Abstract
Background
Numerous studies have shown that high C-reactive protein (CRP) levels predict cardiovascular disease and augur a poor prognosis in patients with acute coronary syndromes. Much in vitro and in vivo data support of a role for CRP in atherogenesis. There is an urgent need to develop inhibitors that specifically block the biological effects of CRP in vivo. The one-bead–one-compound (OBOC) combinatorial library method has been used to discover ligands against several biological targets. In this study, we use a novel fluorescence-based screening method to screen an OBOC combinatorial library for the discovery of peptides against human CRP.
Methods
Human CRP was labeled with fluorescein isothiocyanate (FITC) and human serum albumin (HuSA) was labeled with phycoerythrin (PE) and used for screening. The OBOC library LWH-01 was synthesized on TentaGel resin beads using a standard solid-phase “split/mix” approach.
Results
By subtraction screening, eight peptides that bind specifically to CRP and not to HuSA were identified. In human aortic endothelial cells (HAECs) incubated with CRP, inhibitors CRPi-2, CRPi-3, and CRPi-6 significantly inhibited CRP-induced superoxide, cytokine release, and nuclear factor-κB (NFκB) activity. Molecular docking studies demonstrate that CRPi-2 interacts with the two Ca2+ ions in the single subunit of CRP. The binding of CRPi-2 is reminiscent of choline binding.
Conclusions
Future studies will examine the utility of this inhibitor in animal models and clinical trials.
Introduction
Inflammation is pivotal in all phases of atherosclerosis from the fatty streak lesion to acute coronary syndromes.1 An important downstream marker of inflammation is C-reactive protein (CRP).2 Numerous studies have shown that high CRP levels predict cardiovascular disease in apparently healthy individuals, and high levels of CRP augur a poor prognosis in patients with acute coronary syndromes. More interestingly, much in vitro and in vivo data have now emerged in support of a role for CRP in atherogenesis.3,4 To date, studies largely in endothelial cells, monocyte-macrophages, and vascular smooth muscle cells support a role for CRP in atherogenesis.3,4 CRP is a member of the pentraxin family of proteins, which are nonspecific, acute-phase reactant proteins composed of five identical 23-kD polypeptide subunits arranged in a cyclic pentameter shape.5,6 Each of these subunits contains one binding site for a phosphocholine molecule and two binding sites for calcium. These binding sites allow CRP to recognize and bind to a variety of biological substrates, including phosphocholine and phospholipid components of damaged cell walls and chromatin and nuclear antigens, resulting in the formation of CRP–ligand complexes. There is an urgent need to develop inhibitors that specifically block the biological effects of CRP in vivo. Pepys et al. reported the development of one such inhibitor, bisphosphocholine.7
In 1991, the Lam laboratory first introduced the one-bead–one-compound (OBOC) combinatorial library method and used it to identify short linear peptides that bind to anti-β-endorphin antibody and streptavidin.8 Since then, we and others have applied this powerful method to discover ligands against a number of different biological targets, such as protein kinase substrates and inhibitors,9–11 protease substrates and inhibitors,12–14 ligands of regulators of G-protein signaling (RGS),15,16 G-quadruplex stabilizing ligands,17 foldamer ligands for the BH3-recognition cleft of the antiapoptotic protein Bcl-xL, and ligands for cell-surface receptors.18–23 Most of the published work in OBOC combinatorial libraries involves on-bead screening, in which the library compounds remained tethered to individual beads during screening. Here, we report the use of a novel fluorescence-based screening method to screen OBOC combinatorial peptide libraries for the discovery of novel inhibitors against human CRP.
Materials and Methods
CRP was purified from human ascitic/pleural fluids as described previously.24,25 Fatty acid-free and endotoxin-free human serum albumin (HuSA) was used as a negative control. CRP was labeled with fluorescein isothiocyanate (FITC) and HuSA was labeled with phycoerythrin (PE) using labeling reagents from Pierce Biochemicals; these were used for screening for peptide inhibitors.
Synthesis of the OBOC libraries
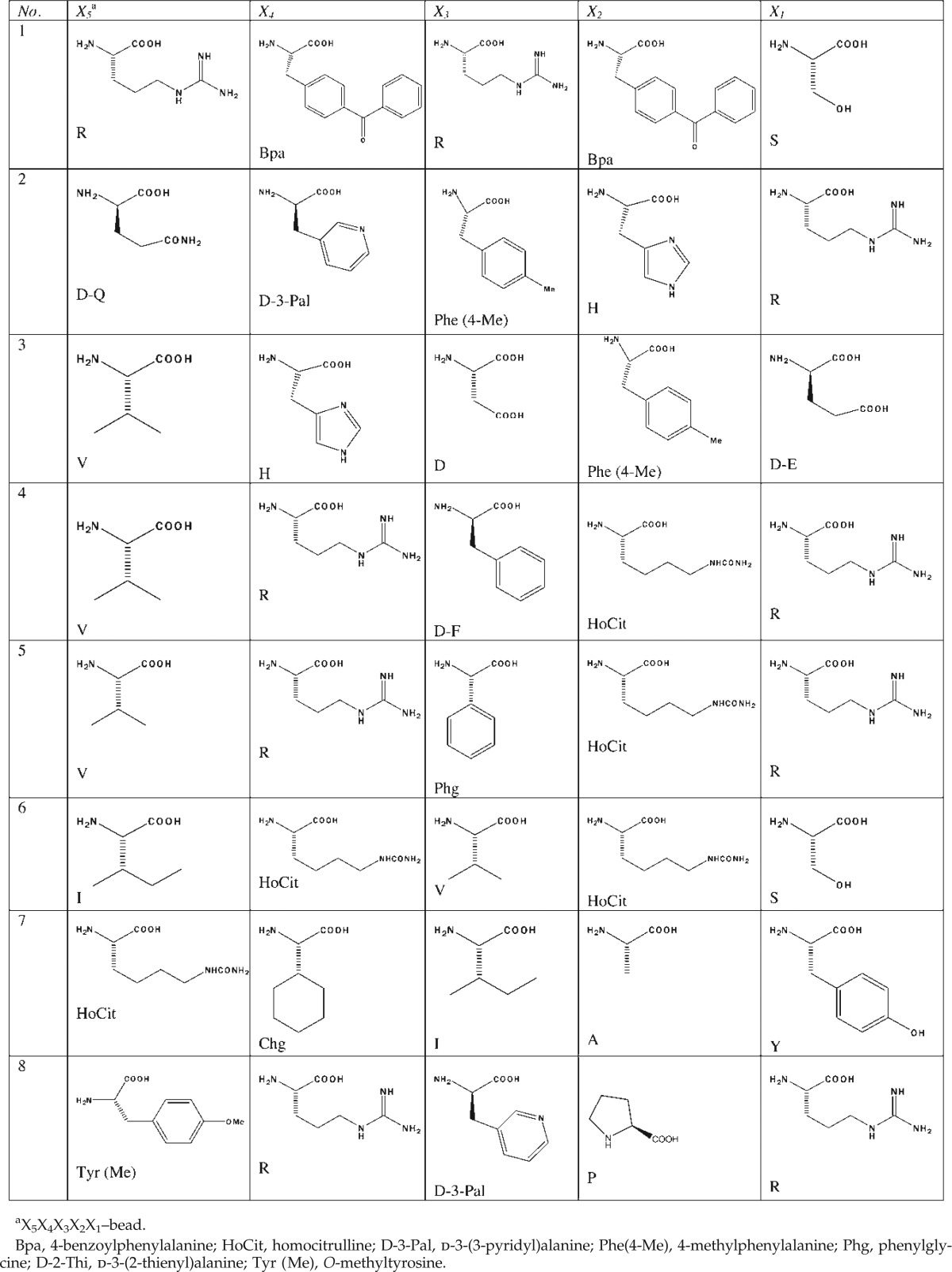
The OBOC library LWH-01 was synthesized on TentaGel resin beads using a standard solid phase “split/mix” approach26–28 (Supplementary Table S1; Supplementary Data are available at www.liebertpub.com/met/.). In brief, TentaGel beads (2.0 grams, at 0.26 mmol/gram) were swollen in N,N′-dimethylformamide (DMF) for 3 hr. The beads were split into 30 equal portions. Thirty different fluorenylmethyloxycarbonyl (Fmoc)-amino acids (Table 1, 3 equiv. to beads) were dissolved separately in a solution of 1-hydroxybenzotriazole (HOBt) (3 equiv.) and diisopropylcarbodiimide (DIC) (3 equiv.) in DMF. They were added to 30 columns and coupled to the beads at position X1, each column receiving one amino acid. The coupling was carried out at room temperature for 2–4 hr. A Kaiser test was used to verify the completion of coupling. After filtration, the beads were combined and washed with DMF (3×20 mL), methanol (MeOH) (3×20 mL), and DMF (3×20 mL), respectively. The Fmoc protecting group was removed with 20% 4-methylpiperidine twice (5 and 15 min, respectively). The beads were washed with DMF (3×20 mL), MeOH (3×20 mL), and DMF (3×20 mL). They were then split into 30 columns again and subjected another cycle of coupling of 30 Fmoc-amino acids at the X2 position. After removal of Fmoc, the beads were then subjected to three additional cycles of coupling for positions X3–X5 in the same manner as described above. Note that only 29 amino acids (without Aic) were used for X3, and 28 amino acids [without Aic and Phe(4-Me)] for X4 and X5, respectively. After removal of Fmoc, the combined beads were washed with DMF (3×20 mL), MeOH (3×20 mL), and dichloromethane (DCM) (3×20 mL). The beads were then dried under vacuum for 1 hr. Side-chain deprotection was achieved using a mixture of 82.5% trifluoroacetic acid (TFA):5% phenol:5% thioanisole:5% water:2.5% triisopropylsilane (TIS) (vol/vol). After neutralization with 10% N,N-diisopropylethylamine (DIEA)/DMF (twice), the beads were washed sequentially with DMF (3×20 mL), MeOH(3×20 mL), DCM (3×20 mL), DMF(3×20 mL), 50% DMF/water(3×20 mL), water(3×20 mL), and phosphate-buffered saline (PBS) (10×20 mL). The bead library was stored in PBS buffer with 0.05% sodium azide until screening. The permutations of LWH-01 are 30×30×29×28×28=2.04624×107. The same procedure was used to synthesize hexameric (B6) and heptameric (B7) libraries where “B” represents 19 natural amino acids except cysteine.
Table 1.
Sequences of Peptides Identified from Library LWH-01
 |
Screening and sequencing for high-potency CRP inhibitors
A linear pentameric library, LWH-01, was synthesized on beads using the split-mix method and was used for screening. A high-stringency, competitive binding assay was performed to identify the CRP inhibitors with modifications, as described by us previously.29 Use of a fluorescence-labeled protein in OBOC screening is greatly limited by the intrinsic fluorescence derived from commonly used solid supports, residual coupling reagents, and library compounds. We have modified the OBOC screening method, which can be used to screen pure protein tagged with fluorescein. We have used FITC-conjugated CRP to screen the libraries to find CRP inhibitors. This method involves a simple fluorescence-based two-stage screening process and enables one to select the desired compound-beads.
Screening was done with a Olympus fluorescence microscope using the filter FITC/TIRC (model no U-M51004A/R-V2). Using this filter allowed us to visualize compounds with excitation spectra at 488 nm and emission spectra either 520 nm or 620 nm. Both PE and FITC can be visualized in the same field. Approximately 1 million beads from each library were first incubated with PE-conjugated HuSA for 3 hr. The beads binding with PE-HuSA fluoresce with red color. This may be specific binding with PE-HuSA or a nonspecific protein–protein interaction. This red background makes screening easier to identify false-positive beads. The autofluorescent beads and FITC-binding beads were removed from the library by manual sorting. The libraries that showed more false positives (>5% of beads) were not included for screening. After removal of false-positive beads, FITC-conjugated CRP was added and incubated for 1 hr at 37°C in a shaker incubator. The beads with outer green fluorescence were due to the CRP-FITC and were isolated. The positive beads were physically picked up and cleaned by incubating with 8 M guanidine to remove proteins. The positive beads were sequenced by Edman chemical degradation.30 By this subtraction screening method, peptides/peptidomimetic compounds that bind specifically to CRP and not to other carrier proteins such as HuSA were identified. The peptides were resynthesized on beads again, and the above experiments were repeated for reconfirmation.
In vitro effects of CRP inhibitors
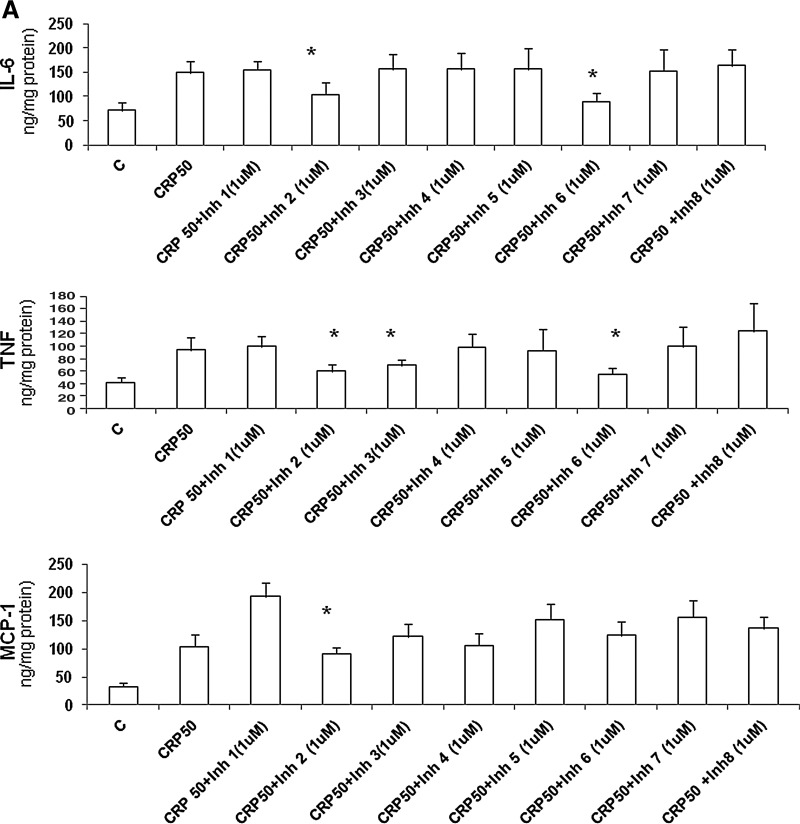
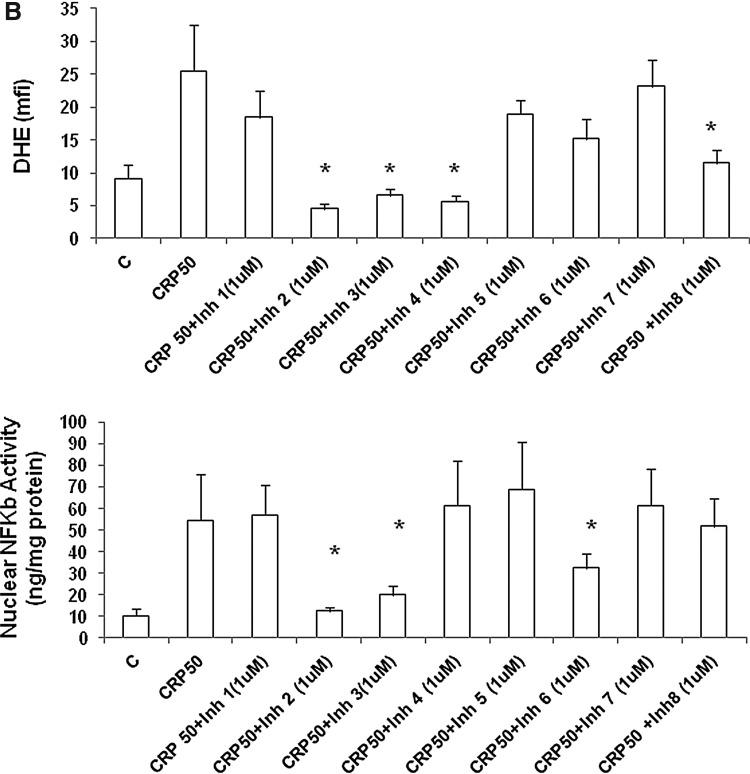
We then tested the effect of the eight peptides in soluble form in vitro. Human aortic endothelial cells (HAECs) (passages 4–8) were incubated with different doses of inhibitors 1–8 for 4 hr before addition of CRP (50 μg/mL) (only data with 1 μM inhibitor are shown). CRP-induced superoxide was assessed by dihydroethidium (DHE) fluorescence, cytokine release [interleukin-6 (IL-6), tumor necrosis factor (TNF), monocyte chemotactic protein (MCP)] were assessed by multiplex bead arrays (Millipore) as described previously, and nuclear factor-κB (NFκB) activity in nuclear extracts was examined using TransAM activity assays following the manufacturer's (Active Motif ) instructions.31–34
The following parameters were assessed. Superoxide anion release was assessed by DHE fluorescence as described previously.31 The second parameter was multiplex protein analyses of cytokines/chemokines. To assess if the inhibitor compounds effectively blocked CRP's proinflammatory effects, cytokines/chemokines were assessed by Bioplex rat cytokine multiplex assay, as described previously.32,33 NFκB activity was examined in nuclear extracts of macrophages, as described previously.34
Molecular docking studies
Molecular docking simulations of the peptides to CRP were performed.35–39 The crystal structure of CRP bound with phosphocholine (PDB ID 1B09)35 was used for docking simulations. The cholines were removed from the protein, and a single protein subunit was used for docking because the binding site for these peptides may not be the same as phosphocholine. AMBER charges were used for the protein and AM1-bond charge correction (BCC) charges36 were assigned to the peptides. The program Antechamber was used to assign the AM1-BCC charges. The docking simulations were performed using Autodock (4.2.2.2).37 A 127×127×127 grid was used with a spacing of 0.375 Å. The grid was centered in the middle of the choline binding site, but it was large enough to encompass the entire protein subunit. A Lamarckian algorithm was used to generate conformations for the peptide within the grid box. The parameters used for the Lamarckian genetic algorithm were the same as in Legge et al.,38 except the maximum number of energy evaluations was 250,000. A total of 256 conformers were generated for each peptide to the protein. The conformers were clustered using a 2.0 Å root mean square deviation (RMSD).39
Results
Identification of peptide inhibitors for CRP
A OBOC combinatorial library method was used to identify small peptide inhibitors against CRP. We have used pentameric, hexameric, and heptameric random linear OBOC libraries for screening, of which the LWH01-pentameric library gave positive hits for CRP binding. In the initial screening of this library (∼1 million beads), only eight positive beads were identified and decoded.30 The sequences of the eight peptides are shown in Table 1. Figure 1 depicts the beads that only bind to CRP and not to HuSA. Using this subtraction screening method, we have identified peptides that bind specifically to CRP and not to other carrier proteins such as HuSA. The eight peptides were resynthesized on TentaGel resin beads to confirm their binding to CRP. These peptides were also synthesized in soluble form by Biotek Synthesis (Ontario, Canada) and used for biological testing. The purity of the soluble peptides were found to be greater than 90% using high-performance liquid chromatography (HPLC), and their identities were confirmed with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS).
FIG. 1.
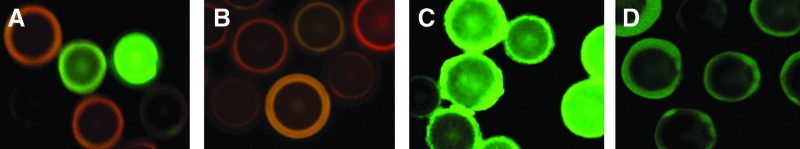
Fluorescence-based screening of one-bead–one-compound (OBOC) library. A linear pentameric library was synthesized on beads using a split-mix method for screening as described in Materials and Methods. Only a small portion of the entire image is presented in A. The positive human serum albumin (HuSA)-phycoerythrin (PE) binding beads are red and C-reactive protein–fluorescein isothiocyanate (CRP-FITC)-binding beads are green. Both HuSA- and CRP-binding beads are orange (B). Some beads have no color, meaning there was no binding to CRP and/or HSA. The green positive beads were resynthesized on bead for reconfirmation. The CRP strong binding CRPi-2 (C) and weakly binding CRPi-6 (D) are shown here. Autofluorescent beads were removed from the library.
In vitro effects of CRP inhibitors
Figure 2 shows the effect of the eight lead peptide inhibitors developed. We incubated HAECs with different doses of inhibitors 1–8 for 4 hr before addition of CRP (50 μg/mL) (only data with 1 μM inhibitor are shown). As seen in Fig. 2A, inhibitors CRPi-2 and CRPi-6 significantly inhibited CRP-induced IL-6 release, CRPi-2, i-3, and i-6 significantly decreased CRP-induced TNF release, and CRPi2 significantly decreased CRP-induced MCP-1 release. As shown in Fig. 2B, CRPi-2, -3, -4, and -8 significantly inhibited CRP-induced superoxide anion release, as assessed by DHE fluorescence, whereas only inhibitors CRP-i2, -i3, and -i6 significantly inhibited CRP-induced nuclear NFκB activity, the master switch of inflammation.
FIG. 2.
Effect of the C-reactive protein (CRP) peptide inhibitors on CRP-induced inflammation (in vitro). Human aortic endothelial cells (HAECs) were incubated with 1 μM doses of CRP inhibitors 1–8 separately for 4 hr before the addition of CRP (50 μg/mL). CRP-induced superoxide was assessed by cytokine release [interleukin-6 (IL-6), tumor necrosis factor (TNF), monocyte chemotactic protein (MCP)] in A and dihydroethidium (DHE) fluorescence and nuclear factor-κB (NFκB) activity in B; (*) P<0.05 compared to CRP (50 μg/mL). mfi, mean fluorescence intensity.
Molecular docking studies
We used an advanced computerized docking model to dock the identified ligands with calcium and the phosphatidyl choline binding site of the CRP. The phosphocholine-binding site of CRP lies within a hydrophobic cavity on the surface of the protein. The two oxygens of the phosphate group of phosphocholine are coordinated to a pair of calcium ions, and the trimethyl ammonium group interacts with the carboxylate side chain of Glu81.
Because the most consistent results were obtained for CRPi-2, molecular docking studies of CRPi-2 were performed to determine possible binding conformations for these peptides. Although these peptides are much larger than phosphocholine, the most important interactions are identical. In the lowest-energy binders to CRP, a carboxylate from the peptide binds to the two calcium ions and either an amine or guanidium group interacts with the carboxylate of Glu81. The carboxylate and amine/guanidium group have to be in close proximity with each other to be able to interact with the two charged moieties on the protein. As shown in Fig. 3, for CRPi-2, we demonstrate that it interacts with the two Ca2+ ions (yellow spheres) in the single subunit of the CRP. The carboxylate terminus is coordinated with each metal ion. The Arg side chain of CRPi-2 (shown in cyan) is able to interact with the carboxylate side chain of Glu81. The binding of CRPi-2 is reminiscent of choline binding (shown as a ball-and-stick model) and helps to explain the prevalence of Arg at the X1 position of the CRP-binding ligands found in this peptide library. An additional interaction of the amide side chain of Asn and the amino-terminal amine coordinate to the carboxylate side chain of Glu147.
FIG. 3.
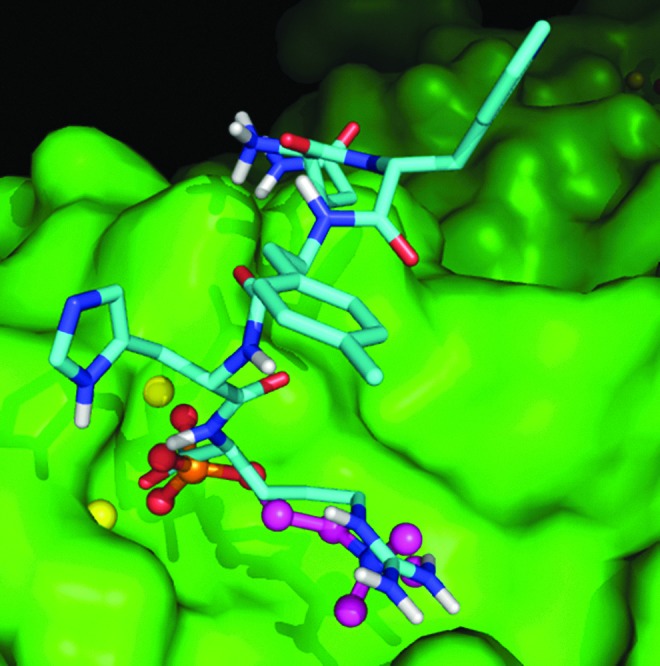
Molecular docking studies of CRPi-2 with C-reactive protein (CRP). Molecular docking studies were performed in the Lawrence Livermore National Laboratory as described in Materials and Methods. CRPi-2 interacts with the two Ca2+ ions (yellow spheres) in the single subunit of CRP. The carboxylate-terminus is coordinated to each metal ion. The Arg side chain of CRPi-2 (shown in cyan) is able to interact with the carboxylate side chain of Glu81. The binding of CRPi-2 is reminiscent of choline binding (shown as a ball-and-stick model). An additional interaction of the amide side chain of Asn and the amino-terminal amine coordinate to the carboxylate side chain of Glu147.
Discussion
High levels of CRP, the prototypic marker of inflammation in humans, predict future cardiovascular events (CVE).1 Furthermore, high CRP levels augur a worse prognosis in patients with acute coronary syndromes.2 In addition to being a cardiovascular risk marker, there is a huge body of evidence that CRP may promote atherothrombosis.3,4 Also, infusion of human CRP into human volunteers results in leukocyte activation and endothelial dysfunction.40,41 Recent clinical studies support the hypothesis that CRP contributes to atherothrombosis and suggest that lowering CRP is beneficial.42,43 To prove convincingly that CRP contributes to atherothrombosis, the perfect strategy would be the use of a drug that selectively targets CRP without an effect on low-density lipoprotein cholesterol (LDL-C). However, no such CRP inhibitor is commercially available at this time. Therefore, targeting human CRP is a valid therapeutic strategy to decrease inflammation and cardiovascular risk. The Pepys group recently reported the rational design, novel synthesis, mode of action, and in vivo efficacy of the first specific CRP inhibitor drug bis(phosphocholine)-hexane, which inhibits ligand binding and complement activation by CRP in vitro and in vivo.7 In a small study, they demonstrated that administration of this compound to rats completely abrogated the increased morbidity, mortality, and infarct size experienced after coronary ligation by rats receiving human CRP alone. This inhibitor is developed by joining phosphocholine, one of its natural substrates. However, no synthetic inhibitor to CRP is as yet available commercially.
In this manuscript, we report on the identification of a new peptide CRP inhibitor (CRPi-2) by screening a random pentameric OBOC combinatorial library. The sequence of this inhibitor is shown in Table 1. It has one d-amino acid, (d-G,d-glutamic acid) and two unnatural amino acids (d-3-Pal, d-3-(3-pyridyl)alanine; Phe(4-Me), 4-methylphenylalanine). It has been shown that peptides made of d-amino acids and unnatural amino acids are mostly resistant to protease digestion and less immunogenic, thus they have more plasma stability than natural peptides.10 This CRPi-2 molecule also significantly reduces proinflammatory cytokines such as IL-1β, MCP-1, TNF, and, IL-8 as well as superoxide anion release in macrophages, which have previously been shown to be induced by high levels of CRP. Also, it inhibits CRP-induced NFκB activity.
CRP contributes to tissue damage in a range of diseases in which CRP levels are greatly increased, thus the inhibition of CRP may have a broad application in medicine. CRP is normally present at trace levels in the blood, but its concentration increases significantly in almost all disease states, including trauma, infection, strokes, and chronic illnesses, such as rheumatoid arthritis and Crohn disease. Thus, a new CRP-targeting inhibitor may also be useful in providing more information about the physiologic and pathologic roles of CRP in humans. For CRPi-2, our molecular docking studies show that the arginine at the carboxyl terminus acts like phosphocholine. The guanidium side chain spans the binding site when interacting with Glu81, and the carboxylate terminus is coordinated to the calciums. Thus, it is possible that by interacting with the phosphocholine and calcium binding sites, this inhibitor exerts its attenuation of CRP's proatherogenic effects. Furthermore, the two amino-terminal residues are comprised of d-amino acids, making it resistant to proteolysis.
Future studies will examine pharmacokinetics of the peptide inhibitor, elucidate its binding affinity to CRP, and examine if long-term administration using osmotic pumps in the rat model will be effective in reducing CRP-induced impaired vasoreactivity and coronary artery ligation–induced myocardial infarction and if it can be used safely in prospective clinical trials.
Supplementary Material
Acknowledgments
This study was supported by grant NIH RO1 HL07436 and had technical support from Jung Mi Yun, PhD.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Libby P. Ridker PM. Inflammation and atherosclerosis: Role of C-reactive protein in risk assessment. Am J Med. 2004;116:9–16. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Bassuk SS. Rifai N. Ridker PM. High-sensitivity C-reactive protein: Clinical importance. Curr Probl Cardiol. 2004;29:439–493. [PubMed] [Google Scholar]
- 3.Verma S. Devaraj S. Jialal I. C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–2150. [PubMed] [Google Scholar]
- 4.Devaraj S. Singh U. Jialal I. The evolving role of C-reactive protein in atherothrombosis. Clin Chem. 2009;55:229–238. doi: 10.1373/clinchem.2008.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson D. Pepys MB. Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Struct Folding Design. 1999;7:69–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 6.Hirschfield GM. Pepys MB. C-reactive protein and cardiovascular disease: New insights from an old molecule. Qjm-an Int J Med. 2003;96:793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- 7.Pepys MB. Hirschfield GM. Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 8.Lam KS. Salmon SE. Hersh EM, et al. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 9.Al-Obeidi FA. Lam KS. Development of inhibitors for protein tyrosine kinases. Oncogene. 2000;19:5690–5701. doi: 10.1038/sj.onc.1203926. [DOI] [PubMed] [Google Scholar]
- 10.Lam KS. Liu R. Miyamoto S, et al. Applications of one-bead one-compound combinatorial libraries and chemical microarrays in signal transduction research. Accounts Chem Res. 2003;36:370–377. doi: 10.1021/ar0201299. [DOI] [PubMed] [Google Scholar]
- 11.Kim M. Park YS. Shin DS, et al. Antibody-free peptide substrate screening of serine/threonine kinase (protein kinase A) with a biotinylated detection probe. Anal Biochem. 2011;413:30–35. doi: 10.1016/j.ab.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Meldal M. Svendsen I. Direct visualization of enzyme-inhibitors using a portion mixing inhibitor library containing a quenched fluorogenic peptide substrate. 1. Inhibitors for subtilisin Carlsberg. J Chem Soc Perkin Transact. 1995;1:1591–1596. [Google Scholar]
- 13.McBride JD. Freeman N. Domingo GJ, et al. Selection of chymotrypsin inhibitors from a conformationally-constrained combinatorial peptide library. J Mol Biol. 1996;259:819–827. doi: 10.1006/jmbi.1996.0360. [DOI] [PubMed] [Google Scholar]
- 14.Kumaresan PR. Natarajan A. Song AM, et al. Development of tissue plasminogen activator specific "on demand cleavable" (ODC) linkers for radioimmunotherapy by screening one-bead-one-compound combinatorial peptide libraries. Bioconj Chem. 2007;18:175–182. doi: 10.1021/bc0602681. [DOI] [PubMed] [Google Scholar]
- 15.Roof RA. Sobczyk-Kojiro K. Turbiak AJ, et al. Novel peptide ligands of RGS4 from a focused one-bead, one-compound library. Chem Biol Drug Design. 2008;72:111–119. doi: 10.1111/j.1747-0285.2008.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruber CW. Muttenthaler M. Freissmuth M. Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors. Curr Pharmaceut Design. 2010;16:3071–3088. doi: 10.2174/138161210793292474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redman JE. Ladame S. Reszka AP, et al. Discovery of G-quadruplex stabilizing ligands through direct ELISA of a one-bead-one-compound library. Org Biomol Chem. 2006;4:4364–4369. doi: 10.1039/b611716c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray JK. Sadowsky JD. Scalf M, et al. Exploration of structure—activity relationships among foldamer ligands for a specific protein binding site via parallel and split-and-mix library synthesis. J Comb Chem. 2008;10:204–215. doi: 10.1021/cc700153z. [DOI] [PubMed] [Google Scholar]
- 19.Peng L. Liu R. Marik J, et al. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha(4)beta(1) integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2:381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- 20.Aina OH. Liu R. Sutcliffe JL, et al. From combinatorial chemistry to cancer-targeting peptides. Mol Pharmaceut. 2007;4:631–651. doi: 10.1021/mp700073y. [DOI] [PubMed] [Google Scholar]
- 21.Kumaresan PR. Ahir V. Liu R, et al. Identification of cancer specific high affinity targeting ligands by screening One-bead-one-compound and small molecule. Mol Cancer Therapeut. 2007;6:3408s–3408s. [Google Scholar]
- 22.Xiao WW. Wang Y. Lau EY, et al. The use of one-bead one-compound combinatorial library technology to discover high-affinity alpha v beta 3 integrin and cancer targeting arginine-glycine-aspartic acid ligands with a built-in handle. Mol Cancer Therapeut. 2010;9:2714–2723. doi: 10.1158/1535-7163.MCT-10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumaresan PR. Wang Y. Saunders M, et al. Rapid discovery of death ligands with one-bead-two-compound combinatorial library methods. ACS Comb Sci. 2011;13:259–264. doi: 10.1021/co100069t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duclos TW. Zlock LT. Marnell L. Definition of a C-reactive protein-binding determinant on histones. J Biol Chem. 1991;266:2167–2171. [PubMed] [Google Scholar]
- 25.Dasu MR. Devaraj S. Du Clos TW, et al. The biological effects of CRP are not attributable to endotoxin contamination: Evidence from TLR4 knockdown human aortic endothelial cells. J Lipid Res. 2007;48:509–512. doi: 10.1194/jlr.C600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Lebl M. Krchnak V. Sepetov NF, et al. Synthetic combinatorial libraries—a new tool for drug design—methods for identifying the composition of compounds from peptide and/or nonpeptide libraries. J Prot Chem. 1994;13:484–486. [Google Scholar]
- 27.Lam KS. Lebl M. Krchnak V. The ''one-bead-one-compound'' combinatorial library method. Chem Rev. 1997;97:411–448. doi: 10.1021/cr9600114. [DOI] [PubMed] [Google Scholar]
- 28.Lam KS. Lehman AL. Song AM, et al. Synthesis and screening of “one-bead one-compound” combinatorial peptide libraries. Comb Chem Pt B. 2003;369:298–322. doi: 10.1016/S0076-6879(03)69017-8. [DOI] [PubMed] [Google Scholar]
- 29.Kumaresan PR. Lam KS. Screening chemical microarrays: methods and applications. Mol Biosyst. 2006;2:259–270. doi: 10.1039/b602004f. [DOI] [PubMed] [Google Scholar]
- 30.Liu R. Lam KS. Automatic Edman microsequencing of peptides containing multiple unnatural amino acids. Anal Biochem. 2001;295:9–16. doi: 10.1006/abio.2001.5172. [DOI] [PubMed] [Google Scholar]
- 31.Devaraj S. Dasu MR. Singh U, et al. C-reactive protein stimulates superoxide anion release and tissue factor activity in vivo. Atherosclerosis. 2009;203:67–74. doi: 10.1016/j.atherosclerosis.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh U. Devaraj S. Dasu MR, et al. C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Arterioscler Thromb Vasc Biol. 2006;26:2469–2475. doi: 10.1161/01.ATV.0000241572.05292.fb. [DOI] [PubMed] [Google Scholar]
- 33.Singh U. Dasu MR. Yancey PG, et al. Human C-reactive protein promotes oxidized low density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats. J of Lipid Res. 2008;49:1015–1023. doi: 10.1194/jlr.M700535-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devaraj S. Kumaresan PR. Jialal I. Effect of C-reactive protein on chemokine expression in human aortic endothelial cells. J Mol Cell Cardiol. 2004;36:405–410. doi: 10.1016/j.yjmcc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Cornell WD. Cieplak P. Bayly CI, et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc. 1996;118:2309–2309. [Google Scholar]
- 36.Jakalian A. Bush BL. Jack DB, et al. Fast, efficient generation of high-quality atomic Charges. AM1-BCC model: I. Method. J Comput Chem. 2000;21:132–146. doi: 10.1002/jcc.10128. [DOI] [PubMed] [Google Scholar]
- 37.Morris GM. Huey R. Lindstrom W, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legge GB. Morris GM. Sanner MF, et al. Model of the alpha L beta 2 integrin I-domain/ICAM-1 DI interface suggests that subtle changes in loop orientation determine ligand specificity. Proteins Struct Funct Genet. 2002;48:151–160. doi: 10.1002/prot.10134. [DOI] [PubMed] [Google Scholar]
- 39.Wang JM. Wang W. Kollman PA, et al. Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model. 2006;25:247–260. doi: 10.1016/j.jmgm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Bisoendial R. Birjmohun R. Keller T, et al. Letter to the editor. Circ Res. 2005;97:E115–E116. doi: 10.1161/01.RES.0000196746.75724.8b. [DOI] [PubMed] [Google Scholar]
- 41.Bisoendial RJ. Kastelein JJP. Peters SLM, et al. Effects of CRP infusion on endothelial function and coagulation in normocholesterolemic and hypercholesterolemic subjects. J Lipid Res. 2007;48:952–960. doi: 10.1194/jlr.P600014-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM. Cannon CP. Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM. Danielson E. Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.