Abstract
Nerve Growth Factor (NGF) augments excitability of isolated rat sensory neurons through activation of the p75 neurotrophin receptor (p75NTR) and its downstream sphingomyelin signaling cascade, wherein neutral sphingomyelinase(s) (nSMase), ceramide, and the atypical PKC (aPKC), PKMζ, are key mediators. Here we examined these same receptor-pathways in vivo for their role in mechanical hyperalgesia from exogenous NGF. Mechanical sensitivity was tested by the number of paw withdrawals in response to 10 stimuli (PWF = n/10) by a 4g von Frey hair (VFH, testing “allodynia”) and by 10g and 15g VFHs (testing “hyperalgesia”). NGF (500 ng/10 µl) injected into the male rat’s plantar hind paw induced long lasting ipsilateral mechanical hypersensitivity. Mechano-hypersensitivity, relative to baseline responses and to those of the contralateral paw, developed by 0.5–1.5h and remained elevated at least for 21–24h, Acute intraplantar pre-treatment with nSMase inhibitors, GSH or GW4869, prevented the acute hyperalgesia from NGF (at 1.5h) but not that at 24h. A single injection of N-acetyl sphingosine (C2-ceramide), simulating the ceramide produced by nSMase activity, induced ipsilateral allodynia that persisted for 24h, and transient hyperalgesia that resolved by 2h. Intraplantar injection of hydrolysis-resistant mPro-NGF, selective for the p75NTR over the TrkA receptor, gave very similar results to NGF and was susceptible to the same inhibitors. Hyperalgesia from both NGF and mPro-NGF was prevented by paw pre-injection with blocking antibodies to rat p75NTR receptor. Finally, intraplantar (1 day before NGF) injection of mPSI, the myristolated pseudosubstrate inhibitor of PKCζ/PKMζ, decreased the hyperalgesia resulting from NGF or C2-ceramide, although scrambled mPSI was ineffective. The findings indicate that mechano-hypersensitivity from peripheral NGF involves the sphingomyelin signaling cascade activated via p75NTR, and that a peripheral aPKC is essential for this sensitization.
Keywords: nerve growth factor, PKMζ, aPKCs, sphingomyelinase, hyperalgesia, pain
1
Nerve growth factor (NGF) plays a critical role in development and growth of peripheral sensory neurons, and also induces thermal and mechanical sensitization of these neurons in adult mammals. Much evidence indicates that in adults NGF may still have some trophic role, but its main function is to initiate and maintain nociceptor hypersensitivity as a part of inflammatory and immune responses after tissue injury (see Pezet and McMahon, 2006). The trophic actions of NGF have been attributed to activation of a receptor tyrosine kinase (TrkA) that is expressed on peripheral and central neurons and distinguished by its high affinity for NGF (Meakin and Shooter, 1992; Barker and Murphy, 1992; Fundin et al., 1997). Nerve growth factor is also known to induce hypersensitivity and pain in humans (Dyck et al., 1997; Svensson et al., 2003; Rukwied et al., 2010) and to decrease nociceptive thresholds in rodent models of pain (Lewin et al., 1993; Woolf et al., 1994; Woolf 1996; McMahon et al., 1995; Hathway and Fitzgerald, 2006; Mills et al., 2013)
Results of a recent study exploring the capacity of NGF to directly and acutely modulate the excitability of isolated sensory neurons suggest that such actions follow activation of the low affinity NGF-binding receptor, p75 neurotrophin receptor (p75NTR), which can trigger activation of the downstream sphingomyelin signaling cascade (for review see Nicol and Vasko, 2007; Zhang et al., 2012). Neutral sphingomyelinase(s) (nSMase), ceramide and the atypical PKC (aPKC), PKMζ, are important effector molecules of this intracellular pathway.
In the present work we aimed to determine the contribution of these mediators of the p75NTR signaling pathway to the nociceptive mechanical hypersensitivity produced by local NGF administration in rats in vivo. The results show that the p75NTR is essential for this response and that inhibition of nSMase, i.e., of ceramide liberation from sphingomyelin, and inhibition of peripheral aPKCs have preventive actions on the development of NGF-dependent mechanical hypersensitivity.
2.0 Experimental Procedures
Experiments were conducted in adult male Sprague-Dawley rats (235–330g). Rats were housed in groups of 2 per cage under a 12:12 h dark-light cycle and were provided with food and water ad libitum. Animals were experimentally treated and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (Guide, 1996) as reviewed and approved by the Harvard Committee on Animals
2.1 Mechanical testing
Unrestrained rats were placed on an elevated plastic mesh floor (28 × 17.5 cm; 9.5 × 9.5mm openings) and allowed to habituate for 25–40 min before initial testing. Paw Withdrawal Frequency to mechanical stimulation was determined using calibrated von Frey hairs (VFH) applied perpendicular to the plantar surface of a hind paw through spacing in the mesh. Each VFH (4g, 10g and 15g) was applied 10 times for 3 sec, separated by a 3 sec interval. Testing with the next VFH started ca. 8–10 min after the beginning of the testing with a previous force. Testing started with a lowest force of 4g, and continued with increasing forces, with all three forces tested with 10 probings in each test period. To avoid stress and to obtain consistent responsiveness to the same force, the rats were habituated and tested on mesh racks over 5–6 days before each experiment (training period). Withdrawal responses were registered initially on the ipsilateral paw (IPSI) in 4 rats, then on the contralateral paw (CLP), for each VFH. The number of paw withdrawals, n, occurring in response to 10 stimuli (range: n=0–10) was used to assess mechanical sensitivity, and graphed as Paw Withdrawal Frequency (n) for each force.
2.2. Injection procedures
NGF, GSH, C2-ceramide, GW4869 or its vehicle, alone or with NGF, were injected subcutaneously (s.c.) in a 20 µL volume into the mid-plantar hind paw, 1 cm distal from the heel. The non-selective atypical myristoylated pseudosubstrate inhibitor (mPSI; also known as ZIP, Eichholtz et al., 1993; Thiam et al., 1999) was injected s.c. into the plantar surface (40 µg/20 µL). Injections occurred under brief general anesthesia from inhalation of the rapidly reversible agent sevoflurane (Abbott Labs, N. Chicago, IL, USA). After anesthesia was discontinued, the righting reflex recovered in <30 sec for intraplantar (i.pl.) injection; 5–10 min later “normal” nocifensive responses (thresholds, latencies) could be assessed.
2.3. Chemicals
NGF-β (rat) (Sigma-Aldrich, St. Louis MO, USA) was made as a stock solution (100 ng/µL of phosphate buffered saline (PBS: pH7.4)) and stored in 40 µL aliquots at −80°C. L-Glutathione (GSH, Sigma-Aldrich) was dissolved in PBS immediately before each injection (fresh made solutions for pre- and co-injections). Prior to the injection, NGF stock aliquots were diluted (1:1) in PBS or mixed with GSH solutions (for co-injections) to the noted final concentration of 50 ng/µL. A non-hydrolyzable mutated homologue of Pro-NGF, mPro-NGF (Alomone Labs, Jerusalem, Israel) was reconstituted in PBS, then centrifuged at 10,000×g for 5 min to remove any undissolved particulates before paw injection. C2-ceramide (Enzo Life Sciences, Farmingdale, NY) was dissolved initially as a 25 µg/µL DMSO stock solution, stored at −80°C in aliquots, then diluted to 20 µg/10 µL DMSO before the injection. GW4869 (Sigma-Aldrich) was dissolved in DMSO as a 2 mM working solution, and aliquots were prepared under an inert gas (nitrogen) and stored at −80°C. The atypical PKC pseudosubstrate inhibitor, mPSI (Calbiochem, USA; Enzo Life Sciences) was dissolved immediately before the experiment, first in H2O to 5 µg /µL and then diluted 1:10 in PBS.
A polyclonal blocking antibody to rat p75NTR was generously donated by Prof. Louis Reichardt (University of California, San Francisco); 20 µL volumes were injected into the plantar paw surface 4 h before the injection of NGF or Pro-NGF. As a control for the p75NTR blocking antibody, a rabbit IgG solution containing approximately the same total protein was used ( rabbit polyclonal IgG; Abcam, MA USA).
2.4. Experimental design
NGF was injected s.c. into the plantar surface of the rat’s hind paw, always at a dose of 500 ng in 10 µL. To test GSH effectiveness against NGF-induced hyperalgesia, GSH was delivered in either of two injection protocols. 1) Acute pre-treatment: the first injection occurred at “15” (15–18) min prior to NGF and the second injection was delivered mixed with the NGF. Two different dosing levels were delivered, one in which GSH was applied at a low total dose (0.6 µmol/paw) and another at a high total dose (1.8 µmol/paw; the concentrations and doses are indicated in the Results and figure legends). 2) Prolonged pre-treatment: the first injection occurred 1.5h prior to the NGF injection, and the second, as in (1), above, mixed with NGF. For these experiments, GSH was delivered at the high total dose (1.8 µmol/paw). In Control experiments NGF-β (rat) was injected: 1) “15” (15–18) min after the injection of the vehicle for GSH (PBS, 10 µl); 2) a single injection of GSH, without NGF, was administered at a dose intermediate (1.2 µmol/paw) between the “high” and the “low” total dose condition, which resulted in the highest concentration (120 mM) of GSH used for either acute or prolonged pre-treatment.
To corroborate the effects of GSH, a structurally different nSMase inhibitor, GW4869 (2 mM, 10 µl) (or its vehicle) was injected once, either 17 min before or 1h after NGF.
In another group of rats, membrane-permeant C2-ceramide alone, or its vehicle, was tested for its ability to affect mechanical sensitivity after either a single i.pl. injection (20 µg/10 µl) or following multiple injections of the same dose, spaced 75 min apart. This separation time was chosen to be long enough to allow the acute, transient effects from a single dose to fully reverse and therefore to minimize the role of a “sensitized state” in altering responses to subsequent doses of the algogen.
To establish the importance of atypical PKCs (e.g., PKMζ) for NGF-dependent hyperalgesia, a non-selective inhibitor of aPKCs, myristoylated pseudosubstrate inhibitor (mPSI, 40 µg/20µL), or its vehicle, were injected s.c. into the plantar surface of the rat’s hind paw. Such injection caused substantial edema, without accompanying changes in mechano-sensitivity, but requiring a delay in the subsequent injection of NGF. The mean value for each force was taken as the baseline paw withdrawal frequency at 1 day after mPSI injection, when the paw size had returned to normal, shortly after which NGF was injected into the paw.
2.5. Analysis
Data are presented as means ± S.E.M and evaluated using GraphPad InStat version 3.0 (GraphPad Software, CA, USA). Since the hind paw withdrawal frequency data presented here do not follow a normal distribution, statistical analysis used nonparametric tests. Friedman test followed by Dunn’s post hoc test was applied to compare repeated measures of Paw Withdrawal Frequency with baseline values. An unpaired, two-tailed Mann-Whitney U-test was used to compare responses, to the same force at the same time point, of the ipsilateral and contralateral paws. Wilcoxon matched pairs (two-tailed) test, with repeated measures correction, was applied for comparison of responses at different times with the baseline response. P values <0.05 were considered to be significant.
3.0. RESULTS
3.1. NGF and C2-ceramide sensitize the paw to mechanical stimulation
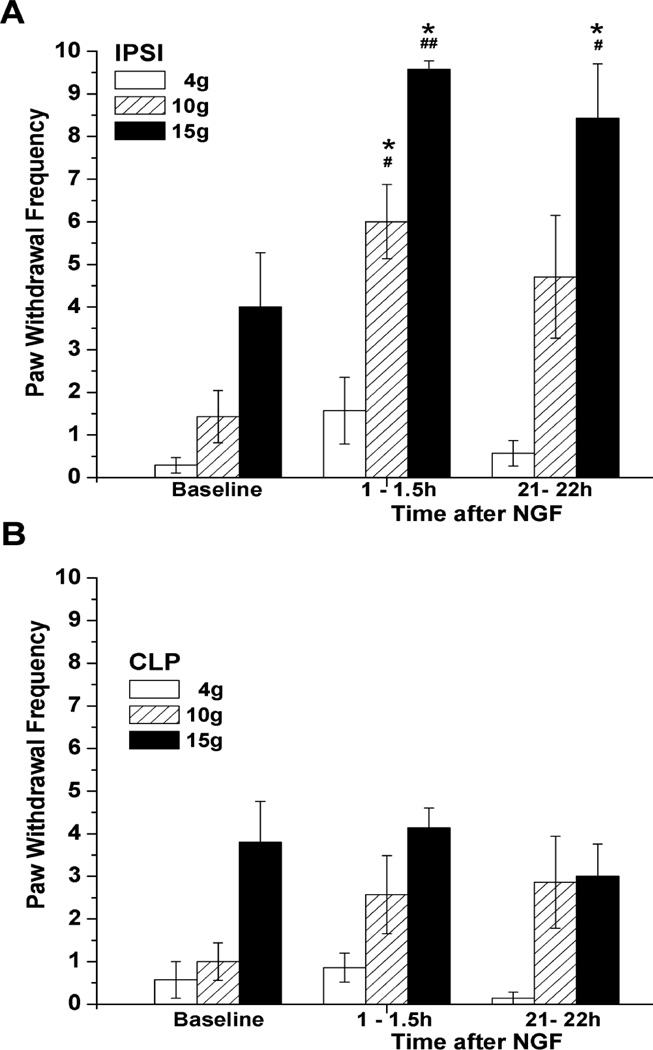
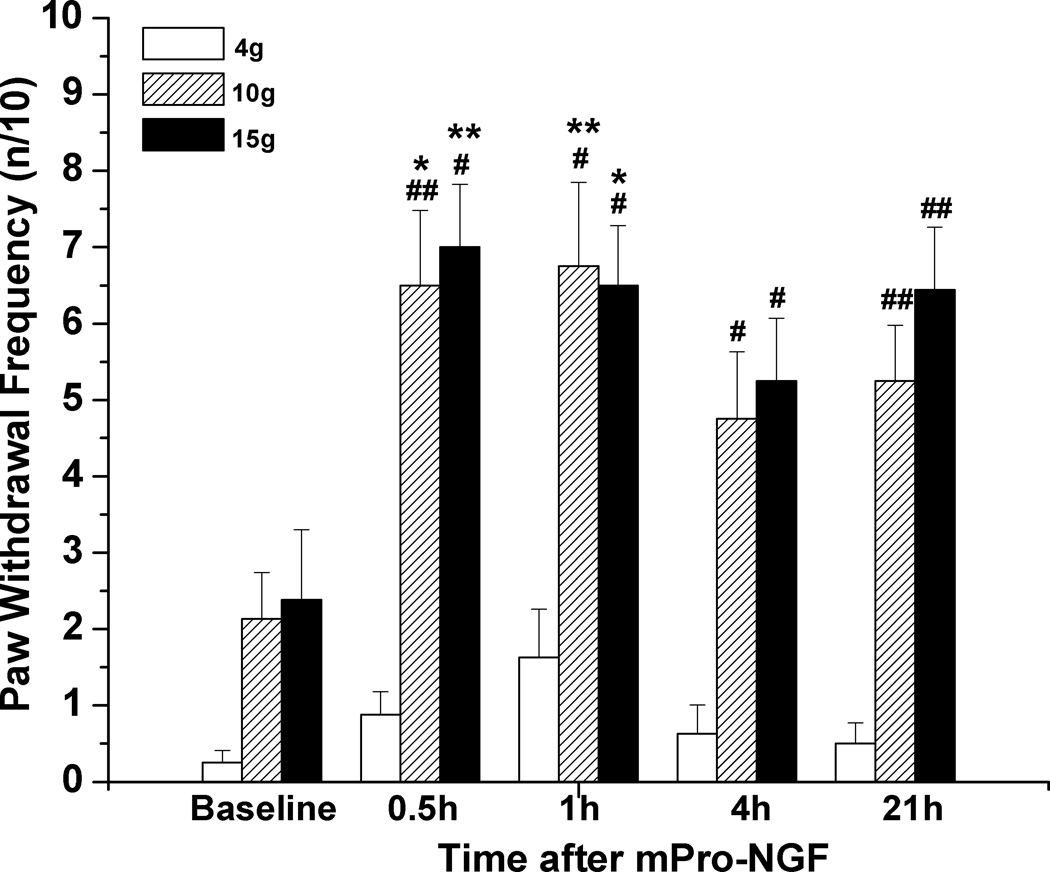
Intraplantar injection of NGF (500 ng/10 µL) induced a long lasting ipsilateral mechanical hyperalgesia. Paw withdrawal frequency for the 10g and 15g VFH, but not the 4g VFH, had significantly increased by 0.5–1.5h after injection and remained elevated at 21–22h, compared to either the baseline responses or the unchanged responses of the contralateral paw (Fig. 1). (Responses to the 4g VFH varied among cohorts of rats and had the largest variance, such that the changes after NGF sometimes reached significance but other times did not. Changes in the response to the 15g VFH stimulation were consistent.) This general pattern of a rapidly developing and persistent mechano-sensitization was observed independently many times when NGF was injected into naïve paws.
Figure 1.
Mechanical hyperalgesia induced by NGF. (A) Paw withdrawal frequency in response to stimulation with 10g and 15g VFH was enhanced following subcutaneous injection of 500 ng/10 µL NGF into the rat plantar hind paw (n=7). *P<0.05 vs. baseline values (Friedman test followed by Dunn's post hoc test), #P<0.05, ##P<0.001 vs. CLP (two-tailed Mann-Whitney test). (B) No changes in mechanical responsiveness occurred in the contralateral plantar hind paw following local administration of NGF.
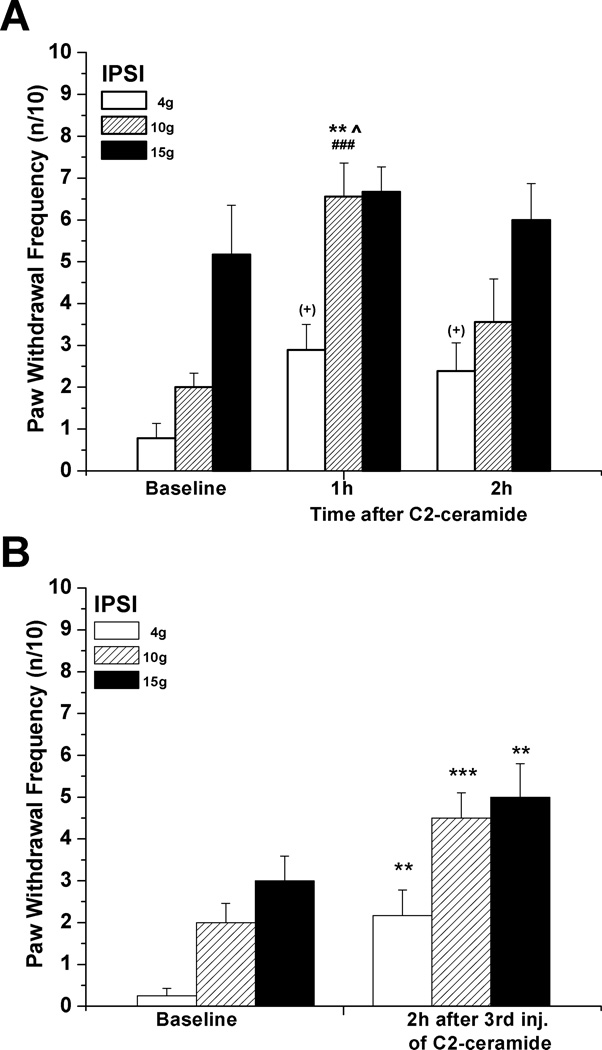
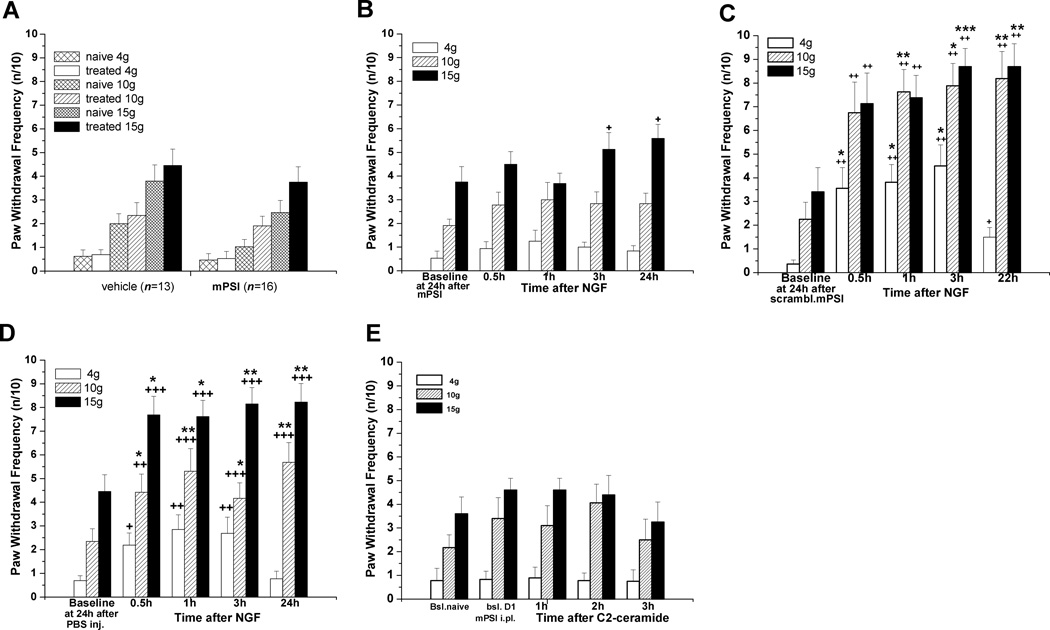
A single injection of N-acetyl sphingosine (C2-ceramide, 20 µg/10 µL), a cell-permeant ceramide analog, caused acute allodynia and hyperalgesia that, unlike that caused by NGF, had usually resolved by 2h (Fig. 2A). In separate experiments, attempting to extend the hypersensitivity by increasing the duration of C2-ceramide’s presence in the paw, three sequential identical doses were injected, each 75 min apart, timed so that the effect from one single dose had resolved before the next injection. This also caused an acute, ipsilateral increase in mechanical responsiveness to stimulation with all three forces, one that was still present at 2h after the last injection (Fig 2B), but had resolved by 24h (data not shown).
Figure 2.
Mechanical hyperalgesia induced by local C2-ceramide. (A) C2-ceramide (20 µg/10 µL) injected s.c. into the rat plantar hind paw caused an acute increase in responsiveness to stimulation with low and medium forces (4g, 10g) (n=9). **P<0.01 vs. baseline, ^P<0.05 vs. responses at 2h (Friedman test followed by Dunn's post hoc test), ###P<0.005 compared to the contralateral values (two-tailed Mann-Whitney test); for allodynia: (+) p=0.0450 (Friedman test), without significant difference between rank sum means (Dunn's post hoc test). (B) In comparison, significantly elevated ipsilateral responsiveness to stimulation by all 3 forces was observed at 2h after the 3rd of 3 consecutive s.c. injections of C2-ceramide (20 µg/10 µl) given every 75min into the same plantar spot (n=12). **P< 0.01, **P< 0.005 (Wilcoxon matched pairs test, two-tailed).
3.2. Antihyperalgesic actions of GSH
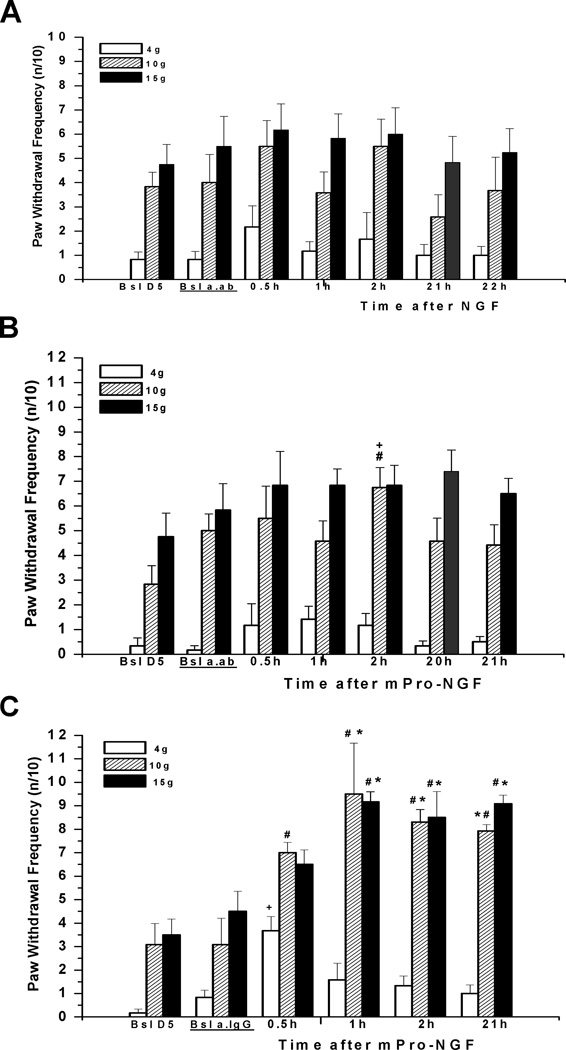
To further evaluate the role of the sphingomyelin cascade, we first tested whether inhibition of nSMase, the enzyme catalyzing the liberation of ceramide, would suppress NGF-induced hyperalgesia. Acute pre-treatment with a semi-selective inhibitor of nSMase activation, GSH (Liu and Hannun, 1997; Liu et al., 1998), pre-injected (120 mM, 10 µl) 15 min before and then co-injected (60 mM, 10 µl) with NGF (total GSH dose = 1.8 µmoles/paw), prevented significant acute mechano-sensitization at 1.5h but did not affect the increase in mechano-sensitivity measured the next day (n=8) (Fig. 3A compare to Fig. 1A). Glutathione applied at a lower total dose of 0.6 µmol (40 mM and 20 mM concentrations for pre- and co-injections, respectively) did not attenuate NGF-induced enhancement of ipsilateral mechanical responsiveness (n=8, data not shown). In contrast to the effects of immediate pretreatment with GSH, when the higher pre-treatment dose (120 mM, 10 µl) was given 1.5h beforehand followed by a co-injection of GSH (60 mM, 10 µl) with NGF, hyperalgesia was not reduced, despite the high total dose of the inhibitor (n=4; Fig. 3B). In controls, injection of GSH into the naïve paw, at a total dose (1.2 µmol; 120 mM, 10 µl) that was intermediate between “high” and “low” doses, evoked swelling of the paw in 50% of rats by 3–4h, but did not change mechanical responsiveness in either ipsilateral, or contralateral paws, as compared to baseline values (n=4). Similarly, no changes in the mechanical responsiveness were observed during the 1.5h pre-treatment of GSH, before the injection of NGF (n=8).
Figure 3.
Glutathione (GSH), an inhibitor of nSMase, prevented the acute hyperalgesia induced by NGF. (A) GSH (1.8 µmoles/paw total dose), when pre-injected (120 mM, 10 µL) 15 min before and then co-injected (60 mM, 10 µL) with NGF (500 ng/10 µL), prevented hyperalgesia measured at 1–1.5h, but did not that measured on the next day (n=8). *P<0.05 vs. baseline IPSI (Friedman test followed by Dunn's post hoc test). (B) When glutathione (1.8 µmoles/ paw total dose) was injected (120 mM, 10 µL) s.c. intraplantar 1.5h before and then co-injected (60 mM, 10 µL) with NGF (500 ng/10 µl) the acute stage of hyperalgesia still occurred (n=4, p=0.0324 for both 4g and 15g forces (Friedman test); #P<0.05 compared to the CLP responses (two-tailed Mann-Whitney test)
3.3. Attenuation of NGF-induced hyperalgesia by GW4869
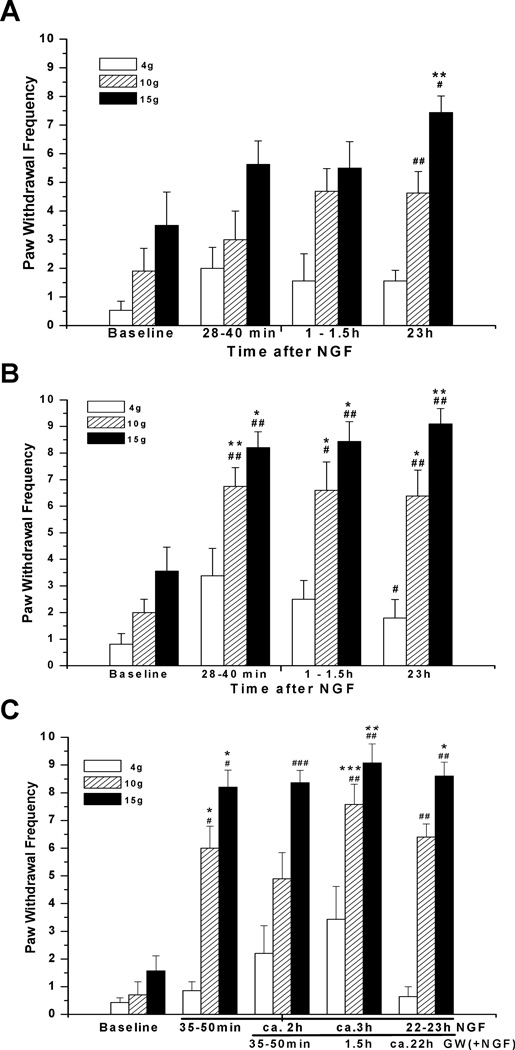
Since GSH was used at a high concentration and affects many other proteins we examined a more selective inhibitor of nSMase, GW4869 (Luberto et al., 2002; Marchesini et al., 2003). This cell-permeant, non-competitive, nSMase inhibitor was injected into the paw 17 min prior to NGF. The effect of this single application (2 mM, 10 µL; dose 20 nmoles) was similar to the actions of the acute pre-treatment with high dose GSH; it prevented the hypersensitivity at 0.5–1.5h, but not at 23h after NGF injection (n=8) (Fig. 4A). In the vehicle control group, wherein NGF was injected following pre-injection of DMSO, hyperalgesia developed as after NGF alone, both responses to 10g and 15g forces being augmented significantly at 0.5–23h (n=8) (Fig. 4B). In neither group of rats were any changes in responsiveness of the contralateral paw observed (data not shown). Therefore the inhibitor of nSMase GW4869 prevented NGF-induced local mechanical hyperalgesia, although this effect lasted less than one day.
Figure 4.
A short-lasting preventive effect of GW4869 against NGF-induced hyperalgesia. (A) A cell-permeant non-competitive, nSMase inhibitor, GW4869 (2 mM, 10 µL; dose 20 nmoles/plantar paw), injected 17 min prior to NGF (500 ng/10 µl), prevented acute hyperalgesia, at 0.5–1.5h, but not the later hyperalgesia, at 23h after injection (n=8). (B) Hyperalgesic actions of NGF in controls, where vehicle (DMSO, 10 µL) injection preceded NGF (n=8). (C) GW4869 (2 mM, 10 µL; dose 20 nmoles/plantar paw) was ineffective against NGF-induced hyperalgesia when it was injected 1h after NGF (500 ng/10 µL) (n=7). Note significantly increased responses at 35–50min after NGF (injection into naïve paw), at 2h (=35–50min post GW4869; for 15g as compared with CLP, Mann-Whitney test), at 3h (=1.5h post GW4869), and at 22–23h (=ca. 22h post GW4869). *P<0.05, **P<0.01 vs. baseline (Friedman test followed by Dunn's post hoc test), #P<0.05, ##P<0.005, ###P<0.001 compared to the CLP values (two-tailed Mann-Whitney test).
Pre-injection of the paw with GW4869 had no effect on the acute hyperalgesia caused by the C2-ceramide injection (n=8, data not shown). This result is consistent with an upstream inhibition of GW4869, at the nSMase location, and also shows that the inhibitor did not alter any reactions of the downstream pathway.
When GW4869 (2 mM, 10µL) was injected 1h after NGF it failed to reverse the already developed mechanical hyperalgesia (n=7) (Fig. 4C). Some tendency to attenuate the hyperalgesia was noticed at 35–50 min after injection of GW4869; the NGF-induced increase in mechanical response to 10 and 15 g VFHs did not reach significance vs. baseline in a group test (Friedman+Dunn’s). These changes vs. ipsilateral baseline, however, were highly significant for 15 g VFH, when compared using the Mann-Whitney test (p=0.0006).
3.4. Hypersensitivity from Pro-NGF
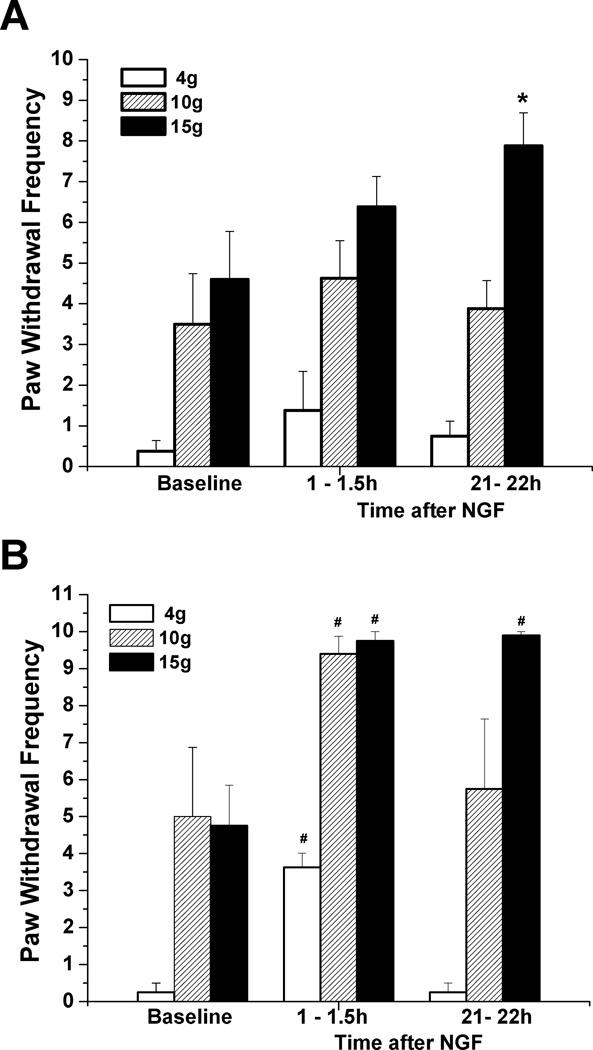
A non-hydrolyzable homologue of Pro-NGF (Pagadala et al., 2006, the larger precursor to mature NGF which has selectivity for the p75NTR over the TrkA receptor (Lee et al., 2001; Beattie et al., 2002; Ibañez, 2002; Teng et al., 2010), also caused hyperalgesia. Injections of this mPro-NGF (500ng/10µL) into the hindpaw increased responsiveness to 10 and 15 g VFH at 0.5 to 21 h, very similar to the time-course following NGF injection (Fig. 5; compare to Fig 1A). Responsiveness to the 4g VFH was not significantly changed.
Figure 5.
Mechanical ipsilateral hyperalgesia is induced by mutated, protease-resistant Pro-NGF. Paw withdrawal frequency in response to stimulation with 10g and 15g VFH was enhanced following s.c. injection of mPro-NGF ( 500 ng/10 µL) into the rat plantar hind paw (n=8). *P<0.05, **P<0.01 vs. baseline values (Friedman test followed by Dunn's post hoc test), #P<0.05, ##P<0.01 vs. baseline values (Wilcoxon matched pairs test, two-tailed).
3.5 A p75NTR blocking antibody abolishes NGF-induced hyperalgesia
To further confirm that the active receptor for NGF’s actions is p75NTR, we used a receptor-specific antibody. Polyclonal rat anti-p75NTR antibodies (generously donated by Prof. Louis Reichardt, UCSF) were injected into the plantar hindpaw 4h before NGF or mPro-NGF injection. The antibody alone did not affect mechano-responsiveness (Fig 6A). Subsequent injection of NGF (Fig. 6A) or mPro-NGF (in separate experiments, Fig. 6B), at the same s.c. location in the paw as the antibody injection, resulted in virtually no increase in paw mechano-sensitivity. By comparison, pre-injections of the same amount of naïve serum-derived IgG did not diminish the hypersensitivity from mPro-NGF (Fig. 6 C) or from NGF (not shown).
Figure 6.
A polyclonal antibody to p75NTR prevented mechanical hyperalgesia induced by NGF and mProNGF. (A) When pre-injected (20 µL) into the paw 4h before NGF (500 ng/10 µl), the antibody prevented any significant hyperalgesia at 0.5h-21h (n=6). (B) A similar inhibition occurred when the antibody was injected 4h before mPro-NGF (500 ng/10 µL) (n=6). Significant elevation of response was found only for the 10g force at 2h after mPro-NGF injection. #P<0.05 vs. baseline-after-antibody values (Wilcoxon matched pairs test, two-tailed), and +P<0.05 vs. baseline-after-antibody values and vs. 21h values for 3 groups comparison: Bsl a.ab., 2h, 21h (Friedman test followed by Dunn's post hoc test). Bsl a.ab. = 2.5h after ab.p75 injection. (C) In controls, where IgG (20 µL) from naïve serum was injected 4h prior to mPro-NGF, hyperalgesia was fully present(n=6; compare with Fig 5). Bsl a.IgG=2.5h after IgG injection.
3.6. The myristoylated pseudosubstrate inhibitor of aPKCs (mPSI) suppresses NGF-induced hyperalgesia
Previously published electrophysiological studies of isolated sensory neurons have shown that NGF-induced hyperexcitability depends on the nSMase > ceramide > S-1-P pathways, with an essential downstream role of aPKCs, specifically PKMζ (Zhang et al., 2002, 2006, 2012; Nicol and Vasko, 2007). To determine whether aPKCs were involved in the local effects of NGF, we pre-treated rats peripherally with mPSI, a cell permeant inhibitor acting non-selectively on all aPKCs (Eicholtz et al., 1993; Thiam et al., 1999; Harishchandran and Nagaraj, 2005; Moscat et al., 2006). Edema of the paw followed acutely after mPSI injection, possibly due to its actions on vascular targets unrelated to aPKCs (Krotova et al., 2006), and this necessitated a delay before the injection of NGF. Therefore, this inhibitor was injected subcutaneously (40 µg/20 µl) into the plantar hind paw 24h before the injection of NGF. Pre-injection of mPSI alone did not increase the responsiveness to mechanical stimulation tested 24h later, the small changes being the same as from vehicle injection (Fig. 7A), and by then there was no edema. Locally administered mPSI strongly suppressed the development of mechanical hyperalgesia from NGF injected into the same plantar area (n=16, Fig. 7B, compare to Figures 1, Fig. 7D). A scrambled version of mPSI did not affect the hypersensitivity from NGF (Fig. 7C), showing the selectivity of this inhibitor for its aPKC inhibitory function (Krotova et al., 2006). In cohort-matched control experiments, a robust hyper-responsiveness was observed at 0.5–24h after NGF injection in the control group of rats, which had been pre-injected with intraplantar vehicle (PBS) 24h before NGF (n=13, Fig 7D).
Figure 7.
Local pre-treatment with myristoylated pseudosubstrate inhibitor of atypical PKCs decreased hyperalgesia from NGF and C2-ceramide. For all panels the inhibitor (40 µg /20 µl) was injected subcutaneously into the plantar hind paw 24h before the intraplantar injection of the algogens. (A) mPSI alone did not change the plantar hind paw responsiveness to mechanical stimulation, when tested at 24h after injection into naïve rats. (B) NGF injected into the plantar area after mPSI induced a much smaller than usual hyperalgesia (n=16): significant changes in responses were found only for 15g force (p=0.0489, Friedman Test for 5 groups; no significance by Dunn's post hoc test). However, even for 15g, P>0.05 was found when the test was applied to 4 same day-groups, excluding 24h time point responses. Analysis by Mann Whitney test revealed a significant difference for comparisons with corresponding baseline values only for 4g and 15g, at 3h and 24h time points, respectively. (C) Injection into the plantar hind paw of scrambled mPSI(40 µg /20 µl), which lacks inhibitory action on aPKCs, 24h before the intraplantar injection of NGF, neither prevented, nor decreased hyperalgesia from NGF (500 ng/10 µl) (n=8). *P<0.05, **P<0.01, ***P<0.001 vs. baseline (Friedman test, 5 groups, followed by Dunn's post hoc test); #P<0.05, ##P<0.01 vs. baseline (two-tailed Wilcoxon test). (D) Vehicle control: pronounced hyperalgesia at 0.5–24h after NGF injection was observed in rats pre-injected with vehicle (PBS) into the same plantar area 24h before (n=13). *P<0.05, **P<0.01 vs. baseline (Friedman test followed by Dunn's post hoc test). Some enhancement was revealed for responses to 4 g p=0.0032 (Friedman test, 5 groups), however, no significance was found by Dunn's post hoc test. +P<0.05, ++P<0.01 compared to baseline values (two-tailed Wilcoxon test). (E) mPSI (40ug/20uL) injected i.pl. prevented mechanical hyperalgesia induced by C2-ceramide (20ug/10uL) (n=9).
As with NGF, intraplantar mPSI blocked the acute hyperalgesia resulting from injection of C2-ceramide into the paw (Fig. 7E). From 0.5 to 3h after C2-ceramide (20µg/10 µL) injection into the paw, the responsiveness was unchanged from the baseline values (n=12), contrasting with the enhanced sensitivity caused by this dose of C2-ceramide in naive rats (cf. Fig. 2).
4.0. DISCUSSION
The mechanism of NGF-induced mechanical hyperalgesia is not completely known. NGF initiates a variety of intracellular signals through activation of its two receptors, TrkA, with a higher NGF affinity and slower dissociation (respectively, Kd ~10−11M, t1/2 ~ 10 min), and a broadly selective neurotrophin receptor, p75NTR, having a lower affinity and faster dissociation for NGF (Kd ~10−9M, t1/2 ~ 3 sec) (Meakin and Shooter, 1992). Both receptors are expressed on sensory neurons and are assumed to be activated by relatively high concentrations of NGF either released endogenously during inflammation, or administered exogenously (Woolf, 1996; Woolf et al., 1996; Nicol and Vasko, 2007). We tested the hypothesis that activation of the p75NTR signaling cascade that has been shown to sensitize isolated peripheral nociceptive neurons (Zhang et al., 2002; Zhang and Nicol, 2004; Zhang et al., 2012) contributes to the sensitization of mechanical responsiveness by NGF in adult rats in vivo. Our findings show a rapidly developing tactile hypersensitivity, present at 1 h and persisting for at least 24h after hindpaw injection of 500 ng of NGF. Injection of the p75NTR-selective homologue Pro-NGF, which had been mutated to mPro-NGF in order to prevent the hydrolytic cleavage that frees mature NGF (Pagadala et al., 2006), produced an identical hypersensitivity. Direct binding studies show that Pro-NGF binds with ~5-fold higher affinity to p75NTR than to TrkA receptors (Lee et al., 2001; Nykjaer et al., 2004), although the predominant pathway in any action will depend on the relative levels of expression of the two receptors (Masoudi et al., 2009) and early evidence suggests that interactions between the two receptors may alter their activities (Benedetti et al., 1993).
Importantly, paw injection of an antibody directed against the rat p75NTR (Weskamp and Reichardt, 1991) prevented tactile hypersensitivity from either NGF or mutant Pro-NGF. This antibody showed a large selectivity for the p75NTR based on direct NGF binding studies and on their ineffectiveness in suppressing neuron survival and neurite outgrowth that are known to be mediated by Trk receptors (Fariñas et al., 1998). Inhibition of NGF- and mPro-NGF induced hyperalgesia by this antibody is consistent with an earlier report wherein treatment with the same antibody prevented the NGF-induced increase in excitability in isolated sensory neurons (Zhang and Nicol, 2004).
In addition, our results demonstrate that NGF/p75NTR – mediated hypersensitivity results from activation of a neutral sphingomyelinase, can be recapitulated by a membrane-permeant ceramide analogue, albeit only briefly compared to that of NGF, and, lastly, a key downstream effector is an atypical PKC in the periphery NGF-induced mechanical hypersensitivity in adult rats develops after a single systemic injection (1 µg/gm, s.c.) with a rapid (0–7h) and a slow (8–30h) phase (Lewin et al., 1993). The authors suggested that the delayed mechanical hypersensitivity in adult rats results from central changes, perhaps due to up-regulation of peptides in the peripheral
NGF-responsive sensory neurons, where the internalized NGF-TrkA complex in the periphery could be transported to central terminals within hours. Thermal hypersensitivity was attributed to both peripheral and central neuron sensitization. NGF’s involvement in prolonged inflammatory hyperalgesia, after injection of Complete Freund’s Adjuvant, appears to require both sensory neurons and mast cells (Woolf et al., 1996), both of which express TrkA receptors. Consistent with our results described above, intradermal injection of NGF (1µg) into the dorsum of the paw after just 10 min, resulted in tactile hypersensitivity that reached its peak change by 3h and lasted for several days (Malik-Hall et al., 2005). Most recently it has been shown that single intraplantar injections of NGF (0.3–5 µg/paw) reduced the mechanical withdrawal threshold maximally by 50–70 % after 1hr, which only slowly reversed over several weeks (Mills et al., 2013).
Previous publications present contradictory findings about the roles of TrkA vs. p75NTR in pain sensitization. Most publications support an essential role of the TrkA receptor (Mantyh et al., 2011). Mutated mice lacking p75NTR still develop mechanical and thermal hyperalgesia after systemic NGF delivery (Bergmann et al., 1998), suggesting that TrkA plays the dominant role. However, these knock-out mice have higher thresholds for acute nocifensive responses to cutaneous thermal and mechanical stimuli and show significant reductions in the density of epidermal neurites (Bergmann et al., 1997). Differences in the distribution of DRG cell sizes between p75NTR −/− mice and the wild-type+/+ controls were modest, and there was no difference in their neurochemical profiles (Bergmann et al., 1997). Others, however, have reported a marked reduction of both peptide-containing neurites in the skin and thermal sensitivity in p75NTR knock-out mice (Lee et al., 1992). Also in support of TrkA involvement, intrathecal delivery of anti-sense oligodeoxynucleotides targeted to TrkA decreased TrkA protein in peripheral nerve and prevented NGF-induced mechanical hyperalgesia (Malik-Hall et al., 2005).
In contrast, other studies suggest that the p75NTR plays a key role in pain. Inflammation resulting from CFA injection into the rat wrist joint induced an upregulation of p75NTR immunoreactivity, and local injections of blocking antibodies against the p75NTR reduced CFA-induced mechano-hyperalgesia (Iwakura et al., 2010). Similarly, intraplantar injections of CFA, NGF or Pro-NGF caused thermal hypersensitivity, and also increased the expression of CGRP in primary sensory neurons, both changes being prevented by the anti-p75 NTR blocking antibody (Watanabe et al., 2008). Signaling through the sphingomyelin pathway is known to be activated by the low-affinity p75NTR, resulting in the liberation of ceramide (Dobrowsky et al., 1994). Consistent with this idea, C2-ceramide injected into the paw induces mechano-hyperalgesia that is mimicked by injection of TNF-α (Woolf et al., 1997), an effect that was blocked by the nSMase inhibitor GW4869 (Joseph and Levine, 2004). Recent findings further show that intraplantar injection of C2-ceramide produced thermal hyperalgesia that peaked 1h post-injection, as well as increased levels of TNF-α (Doyle et al., 2011a,b).
There is compelling evidence that NGF acts directly on sensory neurons. Capsaicin-evoked currents through TRPV1 receptors (Shu and Mendell, 1999, 2001), were enhanced via activation of TrkA (Galoyan et al,, 2003; Zhu and Oxford, 2007). NGF-induced activation and up-regulation of TRPV1 (Winston et al., 2001; Zhang et al., 2005), and this receptor/channel’s transport to peripheral C-fiber terminals of adult rat DRG neurons contributes to the maintenance of inflammatory heat hypersensitivity (Ji et al., 2002). In capsaicin-sensitive, small-diameter DRG neurons isolated from adult rats, both NGF and C2-ceramide acutely increased firing of action potentials (AP) as a result of the rapid augmentation of a tetrodotoxin-resistant sodium current and suppression of a delayed rectifier-type potassium current (Zhang et al., 2002); both actions appear to involve the p75NTR receptor. This effect from NGF, but not the identical one from C2-ceramide, was completely prevented by pretreatment with the same p75NTR blocking antibody used in the current studies (Zhang and Nicol, 2004). Furthermore, inhibitors of tyrosine kinase receptors failed to affect the increased AP firing produced by either NGF or BDNF (Zhang et al., 2008). Involvement of the sphingomyelin signaling pathway is evident in the NGF-induced augmentation of AP firing by its strong inhibition by glutathione, a cell-permeant inhibitor of nSMase, as also shown in our present in vivo study. This increase in excitability could be mimicked by intracellular perfusion with bacterial SMase, simulating the release of native ceramide by endogenous enzymes. Exogenous C2-ceramide enhanced the number of evoked APs, despite the presence of glutathione and NGF, indicating that ceramide’s sensitizing actions lay downstream of NGF (Zhang et al., 2002), identical to the interactions shown here in vivo. Moreover, sphingosine-1-phosphate, a product of intracellular metabolism of ceramide by sphingosine kinase, acted as a second messenger in augmenting neuronal excitability (Zhang et al., 2006).
In the current work both intraplantar NGF and C2-ceramide induced mechanical hypersensitivity. However, NGF - evoked hyperalgesia persisted at least for 24h (also see Mills et al., 2013) whereas that from a single C2-ceramide injection had resolved by 2h. Multiple sequential injections of this dose of C2-ceramide extended the hypersensitivity after the last dose, but not for more than a few hours, still very brief compared to NGF. This difference in the duration of hypersensitivity may be explained by the different abilities of the two agents to persistently activate the common pathway. In isolated cells the two compounds cause essentially identical changes in excitability and its underlying ion currents (Zhang et al., 2002). But the C2-analogue of ceramide might be quite differently disposed of in the rat than the longer fatty acyl chain ceramides that are liberated in vivo by nSMase; these ceramides may be bound to hydrophobic regions of membranes or proteins and thus less mobile for diffusion and less amenable to degradative enzymes (Liu and Hannun, 1997; Liu et al., 1998). Furthermore, other cells near the cutaneous injection site, e.g., keratinocytes, might also respond to NGF and to ceramide, leading to activation of pathways that are absent from isolated sensory neurons.
Two different nSMase inhibitors appeared effective against the acute hyperalgesic actions of NGF. Brief (15 min) pre-treatment with GSH, a selective inhibitor of nSMase activation (Liu and Hannun, 1997; Liu et al., 1998), prevented the acute NGF-induced hyperalgesia, measured 1.5h later, but did not affect mechanical hypersensitivity measured the next day. This finding suggests that there is a limited time during which GSH’s inhibitory actions are effective, implying that the period of p75NTR-induced nSMase activation continues after the readily oxidized GSH has been exhausted or removed from the paw. To test the hypothesis of limited time of protection we injected a high concentration of GSH 1.5h before NGF (which was also co-injected with GSH). This earlier delivery of GSH failed to suppress the NGF-induced tactile hyperalgesia, showing that protection by GSH is indeed a short-lived phenomenon.
The cell-permeant non-competitive nSmase inhibitor GW4869, injected once shortly before NGF, also prevented the NGF-induced hyperalgesia at 0.5–1.5h, but not on the next day. Here, using a more stable inhibitor than GSH (Luberto et al., 2002), a preventive effect of nSMase inhibition also lasted only a short time. It is plausible that the inhibitor could have been removed from the paw by the next day, which may explain the lack of GW4869 anti-hyperalgesic actions at 23h post NGF. However, GW4869 when injected 1h after NGF also appeared to be completely ineffective against the previously developed hyperalgesia. These results imply that the liberation of ceramide by nSMase contributes to the induction rather than the maintenance of mechanical hypersensitivity in rat plantar skin, wherein the downstream effect of ceramide, once initiated, leads to continuous functioning of intracellular machinery to provide a hyperalgesic state. The short term preventive actions of peripheral nSMase inhibitors against NGF-induced hypersensitivity along with the acute hyperalgesic actions of exogenous C2-ceramide suggest an involvement of different signaling events, possibly through different sensory neuron sub-types innervating different spinal targets, to provide a long-lasting hyper-responsiveness from NGF (Kobayashi et al., 2012).
PKCs are profoundly important for cellular responses to extracellular stimulation (Gallegos and Newton, 2008; Gould and Newton, 2008; Newton, 2009) where the atypical PKCs seem to have a specialized role in mediating long-term changes (Moscat and Diaz-Meco, 2000; Hirai and Chida, 2003). Recent studies, either using a pseudosubstrate inhibitor targeting all aPKCs, mPSI (ZIP), or specifically knocking down PKMζ by siRNA, identified this isozyme of PKC as the critical kinase in the NGF-p75NTR pathway driving sensitization of isolated sensory neurons (Zhang et al., 2012). Although the current results do not identify the specific aPKC that is involved, our experiments show that one of these isozymes appears to be essential in the periphery for hypersensitivity mediated through the p75NTR.
Atypical PKCs are claimed by some to be essential contributors supporting chronic hyperalgesia after nerve injury (King et al. 2012; see Price and Ghosh, 2013). Recruited by NGF to enhance the excitability of isolated rat sensory neurons (Zhang et al., 2012), PKMζ also appears to be essential for the development and persistence of a sensitized state in the dorsal horn (Laferriere et al., 2011; Marchand et al., 2011; Asiedu et al., 2011). These papers report an elevation of activated and total aPKCs, especially PKMζ, in the dorsal horn after inflammation, and a reduction of inflammation-induced hyperalgesia by chemical inhibition of spinal PKMζ. The current paper, and related previous in vitro studies (Zhang et al., 2012), suggest that peripheral aPKCs may also contribute to the relatively long lasting changes in hyper-excitability that subserve prolonged hyperalgesia after NGF.
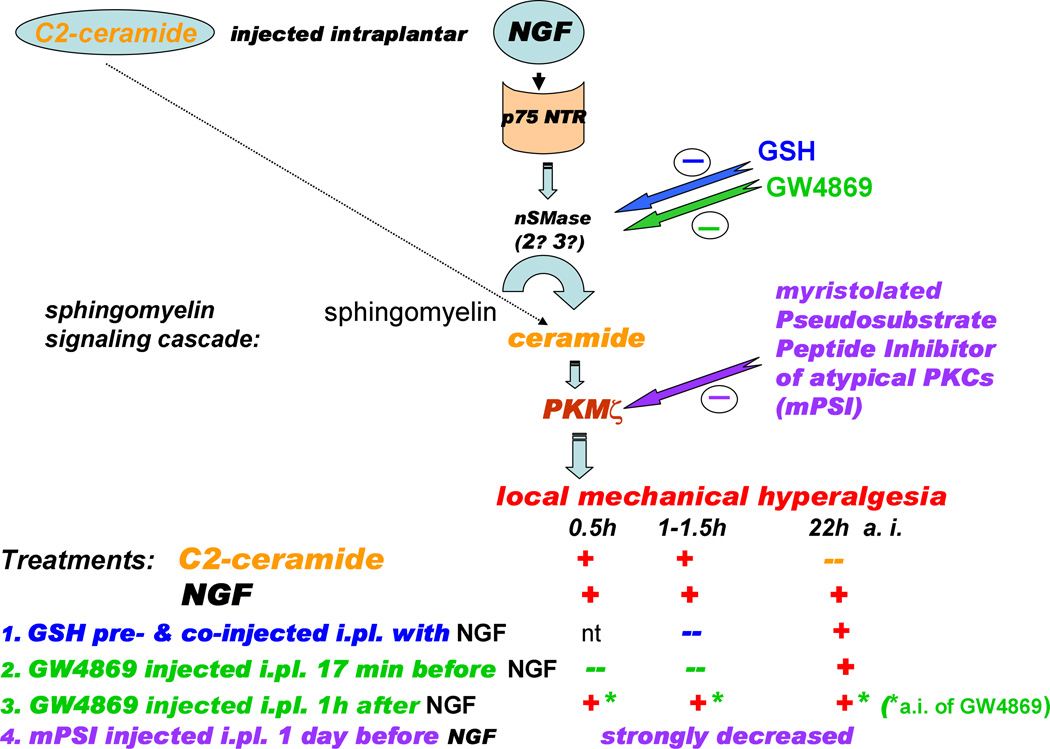
Figure 8 presents a scheme that summarizes this work. Key mediators of the p75NTR signaling pathway contribute to mechanical hyperalgesia induced by NGF, which is consistent with their sensitizing effects demonstrated in the isolated nociceptive DRG neurons. However, accumulating evidence on potential cross-regulation and signaling interactions between p75NTR and TrkA pathways in nociceptive sensory neurons, e.g., common activation of phosphatidylinositol 3-kinase (PI3K) (reviewed by Huang and Reichardt, 2003), or common up-regulation of substance P synthesis by both pathways (Skoff and Adler, 2006), indicate that signaling from both NGF-binding receptors could be involved in mechanical sensitization. The results reported here do not eliminate a role for the TrkA receptor in acute pain processing, but our findings do establish a greater role for p75NTR and its downstream signaling cascade than was previously recognized.
Figure 8.
The intracellular signaling pathway downstream of p75NTR which leads to mechanical hyperalgesia from NGF in rat plantar hind paw, and a summary of the antihyperalgesic effects of the treatments used in the current work. NGF induced a long-lasting hyperalgesia (up to 24h), while the effect from a single injection of C2-ceramide was brief (<2h). Both inhibitors of nSMase, GSH (at a high total dose) and GW4869, prevented the acute hyperalgesia from NGF. A post facto application of GW4869, however, failed to reverse NGF-induced hyperalgesia. Intraplantar pre-treatment with myristoylated pseudosubstrate inhibitor of aPKCs, mPSI, (1 day before) strongly decreased the hyperalgesic actions of NGF. Key: (+) - hyperalgesia was observed; (--) - hyperalgesia was not observed; nt – not tested.
Highlights.
Subcutaneously injected NGF induces tactile hyperalgesia for more than 24 hours.
A p75NTR – selective agonist, Pro-NGF, causes the same effect.
A blocking antibody of the p75NTR prevents the hyperalgesia from NGF and Pro-NGF.
Intermediates of the neutral sphingomyelinase pathway simulate this effect of NGF.
An inhibitor of aPKCs injected beforehand in the same paw suppresses NGF’s effects.
AKNOWLEDGEMENTS
The authors appreciate the excellent assistance of Mr. James Bell, Department of Anesthesiology, Brigham & Women’s Hospital, for support with the graphics. Funding was provided by USPHS grants CA080153 (to GS) and NS078173 (to GDN).
Abbreviations
- p75NTR
p75 neurotrophin receptor
- nSMase
neutral sphingomyelinase
- aPKC
atypical PKC
- DMSO
dimethyl sulfoxide
- DRG
dorsal root ganglia
- MAPK
Mitogen activated protein kinase
- COX2
Cyclo-oxygenase 2
- PLC
phospholipase C
- PGE2
prostaglandin E2
- VFH
von Frey hair
- NGF
nerve growth factor
- C2-ceramide
N-acetyl sphingosine
- GSH
glutathione
- PKCζ/PKMζ
protein-kinase C zeta or protein-kinase M zeta
- TrkA
tyrosine kinase receptor A
- IPSI
ipsilateral paw
- CLP
contralateral paw
- s.c.
subcutaneously
- mPSI
myristoylated pseudosubstrate inhibitor
- PBS
phosphate buffered saline
- ODN
oligodeoxyribonucleotides
- TRPV1
transient receptor potential cation channel subfamily V member 1
- AP
action potential
- siRNA
small interfering RNA
- PI3K
phosphatidylinositol 3-kinase
- BDNF
brain derived neurotrophic factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M æ underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PA, Murphy RA. The nerve growth factor receptor: a multicomponent system that mediates the actions of the neurotrophin family of proteins. Mol Cell Biochem. 1992;110:1–15. doi: 10.1007/BF02385000. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci USA. 90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann I, Priestley JV, McMahon SB, Brocker EB, Toyka KV, Koltzenburg M. Analysis of Cutaneous Sensory Neurons in Transgenic Mice Lacking the Low Affinity Neurotrophin Receptor p75. Eur J Neurosci. 1997;9:18–28. doi: 10.1111/j.1460-9568.1997.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Bergmann I, Reiter R, Toyka KV, Koltzenburg M. Nerve growth factor evokes hyperalgesia in mice lacking the low-affinity neurotrophin receptor p75. Neurosci Lett. 1998;255:87–90. doi: 10.1016/s0304-3940(98)00713-7. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the Sphingomyelin Cycle Through the Low-Affinity Neurotrophin Receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Doyle T, Chen Z, Muscoli C, Obeid LM, Salvemini D. Intraplantar-injected ceramide in rats induces hyperalgesia through an NF-kappaB- and p38 kinase-dependent cyclooxygenase 2/prostaglandin E2 pathway. FASEB J. 2011;25:2782–2791. doi: 10.1096/fj.10-178095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T, Chen Z, Obeid LM, Salvemini D. Sphingosine-1-phosphate acting via the S1P(1) receptor is a downstream signaling pathway in ceramide-induced hyperalgesia. Neurosci Lett. 2011;499:4–8. doi: 10.1016/j.neulet.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O'Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48:501–505. doi: 10.1212/wnl.48.2.501. [DOI] [PubMed] [Google Scholar]
- Eichholtz T, de Bont DB, de Widt J, Liskamp RM, Ploegh HL. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J Biol Chem. 1993;268:1982–1986. [PubMed] [Google Scholar]
- Farinas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A. Characterization of neurotrophin and Trk recepter functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron. 21(2):325–334. doi: 10.1016/s0896-6273(00)80542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Dev Biol. 1997;190:94–116. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- Gallegos LL, Newton AC. Spatiotemporal dynamics of lipid signaling: protein kinase C as a paradigm. IUBMB Life. 2008;60:782–789. doi: 10.1002/iub.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galoyan SM, Petruska JC, Mendell LM. Mechanisms of sensitization of the response of single dorsal root ganglion cells from adult rat to noxious heat. Eur J Neurosci. 2003;18:535–541. doi: 10.1046/j.1460-9568.2003.02775.x. [DOI] [PubMed] [Google Scholar]
- Gould CM, Newton AC. The life and death of protein kinase C. Curr Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Washington, DC, U.S.A; National Academy Press; 1996. [Google Scholar]
- Harishchandran A, Nagaraj R. Interaction of a pseudosubstrate peptide of protein kinase C and its myristoylated form with lipid vesicles: only the myristoylated form translocates into the lipid bilayer. Biochim Biophys Acta. 2005;1713(2):73–82. doi: 10.1016/j.bbamem.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Fitzgerald M. Time course and dose-dependence of nerve growth factor-induced secondary hyperalgesia in the mouse. J Pain. 2006;7:57–61. doi: 10.1016/j.jpain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem. 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Ann Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Ibáñez CF. Jekyll-Hyde neurotrophins: the story of proNGF. Trends Neurosci. 2002;25:284–286. doi: 10.1016/s0166-2236(02)02169-0. Review. Erratum in: Trends Neurosci 2002 Jul;25(7):378. PubMed PMID: 12086739. [DOI] [PubMed] [Google Scholar]
- Iwakura N, Ohtori S, Orita S, Yamashita M, Takahashi K, Kuniyoshi K. Role of Low-Affinity Nerve Growth Factor Receptor Inhibitory Antibody in Reducing Pain Behavior and Calcitonin Gene-Related Peptide Expression in a Rat Model of Wrist Joint Inflammatory Pain. J Hand Surg Am. 2010;35:267–273. doi: 10.1016/j.jhsa.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Caspase signalling in neuropathic and inflammatory pain in the rat. Eur J Neurosci. 2004;20:2896–2902. doi: 10.1111/j.1460-9568.2004.03750.x. [DOI] [PubMed] [Google Scholar]
- King T, Qu C, Okunn A, Melemedjian OK, Mandell EK, Maskaykina IY, Navratilova E, Dussor GO, Ghosh S, Price TJ, Porreca F. Contribution of PKMζ-dependent and independent amplification to components of experimental neuropathic pain. Pain. 2012;153:1263–1273. doi: 10.1016/j.pain.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kiguchi N, Maeda T, Ozaki M, Kishioka S. The critical role of spinal ceramide in the development of partial sciatic nerve ligation-induced neuropathic pain in mice. Biochem Biophys Res Commun. 2012;421:318–322. doi: 10.1016/j.bbrc.2012.03.153. [DOI] [PubMed] [Google Scholar]
- Krotova K, Hu H, Xia SL, Belayev L, Patel JM, Block ER, Zharikov S. Peptides modified by myristoylation activate eNOS in edothelial cells through Akt phosphorylation. Brit J Pharm. 148:732–740. doi: 10.1038/sj.bjp.0706777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferriere A, Pitcher MH, Haldane A, Huang Y, Cornea V, Kumar N, Sacktor TC, Cervero F, Coderre TJ. PKMzeta is essential for spinal plasticity underlying the maintenance of persistent pain. Mol Pain. 2011;7:99. doi: 10.1186/1744-8069-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber J, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted Mutation of the Gene Encoding the Low Affinity NGF Receptor p75 Leads to Deficits in the Peripheral Sensory Nervous System. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- Liu B, Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, Hazen-Martin DJ, Obeid LM, Hannun YA, Smith GK. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of Nerve Growth Factor-TrkA Signaling and the Relief of Pain. Anesthesiology. 2011;115:189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F, D'Mello R, Yip PK, Calvo M, Muller E, Pezet S, Dickenson AH, McMahon SB. Specific involvement of atypical PKCzeta/PKMzeta in spinal persistent nociceptive processing following peripheral inflammation in rat. Mol Pain. 2011;7:86. doi: 10.1186/1744-8069-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol Chem. 2003;278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- Masoudi R, Ioannou MS, Coughlin MD, Pagadala P, Neet KE, Clewes O, Allen SJ, Dawbarn D, Fahnestock M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J Biol Chem. 284(27):18424–18433. doi: 10.1074/jbc.M109.007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1:774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- Mills CD, Nguyen T, Tanga FY, Zhong C, Gauvin DM, Mikusa J, Gomez EJ, Salyers AK, Bannon AW. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain. 2013;17:469–479. doi: 10.1002/j.1532-2149.2012.00202.x. [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1:399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Lipid activation of protein kinases. J Lipid Res. 2009;50(Suppl):S266–S271. doi: 10.1194/jlr.R800064-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv. 2007;7:26–41. doi: 10.1124/mi.7.1.6. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Peterson CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Pagadala PC, Dvorak LA, Neet KE. Construction of a mutated pro-nerve growth factor resistant to degradation and suitable for biophysical and cellular utilization. Proc Natl Acad Sci U S A. 2006;103:17939–17943. doi: 10.1073/pnas.0604139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Price TJ, Ghosh S. ZIPping to pain relief: the role (or not) of PKMζ in chronic pain. Mol Pain. 2013;9:6. doi: 10.1186/1744-8069-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol. 2006;197:430–436. doi: 10.1016/j.expneurol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinse C-zeta is a downstream effector of phosphatidyl-3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol. 2010;70:350–359. doi: 10.1002/dneu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam K, Loing E, Zoukhri D, Rommens C, Hodges R, Dartt D, Verwaerde C, Auriault C, Gras-Masse H, Sergheraert C. Direct evidence of cytoplasmic delivery of PKC-alpha, -epsilon and -zeta pseudosubstrate lipopeptides: study of their implication in the induction of apoptosis. FEBS Lett. 1999;459:285–290. doi: 10.1016/s0014-5793(99)01240-5. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Ito T, Inoue G, Ohtori S, Kitajo K, Doya H, Takahashi K, Yamashita T. The p75 Receptor Is Associated With Inflammatory Thermal Hypersensitivity. J NeuroSci Research. 2008;86:3566–3574. doi: 10.1002/jnr.21808. [DOI] [PubMed] [Google Scholar]
- Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001;89:181–186. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Reichardt L. Evidence That Biological Activity of NGF Is Mediated through a Novel Subclass of High Affinity Receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441–448. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral Cell Types Contributing to the Hyperalgesic Action of Nerve Growth Factor in Inflammation. J Neurosci. 1996;16:2716–2723. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor α. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Chi XX, Nicol GD. Brain-derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. J Physiol. 2008;586:3113–3127. doi: 10.1113/jphysiol.2008.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Kays J, Hodgdon KE, Sacktor TC, Nicol GD. Nerve growth factor enhances the excitability of rat sensory neurons through activation of the atypical protein kinase C isoform, PKMζ. J Neurophysiol. 2012;107:315–335. doi: 10.1152/jn.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Nicol GD. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci Lett. 2004;366:187–192. doi: 10.1016/j.neulet.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol. 2006;575:101–113. doi: 10.1113/jphysiol.2006.111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34:689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]