetrazole-induced seizures in rats. Peptides. 2007;28(6):1214–9. doi: 10.1016/j.peptides.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Ghahramanian Golzar M, Ataei Z, Babri Sh, Ebrahimi H, Mirzaie F, Mohaddes G. Effect of acute and chronic intrahippocampal microinjection of ghrelin on pentylenetetrazole-induced seizures in rats. Pharmaceut Sci. 2011;17(1):11–8. [Google Scholar]
- 15.Aslan A, Yildirim M, Ayyildiz M, Guven A, Agar E. The role of nitric oxide in the inhibitory effect of ghrelin against penicillin-induced epileptiform activity in rat. Neuropeptides. 2009;43(4):295–302. doi: 10.1016/j.npep.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Michalkiewicz M, Zhao G, Jia Z, Michalkiewicz T, Racadio MJ. Central neuropeptide Y signaling ameliorates N(omega)-nitro-L-arginine methyl ester hypertension in the rat through a Y1 receptor mechanism. Hypertension . 2005;45(4):780–5. doi: 10.1161/01.HYP.0000153953.69799.f2. [DOI] [PubMed] [Google Scholar]
- 17.Baraban SC. Antiepileptic actions of neuropeptide Y in the mouse hippocampus require Y5 receptors. Epilepsia. 2002;43(Suppl 5):9–13. doi: 10.1046/j.1528-1157.43.s.5.13.x. [DOI] [PubMed] [Google Scholar]
- 18.Baraban SC. Neuropeptide Y and epilepsy: recent progress, prospects and controversies. Neuropeptides. 2004;38(4):261–5. doi: 10.1016/j.npep.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Gualillo O, Lago F, Gomez-Reino J, Casanueva FF, Dieguez C. Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003;552(2-3):105–9. doi: 10.1016/s0014-5793(03)00965-7. [DOI] [PubMed] [Google Scholar]
- 20.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG. et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 21.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL. et al. The distribution and mechanism of action of ghrelin in the cns demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 22.Horvath TL, Diano S, Sotonyi P, Heiman M, Tschop M. Minireview: Ghrelin and the regulation of energy balance--a hypothalamic perspective. Endocrinology. 2001;142(10):4163–9. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 23.Wren AM, Small CJ, Fribbens CV, Neary NM, Ward HL, Seal LJ. et al. The hypothalamic mechanisms of the hypophysiotropic action of ghrelin. Neuroendocrinology. 2002;76(5):316–24. doi: 10.1159/000066629. [DOI] [PubMed] [Google Scholar]
- 24.Fujimiya M, Asakawa A, Ataka K, Chen CY, Kato I, Inui A. Ghrelin, des-acyl ghrelin, and obestatin: Regulatory roles on the gastrointestinal motility. Int J Pept 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohaddes G, Rasi S, Naghdi N. Evaluation of the effect of intrahippocampal injection of leptin on spatial memory. Afr J Pharm Pharmacol. 2009;3:443–8. [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic press; 2004. [Google Scholar]
- 27.Ishihara A, Tanaka T, Kanatani A, Fukami T, Ihara M, Fukuroda T. A potent neuropeptide Y antagonist, 1229U91, suppressed spontaneous food intake in Zucker fatty rats. Am J Physiol. 1998;274(5 Pt 2):R1500–4. doi: 10.1152/ajpregu.1998.274.5.R1500. [DOI] [PubMed] [Google Scholar]
- 28.Smialowska M, Wieronska JM, Domin H, Zieba B. The effect of intrahippocampal injection of group II and III metobotropic glutamate receptor agonists on anxiety; the role of neuropeptide Y. Neuropsychopharmacology. 2007;32(6):1242–50. doi: 10.1038/sj.npp.1301258. [DOI] [PubMed] [Google Scholar]
- 29.Westfall TC, Naes L, Gardner A, Yang CL. Neuropeptide Y induced attenuation of catecholamine synthesis in the rat mesenteric arterial bed. J Cardiovasc Pharmacol. 2006;47(6):723–8. doi: 10.1097/01.fjc.0000211761.06271.15. [DOI] [PubMed] [Google Scholar]
- 30.Meurs A, Clinckers R, Ebinger G, Michotte Y, Smolders I. Seizure activity and changes in hippocampal extracellular glutamate, GABA, dopamine and serotonin. Epilepsy Res. 2008;78(1):50–9. doi: 10.1016/j.eplepsyres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Toscano CD, Ueda Y, Tomita YA, Vicini S, Bosetti F. Altered GABAergic neurotransmission is associated with increased kainate-induced seizure in prostaglandin-endoperoxide synthase-2 deficient mice. Brain Res Bull. 2008;75:598–609. doi: 10.1016/j.brainresbull.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lian XY, Zhang ZZ, Stringer JL. Anticonvulsant activity of ginseng on seizures induced by chemical convulsants. Epilepsia. 2005;46(1):15–22. doi: 10.1111/j.0013-9580.2005.40904.x. [DOI] [PubMed] [Google Scholar]
- 33.Della Zuana O, Sadlo M, Germain M, Feletou M, Chamorro S, Tisserand F. et al. Reduced food intake in response to cgp 71683a may be due to mechanisms other than npy y5 receptor blockade. Int J Obes Relat Metab Disord. 2001;25(1):84–94. doi: 10.1038/sj.ijo.0801472. [DOI] [PubMed] [Google Scholar]
- 34.Portelli J, Aourz N, Ver Donck L, Moechars D, Schallier A, Michotte Y, et al. Anticonvulsant effects of ghrelin receptor ligands against pilocarpine-induced limbic seizures. 13th International Conference on In Vivo Methods; 12 - 16 September; Brussels, Belgium 2010.
- 35.Lee J, Lim E, Kim Y, Li E, Park S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J Endocrinol. 2010;205(3):263–70. doi: 10.1677/JOE-10-0040. [DOI] [PubMed] [Google Scholar]
- 36.Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84(4):363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K. et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes . 2001;50(2):227–32. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 38.Colmers WF, El Bahh B. Neuropeptide Y and Epilepsy. Epilepsy Curr. 2003;3(2):53–8. doi: 10.1046/j.1535-7597.2003.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Bahh B, Cao JQ, Beck-Sickinger AG, Colmers WF. Blockade of neuropeptide Y(2) receptors and suppression of NPY's anti-epileptic actions in the rat hippocampal slice by BIIE0246. Br J Pharmacol. 2002;136(4):502–9. doi: 10.1038/sj.bjp.0704751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colmers WF, Lukowiak K, Pittman QJ. Presynaptic action of neuropeptide Y in area CA1 of the rat hippocampal slice. J Physiol. 1987;383:285–99. doi: 10.1113/jphysiol.1987.sp016409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Bahh B, Balosso S, Hamilton T, Herzog H, Beck-Sickinger AG, Sperk G. et al. The anti-epileptic actions of neuropeptide Y in the hippocampus are mediated by Y and not Y receptors. Eur J Neurosci. 2005;22(6):1417–30. doi: 10.1111/j.1460-9568.2005.04338.x. [DOI] [PubMed] [Google Scholar]
- 42.Gariboldi M, Conti M, Cavaleri D, Samanin R, Vezzani A. Anticonvulsant properties of BIBP3226, a non-peptide selective antagonist at neuropeptide Y Y1 receptors. Eur J Neurosci. 1998;10(2):757–9. doi: 10.1046/j.1460-9568.1998.00061.x. [DOI] [PubMed] [Google Scholar]
- 43.Reibel S, Nadi S, Benmaamar R, Larmet Y, Carnahan J, Marescaux C. et al. Neuropeptide Y and epilepsy: Varying effects according to seizure type and receptor activation. Peptides. 2001;22(3):529–39. doi: 10.1016/s0196-9781(01)00347-3. [DOI] [PubMed] [Google Scholar]
- 44.Morris MJ, Gannan E, Stroud LM, Beck-Sickinger AG, O'Brien TJ. Neuropeptide Y suppresses absence seizures in a genetic rat model primarily through effects on Y receptors. Eur J Neurosci. 2007;25(4):1136–43. doi: 10.1111/j.1460-9568.2007.05348.x. [DOI] [PubMed] [Google Scholar]
- 45.Silva AP, Xapelli S, Pinheiro PS, Ferreira R, Lourenço J, Cristovao A. et al. Up-regulation of neuropeptide Y levels and modulation of glutamate release through neuropeptide Y receptors in the hippocampus of kainate-induced epileptic rats. J Neurochem. 2005;93(1):163–70. doi: 10.1111/j.1471-4159.2004.03005.x. [DOI] [PubMed] [Google Scholar]
- 46.Bijak M. Neuropeptide Y suppresses epileptiform activity in rat frontal cortex and hippocampus in vitro via different NPY receptor subtypes. Neurosci Lett . 1999;268(3):115–8. doi: 10.1016/s0304-3940(99)00381-x. [DOI] [PubMed] [Google Scholar]
- 47.Benmaamar R, Pham-Le BT, Marescaux C, Pedrazzini T, Depaulis A. Induced down-regulation of neuropeptide Y-Y1 receptors delays initiation of kindling. Eur J Neurosci. 2003;18(4):768–74. doi: 10.1046/j.1460-9568.2003.02810.x. [DOI] [PubMed] [Google Scholar]
- 48.Dumont Y, St-Pierre JA, Quirion R. Comparative autoradiographic distribution of neuropeptide Y Y1 receptors visualized with the Y1 receptor agonist [125I][Leu31,Pro34]PYY and the non-peptide antagonist [3H]BIBP3226. Neuroreport. 1996;7(4):901–4. doi: 10.1097/00001756-199603220-00013. [DOI] [PubMed] [Google Scholar]
- 49.Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21(15):5804–12. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benmaamar R, Richichi C, Gobbi M, Daniels AJ, Beck-Sickinger AG, Vezzani A. Neuropeptide Y Y5 receptors inhibit kindling acquisition in rats. Regul Pept. 2005;125(1-3):79–83. doi: 10.1016/j.regpep.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Ho MW, Beck-Sickinger AG, Colmers WF. Neuropeptide Y (5) receptors reduce synaptic excitation in proximal subiculum, but not epileptiform activity in rat hippocampal slices. J Neurophysiol. 2000;83(2):723–34. doi: 10.1152/jn.2000.83.2.723. [DOI] [PubMed] [Google Scholar]
- 52.Rodi D, Mazzuferi M, Bregola G, Dumont Y, Fournier A, Quirion R. et al. Changes in NPY-mediated modulation of hippocampal [3H]D-aspartate outflow in the kindling model of epilepsy. Synapse. 2003;49(2):116–24. doi: 10.1002/syn.10216. [DOI] [PubMed] [Google Scholar]
- 53.Woldbye DP, Nanobashvili A, Sorensen AT, Husum H, Bolwig TG, Sorensen G. et al. Differential suppression of seizures via Y2 and Y5 neuropeptide Y receptors. Neurobiol Dis. 2005;20(3):760–72. doi: 10.1016/j.nbd.2005.05.010. [DOI] [PubMed] [Google Scholar]


