Abstract
Background
Chronic anticoagulation has been demonstrated to be a risk factor for GI bleeding (GIB) in patients undergoing endoscopic procedures.
Objective
The aim of this study was to determine the incidence of GIB prospectively in a large cohort of patients enrolled in the Clinical Outcomes Research Initiative (CORI) database.
Design
Anticoagulated patients undergoing endoscopic procedures were interviewed by phone 30 to 45 days after the procedure to determine potential adverse events and management of warfarin therapy in the perien-doscopic period.
Setting
Participating CORI sites, Stanford University Hospital, Veterans Administration Palo Alto Health Care System.
Main Outcome Measurement
Postprocedural hemorrhagic or thrombotic events.
Results
Thirteen CORI sites agreed to participate, including 120,886 procedures in 95,807 patients. We contacted 929 patients on warfarin therapy and enrolled 483 patients (52%). The majority of the patients were men with atrial fibrillation undergoing colonoscopy. Warfarin was temporarily suspended in 437 (90%) of the patients before the procedure, and 114 (22%) received periprocedural heparin therapy. There were 10 major hemorrhagic events (2%), and the rate of hemorrhage was not higher in the patients receiving periprocedural heparin therapy (P .1). However, polypectomy was a risk factor for postprocedural hemorrhage (P .02). One fatal stroke (0.2%) occurred in a patient 2 weeks after endoscopy; however, information regarding warfarin management was not available.
Limitations
Small number of enrolled patients and lack of control group. Lack of information regarding prothrom-bin time before procedure, concurrent antiplatelet agents, and timing of bleeding in 50% of the cases. The study was underpowered to definitively conclude benefits of current guidelines regarding thrombosis or bleeding.
Conclusions
Postprocedural hemorrhagic events were not increased in anticoagulated patients. Most patients receiving bridging therapy were managed according to current society guidelines.
GI hemorrhage is a common and clinically significant health care problem in the United States, associated with hospitalization rates of approximately 100 per 100,000 hospital admissions per year for upper GI hemorrhage,1 and 20 per 100,000 for lower GI bleeding.2
Risk factors for GI hemorrhage have included advanced age, male gender, cardiovascular disease, use of multiple medications, and oral anticoagulants.3 Patients requiring warfarin therapy have an increased risk of hemorrhage, especially if their international normalized ratio (INR) is above the therapeutic range.4 For example, in the U.S. Cardiovascular Health Study that included 5888 patients aged 65 years, the use of oral anticoagulants was a strong risk factor for hospitalized upper or lower GI bleeding (GIB) with an age- and gender-adjusted hazard ratio of 2.59 (95% CI, 1.71–3.93; P .001).3
Recently, guidelines for the periendoscopic management of patients on anticoagulant therapy have been updated by the British Society of Gastroenterology and the American Society for Gastrointestinal Endoscopy (ASGE)5,6 ( Table 1). Guidelines from both societies advocate continuing therapeutic warfarin in patients who undergo low-hemorrhage-risk procedures, such as upper or lower endoscopy with or without biopsy, ERCP without sphincterotomy, enteroscopy and diagnostic balloon-assisted enteroscopy, enteral stent deployment without dilation, capsule endoscopy, and endosonography without FNA. In contrast, the guidelines recommend holding oral anticoagulants for 3 to 5 days in patients who undergo high-hemorrhage-risk procedures, such as polypectomy, biliary sphincterotomy, PEG placement, EUS with FNA, endoscopic hemostasis, and tumor ablation. There are recommendations to substitute heparin for warfarin before high-hemorrhage-risk procedures in patients with highly thrombotic conditions, such as patients with mechanical valves and/or history of cerebrovascular accident (CVA), transient ischemic attack, or systemic embolism. For these patients, warfarin is restarted in the evening after endoscopy, and unfractionated or low-molecular-weight heparin can be administered before and after the endoscopic procedure.
Table 1.
American Society for Gastrointestinal Endoscopy and British Society of Gastroenterology guidelines for management of patients on warfarin.
| Condition-specific thromboembolic risk | ||
|---|---|---|
| High | Low | |
| Procedure-related hemorrhage | No change in anticoagulation, but delay elective procedures if INR is supratherapeutic. | |
| Stop warfarin 3–5 days before procedure. Consider bridging therapy* before and after procedure until INR is therapeutic. | Stop warfarin 3–5 days before and restart after procedure. | |
INR, International normalized ratio (prothrombin time).
Bridging therapy with either low-molecular-weight heparin or unfractionated heparin.
Large studies of anticoagulated patients undergoing endoscopic procedures have not been conducted to validate the current ASGE guidelines for anticoagulant management in the periendoscopic period. The purpose of the present prospective study was to collect information regarding postendoscopic outcomes using a large U.S. database of anticoagulated patients.
MATERIALS AND METHODS
Study population and questionnaire
The Clinical Outcomes Research Initiative (CORI) data-base, the largest endoscopic database in the United States, has the potential of recruiting large numbers of anticoagulated patients undergoing endoscopic procedures. We recruited anticoagulated patients for the study who were undergoing endoscopic procedures in a center participating in the CORI project. Patients using warfarin therapy were identified when the gastroenterologist performing the endoscopic procedure checked a box on the preprocedural screening for use of warfarin in the past 30 days.
This checkbox regarding the use of anticoagulant therapy was added to the CORI software program after receipt of funding for the project and approval by the CORI executive staff. For sites agreeing to participate in the study, this yes/no checkbox became a mandatory field for complete-ness of the CORI report. We only enrolled patients taking coumadin and did not collect information regarding use of clopidogrel or other antiplatelet agents.
Sites participating in this study asked every patient presenting for endoscopy to consider providing consent to be contacted by the CORI staff. Only CORI staff had access to identifying information on these subjects. Dr. Gerson served as the principal investigator for this project. Dr. Lieberman and Dr. Eisen from the CORI project served as co-investigators.
We designed a telephone questionnaire for the CORI staff to interview patients 1 month (30–45 days) after the endoscopic procedure (Appendix). The questionnaire was initially implemented in 20 patients at Stanford University Hospital and the Veterans Administration (VA) Palo Alto Health Care System. The preliminary data collected by Dr. Gerson was included in the final data analysis, because no changes were required for the questionnaire and data collection methods. Most patients provided initial verbal consent to respond to the survey and then were allowed to follow-up later with the written consent, which could occur months after the interview.
In the event of an adverse event, we obtained medical records. A major adverse hemorrhagic or thromboembolic event was defined as an event requiring hospitalization, whereas a minor event was bleeding or other symptoms not requiring hospital admission. We enrolled anticoagulated patients undergoing procedures at Stanford University Hospital, the VA Palo Alto Health Care System, and patients who consented in academic or private centers enrolled through the CORI database. Patients were required to be between the ages of 18 and 90 years, under-going an endoscopic procedure, capable of providing in-formed consent, and using chronic warfarin therapy for at least 30 days before the procedure. CORI patients were further required to have provided consent to be contacted.
CORI database center enrollment
At the time of initial study recruitment, there were 67 endoscopy centers registered with CORI. Dr. Gerson called each center, invited participation of the principal investigator, mailed the necessary documents for Institutional Review Board (IRB) submission, or arranged IRB submission through CORI and the Oregon Health and Science University (IRB no. 1044). By the end of the study, the number of centers in CORI had increased to 75, and 13 of the centers had agreed to participate in this study and enrolled at least 1 patient between May 2004 and October 2006 (Table 2).
Table 2.
Enrollment of CORI centers into study
| CORI sites for study | n = 75 |
| Sites enrolling patients | 13 |
| Sites agreeing to enroll* | 12 |
| No response† | 34 |
| Sites declined | 7 |
| No longer using CORI software | 5 |
| Investigator left site | 4 |
CORI, Clinical Outcomes Research Initiative.
Sites agreeing to enroll patients but principal investigators had not completed required paperwork via CORI.
Sites contacted on at least 5 occasions by phone or email who did not respond to contact.
Statistical analysis
The primary hypothesis of the study was that patients on chronic anticoagulant therapy would face both higher thrombotic and higher hemorrhagic risks depending on the management of the warfarin therapy in the periendoscopic period. We anticipated that temporary cessation of warfarin may be associated with a higher thrombotic risk, whereas a supratherapeutic INR or heparin substitution would be associated with a higher rate of hemorrhagic events.
Based on preliminary data from CORI, we estimated that 16,000 procedures would be recorded per month and 0.7% in patients on warfarin therapy (112 patients per month). Because we were able to recruit patients from only 13 centers enrolled in CORI, and historically 50% of patients provide consent to be contacted, we calculated that we would be able to accrue data from approximately 50–80 anticoagulated patients per month. We hypothesized that 30% of the patients would be managed with heparin therapy or continuation of warfarin therapy, and that temporary cessation of anticoagulant therapy would occur in 70% of patients. We expected that the risk of hemorrhage during the 30-day period associated with the endoscopic procedure in patients off warfarin would be 0.4%7, 8 plus 1% associated with the endoscopic proce-dure.9 For patients on warfarin or heparin therapy, the relative risk of a hemorrhagic event would increase by 2.4. When the z-statistic is used to compare proportions, a 2-tailed alpha of .05, and power of 80%, 1964 patients would be required to detect such a difference between the 2 arms.
Based on our earlier decision model,10 we anticipated that patients undergoing heparin substitution for warfarin would have a higher rate of hemorrhage but a lower rate of thrombosis than patients who simply stay on warfarin. We projected that the risk of stroke for a patient who is temporarily off warfarin would be 0.003 during the 30-day period around the endoscopic procedure.11 This estimate was obtained assuming a baseline risk of stroke of 4% per year for patients with nonvalvular atrial fibrillation. We also assumed that warfarin or heparin therapy would reduce the relative risk of stroke by 62% (for a 30-day risk of 0.001). Therefore, if 1375 (70%) of the 1964 patients have temporary cessation of warfarin therapy, we would expect 0.003 1375, or 4 thromboembolic events to occur in this group, compared with no events in the group receiving either continuous warfarin or heparin therapy. To detect such a difference between the 2 groups using a 2-tailed alpha of .05 and power of 80%, we would require 6838 patients in the study.
The cost to enroll such a high number of subjects was not supported by the career award. Therefore, enrollment did not allow the analysis to be sufficiently powered to detect significant differences in bleeding or stroke rates according to management of warfarin therapy. However, we determined that the CORI database would allow us to calculate a reliable estimate of bleeding in anticoagulated patients undergoing endoscopy that could be used to shape future policy regarding anticoagulant management in the periendoscopic period.
Categoric data analysis was conducted using Fisher exact testing performed with Statistical Analyzing System software (SAS 9.1 for Windows; Cary Institute, Cary, NC). Continuous data were analyzed using the Student t test in Microsoft Excel 2000. The level of significance was set at P .05. All tests were 2 tailed.
RESULTS
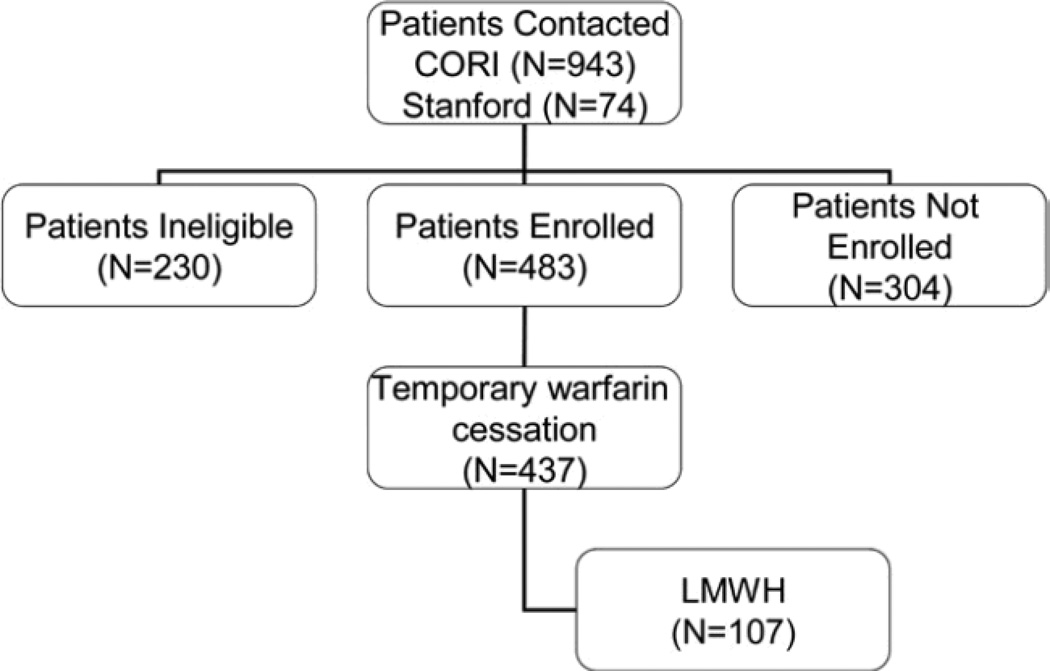
The flow of patients through the study is demonstrated in Figure 1. From the 13 sites recruited to participate in the CORI study, 120,886 procedures were performed in 95,807 patients: 76,821 (63.5%) of the procedures were per-formed in a community setting, 17,828 (15%) in an academic setting, and 26,237 (22%) in a VA setting. We contacted 1017 patients reporting to use warfarin therapy for participation, including 943 patients (93%) via the CORI database and 74 patients undergoing procedures at Stan-ford or the Palo Alto VA Medical Center. All of the patients at Stanford agreed to participate, and none of the patients refused or declined participation. Of the 943 CORI patients, 230 (24%) were not eligible (reasons for ineligibility included not using warfarin therapy [n 88], no phone number [n 50], duplicate entries [n 41], death or hospitalization [n 21], not speaking English [n 13], and other reasons [n 17]), and 320 subjects (34%) were not enrolled. A total of 569 interviews were conducted by the CORI staff, but only 409 subjects returned consent to be included in the final analysis. The final number included in the results section was therefore 483 patients. Although the mandatory fields for use of anticoagulant therapy in CORI should have captured all patients using warfarin therapy, it is possible that some patients using chronic warfarin therapy could have been missed.
Figure 1.
Flow diagram of patients in the study. LMWH, low-molecular weight heparin.
Demographics for the cohort are shown in Table 3. The majority of the patients were men with atrial fibrillation requiring warfarin for a mean of 5 years. Two-thirds of the patients were undergoing colonoscopy, and warfarin was held in 90% of the patients. Unfractionated heparin was administered in 7 patients (2 also received enoxaparin), and 107 (22%) received periendoscopic low-molecular-weight heparin in the periprocedural period. Six of the patients received preprocedure vitamin K. One hundred six patients (22%) were taking aspirin therapy, and 92% had ceased before the endoscopic procedure; 9 patients were managed by 50% dose reduction 5 days before the endoscopic procedure.
Table 3.
Demographic features of study participants (n = 483)
| Age, mean ( SD) years (range) | 68.3 9.7 (35–88) |
| Male gender (%) | 385 (80%) |
| Mean duration of warfarin usage, years | 5.8 6.1 (1 month–39 years) |
| Reason for warfarin | |
| Atrial fibrillation | 217 (45%) |
| Aortic or mitral valve replacement | 48 (10%) |
| Deep vein thrombosis and/or pulmonary embolism | 122 (25%) |
| Cerebrovascular event | 38 (8%) |
| Other* | 58 (12%) |
| Endoscopic procedures | |
| Colonoscopy | 347 (72%) |
| Polypectomy (snare, hot or cold biopsy) | 161 (47%) |
| Upper endoscopy (EGD) | 145 (30%) |
| EGD and colonoscopy | 28 (6%) |
| Endoscopic ultrasound | 7 (1%) |
| Flexible sigmoidoscopy | 9 (2%) |
| Endoscopic retrograde pancreatography | 3 (0.6%) |
| Patients with change in warfarin dose | 346 (72%) |
| Temporary cessation, mean (SD) days (range) | 437 (90%), 5 2.4 days (1–30) |
| Unfractionated heparin | 7 (1%) |
| Low-molecular-weight heparin | 107 (22%) |
| Mean no. of days (before, after procedure) | 4.4, 4.8 |
| Dose reduction by 50% before procedure | 9 (2%) |
| Aspirin usage | 106 (22%) |
| Temporary cessation | 70 (66%) |
Includes factor 5 Leiden deficiency (8 patients), protein C or S deficiency (4 patients), myocardial infarction or CABG (9 patients), congestive heart failure and/or cardiomyopathy (3 patients), lupus anticoagulant (2 patient), primary pulmonary hypertension (1 patient), and other causes (31 patients).
Of the 112 patients receiving periprocedural heparin therapy, the indication was current or earlier deep vein thrombosis/pulmonary embolism in 51 patients (46%), atrial fibrillation in 18 (16%), aortic valve replacement in 14 (12.5%), mitral valve replacement in 9 (8%), atrial fibrillation and prior CVA in 8 (7%), factor 5 Leiden deficiency or protein S deficiency in 5 (5%), and others in 7.
There were 11 deaths occurring within a range of 7 days to 8 months after the procedure. In 2 patients, no cause of death was available. One patient died 7 days after the procedure because of heart failure and sepsis not related to the procedure. Seven other patients died of causes not related to the procedure including myocardial infarction, renal failure, or hospitalization for other reasons. One patient died of pneumonia after undergoing colonic resection for cancer. In 1 patient, a CVA occurred 2 weeks after an upper endoscopy. In the recovery process, the patient fell at home and did not recover. Information about management of warfarin was not available. The National Death Index, a central computerized index of death record in-formation at the Centers for Disease Control and Prevention, was queried with the names of the 230 subjects not enrolled to determine if any had died within the study recruitment window. None of the nonenrolled subjects died during this period.
There were 10 major hemorrhagic events (2%), 5 (50%) of which occurred in patients receiving periprocedural enoxaparin therapy. Details regarding these events are shown in Table 4. Usage of pre- or postprocedural enoxaparin was not significantly associated with a risk of post-procedural hemorrhage (P .1). However, all of the patients undergoing colonoscopy with subsequent bleeding underwent polypectomy via combination of hot snare and hot biopsy. There were 5 out of the 161 patients (3%) in the polypectomy cohort with postprocedural hemorrhage, compared with none out of the 186 patients undergoing colonoscopy who did not have polypectomy (P .02). Warfarin was temporarily suspended in all 5 of the patients undergoing colonoscopy with polypectomy who experienced postprocedural bleeding, and 3 of these 5 patients received periprocedural enoxaparin therapy.
Table 4.
Patients with major hemorrhagic events after procedure
| Age (y), gender, procedure |
Reason for warfarin |
Management | Event | Outcome |
|---|---|---|---|---|
| 76, M, Colo* | TIAs, 4 mg alt with 6 mg daily | Warfarin stopped 1 day before, INR 1.1; LMWH 4 & 11 days after procedure, no ASA | Postpolypectomy 7 days after Colo; Hct drop 38% to 31% | Spontaneous cessation |
| 75, F, ERCP | AVR, 2.5 mg daily | Warfarin held 5 days, INR 1.8, no ASA | Bleeding from sphincterotomy 7 days after ERCP, INR 6, Hct 29% | Endoscopic therapy |
| 78, M, EGD | AF, 2.5 mg alt with 3.5 mg | Warfarin held 5 days, LMWH 4 days before & after, off ASA | Hematemesis 7 days after EGD, Hct 38% to 32% | Spontaneous resolution |
| 71, M Colo* | AF, CHF; 5 mg 6 days, 2.5 mg 1 day | Warfarin held 3 days, 81 mg ASA maintained 5 days | Postpolypectomy bleeding 8 days after Colo, required 4 units and ICU admission | Spontaneous resolution |
| 53, M, Colo* | PE, 6 mg 2 days, 5 mg 5 days | Warfarin suspended 3 days, LMWH 7 days before and 29 days after, no ASA | Hospitalization for rectal bleeding, no transfusions | Endoscopic therapy |
| 77, M, EGD and Colo* | AF, MVR; 5 mg 6 days, 2.5 mg 1 day | Held warfarin 5 days, off ASA 7 days | Hospitalized for rectal bleeding | N/A |
| 65, M, EGD | AF, 5 mg 3 days, 2.5 mg 4 days | Held warfarin 7 days, no ASA | Hospitalized for melena | Conservative |
| 83, M, EGD† | AF, 2.5 mg 4 days, 5 mg 3 days | Held warfarin 6 days, no ASA | Hospitalized for hematochezia | Conservative |
| 77, M, Colo* | AVR, 5 mg daily | Held warfarin 3 days, enoxaparin (no details), no ASA | Hematochezia | Conservative |
| 50, M, Colo* | AVR, 5 mg daily | Held warfarin 3 days, enoxaparin 3 days before and 8 days after | Hematochezia days 1–5 after procedure | Conservative |
AF, Atrial fibrillation; ASA, aspirin; AVR, aortic valve replacement; CHV, congestive heart failure; Colo, colonoscopy; Hct, hematocrit; ICU, intensive care unit; LMWH, low-molecular-weight heparin; MVR, mitral valve replacement; N/A, not available; PE, pulmonary embolism; TIA, transient ischemic attack.
All patients underwent snare polypectomy with or without hot biopsy during colonoscopy.
Upper endoscopy with biopsy.
One patient sustained a fatal thromboembolic stroke (0.2%) 2 weeks after colonoscopy. Unfortunately, information regarding warfarin management was not available from the primary physician and no family contacts were available.
Other postprocedural symptoms were reported in 84 patients (17%) during the postprocedural interview, including dyspnea in 20 (4%), peripheral edema in 19 (4%), self-resolving hematochezia in 12 (2.5%), visual changes in 8 (2%), chest pain in 9 (2%), epistaxis in 4 (0.8%), chest pain in 3 (0.6%), and abdominal pain in 2 (0.2%).
DISCUSSION
The purpose of this prospective study was to use the CORI database to collect outcome data in patients on anticoagulant therapy in the periendoscopic period. We demonstrated that the rate of postprocedural hemorrhage was not elevated among anticoagulated patients. How-ever, the study was underpowered to make definitive conclusions about benefits from current society guidelines regarding postprocedural bleeding. We were unable to make observations about the incidence of thromboembolic events in anticoagulated patients, which would have required a very large sample size, as demonstrated in the statistical calculations for this manuscript. One thromboembolic event (0.2%) did occur, consistent with previous rates in the literature. Other limitations of the study included lack of a control group of patients not taking anticoagulants, no information regarding use of clopidogrel, or data regarding INR levels before the procedure. In addition, there was a possibility of drop-out bias due to missing information about patients who could have died from bleeding or thrombosis with relatives who were unable to be contacted.
The prevalence of postprocedural hemorrhagic events was 1%, a rate similar to that which would be expected in a population of patients undergoing endoscopic procedures who were not taking warfarin therapy.12,13 Our hypothesis was that the usage of periprocedural enoxaparin therapy was associated with higher postendoscopic bleeding rates. We were unable to demonstrate from the enrolled cohort that the usage of periprocedural enoxaparin was associated with high rates of postprocedural hemorrhage. Although 22% of patients were administered low-molecular-weight heparin therapy, we estimated a sample size of 1900 patients would be required to demonstrate an increased risk of hemorrhage compared with patients managed with temporary cessation of warfarin therapy without heparin substitution.
Earlier data regarding therapeutic endoscopic procedures such as polypectomy and/or sphincterotomy in anticoagulated patients are limited, but they suggest an in-creased risk of postendoscopic hemorrhagic events in anticoagulated patients. In a retrospective analysis of 1657 polypectomies in China, the use of antiplatelet agents during polypectomy was not associated with an increase in postpolypectomy bleeding; however, patients on chronic warfarin therapy were shown to have a significant increase in postpolypectomy bleeding.14 A study in patients after sphincterotomy similarly did not demonstrate that antiplatelet agents were a risk factor for postprocedural hemorrhage.15 The data from the present study demonstrated that anticoagulated patients undergoing polypectomy were at increased risk for postprocedural hemorrhage compared with patients undergoing diagnostic colonoscopy (P.2), despite temporary cessation of warfarin therapy. This observation may justify the usage of prophylactic hemoclip application after polypectomy in anticoagulated patients,16 although this practice is not recommended by the current ASGE guidelines, owing to lack of evidence from any randomized controlled trials.6 Al-though bleeding occurred a week after the procedure in most patients, we were unable to collect data about the timing of postprocedural bleeding in 50% of the patients.
Data regarding adherence to the ASGE guidelines have been limited. In 2000, we published a retrospective analysis of 104 anticoagulated patients at the VA Palo Alto Health Care System undergoing endoscopic procedures and demonstrated that 90% of the patients receiving periprocedural heparin therapy were not managed ac-cording to the ASGE guidelines.15 In the present study, enoxaparin was administered appropriately to the 77 members (69%) of the cohort with high-risk conditions, including deep venous thrombosis, pulmonary embolism, earlier CVA, mechanical mitral valve, and atrial fibrillation with earlier CVA. In the 32 patients (28%) with lone atrial fibrillation, or aortic valve replacement, heparin therapy would not be indicated unless those patients were at high risk for a thromboembolic event owing to an earlier CVA or other event. Based on our analysis, the majority of patients were managed appropriately according to the ASGE guidelines in the present study.
In summary, this prospective study using the CORI database did not demonstrate an increased risk of post-procedural hemorrhage in patients using anticoagulant therapy who were managed according to the ASGE guidelines.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the following CORI centers and investigators participating in the study: Sheldon Berger, MD (Berger-St. Francis, Okla); Ralph Breitenstein, MD (Klamath, Ore); Paul Craig, MD (Spokane, Wash); Glenn Eisen (Portland VAMC, Ore); Richard Folan, MD (Colorado Springs, Colo); Patrick Gerstenberger, MD (Durango, Colo); Patrick Quinn, MD (Santa Fe, NM); Francisco Ramirez, MD (Phoenix VA, Ariz); Richard Sampliner, MD (Tucson VAMC, Ariz); Robert Sudduth, MD (Bremerto, Wash); Arjun Venkataramani, MD (Akron, Ohio); Diane Williams, MD (Pinehurst, NC); Thomas Zarchy, MD (Los Angeles, Calif).
Supported by an American Society for Gastrointestinal Endoscopy Career Development Award to Dr. Gerson. The ASGE did not provide any role in regard to the study design, data collection, or data interpretation.
Abbreviations
- ASGE
American Society for Gastrointestinal Endoscopy
- CORI
Clinical Outcomes Research Initiative
- CVA
cerebrovascular ac-cident
- GIB
gastrointestinal bleeding
Footnotes
DISCLOSURE: All other authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Longstreth GF. Epidemiology of hospitalization for acute upper gastro-intestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90:206–210. [PubMed] [Google Scholar]
- 2.Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997;92:419–424. [PubMed] [Google Scholar]
- 3.Kaplan RC, Heckbert SR, Koepsell TD, et al. Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators. J Am Geriatr Soc. 2001;49:126–133. doi: 10.1046/j.1532-5415.2001.49032.x. [DOI] [PubMed] [Google Scholar]
- 4.Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal haemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut. 1994;35:464–466. doi: 10.1136/gut.35.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veitch AM, Baglin TP, Gershlick AH, et al. Guidelines for the management of anticoagulant and antiplatelet therapy in patients undergoing endoscopic procedures. Gut. 2008;57:1322–1329. doi: 10.1136/gut.2007.142497. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MA, Ben-Menachem T, Gan SI, et al. Management of anti-thrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060–1070. doi: 10.1016/j.gie.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 7.Feinberg WM, Kronmal RA, Newman AB, et al. Stroke risk in an elderly population with atrial fibrillation. J Gen Intern Med. 1999;14:56–59. doi: 10.1046/j.1525-1497.1999.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to pre-vent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 9.Sorbi D, Norton I, Conio M, Balm R, Zinsmeister A, Gostout CJ. Post-polypectomy lower GI bleeding: descriptive analysis. Gastrointest Endosc. 2000;51:690–696. doi: 10.1067/mge.2000.105773. [DOI] [PubMed] [Google Scholar]
- 10.Gerson LB, Triadafilopoulos G, Gage BF. The management of anticoagulants in the periendoscopic period for patients with atrial fibrillation: a decision analysis. Am J Med. 2004;116:451–459. doi: 10.1016/j.amjmed.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 12.Rosen L, Bub DS, Reed JF, 3rd, et al. Hemorrhage following colonoscopic polypectomy. Dis Colon Rectum. 1993;36:1126–1131. doi: 10.1007/BF02052261. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs DH, Opelka FG, Beck DE, et al. Postpolypectomy colonic hemorrhage. Dis Colon Rectum. 1996;39:806–810. doi: 10.1007/BF02054448. [DOI] [PubMed] [Google Scholar]
- 14.Hui AJ, Wong RM, Ching JY, et al. Risk of colonoscopic polypectomy bleeding with anticoagulants and antiplatelet agents: analysis of 1657 cases. Gastrointest Endosc. 2004;59:44–48. doi: 10.1016/s0016-5107(03)02307-1. [DOI] [PubMed] [Google Scholar]
- 15.Hussain N, Alsulaiman R, Burtin P, et al. The safety of endoscopic sphincterotomy in patients receiving antiplatelet agents: a case-control study. Aliment Pharmacol Ther. 2007;25:579–584. doi: 10.1111/j.1365-2036.2006.03225.x. [DOI] [PubMed] [Google Scholar]
- 16.Friedland S, Soetikno R. Colonoscopy with polypectomy in anticoagulated patients. Gastrointest Endosc. 2006;64:98–100. doi: 10.1016/j.gie.2006.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.