Abstract
Background and objective
A single dose of 10 Gy radiation to the thorax of rats results in decreased total lung angiotensin-converting enzyme (ACE) activity, pulmonary artery distensibility and distal vascular density while increasing pulmonary vascular resistance (PVR) at 2-months post-exposure. In this study we evaluate the potential of a renin-angiotensin system (RAS) modulator, the ACE inhibitor captopril, to mitigate this pulmonary vascular damage.
Methods
Rats exposed to 10 Gy thorax only irradiation and age-matched controls were studied 2-months after exposure, during the development of radiation pneumonitis. Rats were treated, either immediately or 2-weeks after radiation exposure, with 2 doses of the ACE inhibitor, captopril, dissolved in their drinking water. To determine pulmonary vascular responses, we measured pulmonary hemodynamics, lung ACE activity, pulmonary arterial distensibility, and peripheral vessel density.
Results
Captopril, given at a vasoactive but not a lower dose, mitigated radiation-induced pulmonary vascular injury. More importantly these beneficial effects were observed even if drug therapy was delayed for up to two weeks after exposure.
Conclusions
Captopril resulted in a reduction in pulmonary vascular injury that supports its use as a radiomitigator after an unexpected radiological event such as a nuclear accident.
Keywords: captopril, injury, lung, therapeutics, radiation, pneumonitis
INTRODUCTION
The lung is relatively sensitive to radiation-induced injury (1–4). Depending on the radiation dose, injury to the lung generally manifests in two phases, an acute pneumonitis (after 6–12 weeks in rats) and a delayed fibrosis (from months to years later) (5–7). These injuries are thought to be a result of damage to the vasculature and lung parenchyma in combination with inflammatory mediators (8–12) and possibly effects on other organs, such as the heart (13). We have reported that a number of hemodynamic and vascular parameters in the lung are altered during pneumonitis (13). Others have reported severe endothelial dysfunction (10, 14). Thus vascular damage is substantial and contributes to the symptomatic injuries induced by radiation such as tachypnea and pulmonary inflammation.
Interestingly post-radiation injury has been shown to be reduced by suppression of the renin angiotensin system (RAS) with ACE inhibitors such as captopril or angiotensin receptor blockers (15), even though ACE activity itself is reduced by radiation. In spite of these promising results as a radiomitigator the exact mechanism of action of ACE inhibitors remain elusive, and the effect of captopril on pulmonary hemodynamics and vascular remodeling in the intact irradiated lung has not been examined.
With the threat of nuclear accidents or radiological terrorism and the broadening role of radiation therapy in oncology there are medical imperatives to develop countermeasures and protocols for mitigating radiation injury. Pneumonitis manifests in humans within 3–6 months of irradiation (16). Under the preparedness and response paradigm, agents that work only when given before radiation exposure are of little or no value. The present study was designed to investigate the possibility that therapy with an ACE inhibitor is effective in mitigating pulmonary vascular injury associated with radiation exposure when started 2 weeks after irradiation. Our group have developed a rat model of thoracic irradiation and we have previously reported on the role of modulators of the RAS as effective as post-irradiation mitigators (4, 13, 17). Ghosh et al. have reported improved survival, reduced tachypnea and milder pulmonary fibrosis in rats given 10, 12 and 15 Gy thoracic irradiation and subsequently given captopril therapy (13). In the present study, we evaluated the effects of captopril on mitigating radiation-induced changes on pulmonary vascular hemodynamics, indices that we believe are vital for regulation of normal structure and function in the lung.
METHODS
Animals
All the studies were done under approval of the Medical College of Wisconsin and Zablocki VA Medical Center IACUC review boards and in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Blood Pressure and Plasma renin activity
Female WAG/Rij/Cmcr rats (126±6 gm±SD, N=36) were studied to determine the bioactive effects of captopril. Plasma renin activity (PRA) was measured by radioimmunoassay using a modification of the method of Sealey and Laragh (18). Systolic blood pressure (SBP) was measured using a non-invasive tail-cuff analyzer (Visitec Systems, Apex, North Carolina, USA) as described (19). Rats were conditioned to the apparatus and treated with captopril for a minimum of 10-days before measurements were made.
Injury model
Unanesthetized female rats (110–170 gm, N=63) were placed in a plexiglass jig and exposed to a 10 Gy dose of radiation limited to the thorax as previously described (17). The whole lung was in the field, plus the heart and a small amount of the liver. Irradiation and subsequent housing of the rats took place in a moderate barrier containment facility. The WAG/Rij/Cmcr strain of rat has been developed and inbred in our moderate security barrier facility to maximize the stability of the strain and minimize exposure to pathogens and risk of infection.
Drug Therapy
Captopril (USP grade, Sigma Chemical, St. Louis, Missouri, USA) was added to the drinking water (ad libitum) of the selected groups immediately or after a delay of 2-weeks post-exposure. Captopril was dissolved in water at concentrations of 50, 150, 300, and 500 mg/L to approximate 5.5, 17, 34 and 56 mg/kg/day or 67–333 mg/m2/day as previously described (13).
Lung preparation
Irradiated rats and their age-matched controls were studied at 2-months post-irradiation during the development of radiation pneumonitis (13, 17, 20) using lung preparation and imaging methods as described previously (4, 20, 21).
Hemodynamics/pulmonary vascular resistance (PVR)
Multipoint pressure versus flow measurements were made in the isolated lungs with the airway pressure held constant at 6 mmHg (21, 22). Individual rat data were fit to the model and PVR at a flow rate of 100 ml/min/kg was calculated as a single parameter for comparative analysis.
Total lung Angiotensin Converting Enzyme (ACE) activity
Total lung ACE activity was measured by perfusing a pseudo substrate of the enzyme ACE (N-[3-(2-furyl)-acryloyl]-L-phenyl-alanylglycylglycine) followed by spectrophotometric analysis of the effluent as previously described (4, 23). ACE activity is presented as relative activity units.
Lung Imaging
Microfocal angiographic imaging (including volumetric CT) was performed at 4 perfusion pressures; 30,21,12 and 5 mm Hg. For further details see (21).
Pulmonary arterial distensibility (PAD) was measured using previously published methods (21, 24). Briefly, isotropic CT volumes were used to map and measure the main pulmonary trunk at each intravascular pressure. The data were fit, using an unconstrained multivariable non-linear optimization function ‘fminunc’ in the Matlab optimization toolbox (MathWorks, Natick, Massachusetts, USA), to a biomechanical model of the pulmonary arterial tree, which incorporates PAD.
Microvascular density was quantified as previously described (4, 20) using high-magnification planar angiograms of the right, lower lung lobe imaged at an intravascular pressure of 30 mmHg. The number of pixels segmented as vessel was divided by the total number of pixels in a region of interest to determine a digitized index of peripheral microvascular density.
Histology
Lung tissue was harvested at 2 months after irradiation and fixed in zinc formalin for 24–48 hours as described (10, 13). Paraffin embedded sections (4 μm thick) were stained with hematoxylin and eosin (H&E).
Statistical analysis
Data were calculated and presented as mean ± standard error of the mean (SEM). All statistical comparisons were performed in SigmaStat 3.0 (SPSS, Chicago, Illinois, USA). Comparisons of SBP and PRA were performed using one-way analysis of variance. Pair-wise comparisons procedures used the Holm-Sidak method. Other data were analyzed using an unpaired t-test between experimental groups as indicated in figure legends. If the normality test failed a Mann-Whitney Rank Sum test was executed to measure significance. P values are included in figure legends.
RESULTS
Effects of different doses of captopril on systolic blood pressure (SBP) and plasma renin activity (PRA)
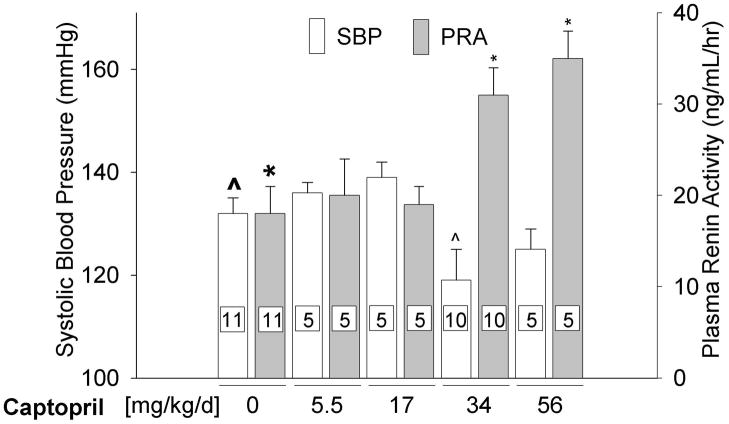
We studied 4 doses of captopril in rats to examine bioactivity. Doses above 17 mg/kg/day elevated PRA and induced mild hypotension, whereas doses at or below 17 mg/kg/day did not (Fig. 1).
Figure 1.
Systolic blood pressure (SBP) measured via tail-cuff and plasma renin activity (PRA) measured using radioimmunoassay in control and captopril treated unirradiated rats. Captopril therapy was provided for a minimum of 10-days before measurements were made. (Data represent mean±sem. Number of rats in individual groups indicated on the bars, ^ p = 0.023, * p ≤ 0.002, difference from bold marker).
Mitigation of radiation-induced increase in PVR by captopril
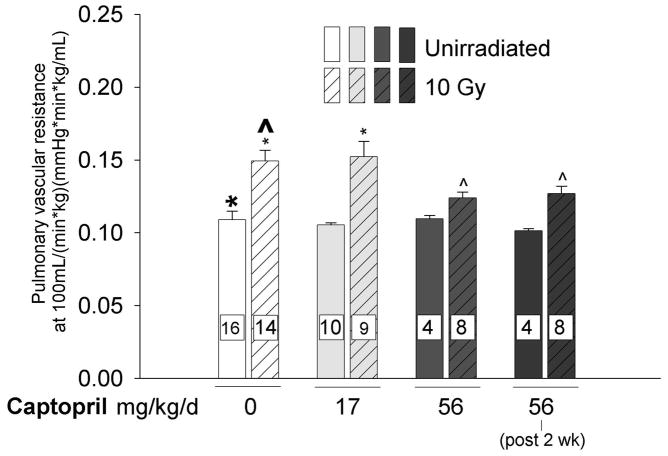
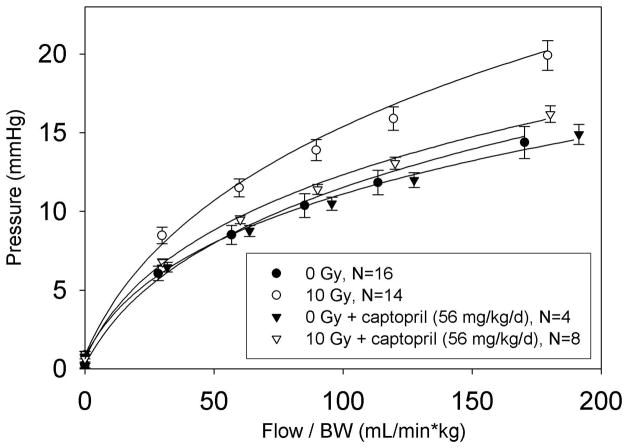
We found irradiation (10 Gy) to the thorax resulted in increased pulmonary vascular resistance (PVR, see Fig. 2a) calculated at a normalized flow rate of 100 mL/min/kg. Captopril therapy was given at 2 doses, 17 and 56 mg/kg/day to a cohort of rats immediately after irradiation. Another group was given captopril at 56 mg/kg/day starting 2 weeks after irradiation. Captopril treatment did not affect PVR in unirradiated rats. Irradiated rats that were treated with 56 mg/kg/day (immediately or after 2 weeks) but not 17 mg/kg/day of captopril had decreased PVR, as shown in (Fig. 2a). Fig. 2b depicts the effect of captopril with or without irradiation, on lowering PVR over a range of flow rates (29).
Figure 2.
a) Effect of thoracic irradiation and captopril therapy on pulmonary vascular resistance (PVR). Values of PVR (mean±sem) calculated at a normalized flow of 100 mL/min/kg. Drug therapy groups received captopril (17 or 56 mg/kg/d) immediately or delayed 2-weeks after irradiation. Irradiation resulted in increased PVR. The vasoactive dose of captopril (56 mg/kg/d) mitigated the radiation-induced increase in PVR. (Number of rats in individual groups indicated on the bars, * p ≤ 0.033, ^ p ≤ 0.048, difference from bold marker). b) Example of the effect of thoracic irradiation and immediate captopril therapy (56 mg/kg/d) on PVR. The graph demonstrates multipoint pulmonary artery pressure (mean±sem) versus flow rate normalized to body weight. Measurements were made 2-months after irradiation (10 Gy limited to the thorax) in isolated perfused lungs. Combined data for each experimental group are fit to a simple distensibility model of PVR over the range of flow rates.
Fall in lung ACE activity by irradiation and by captopril
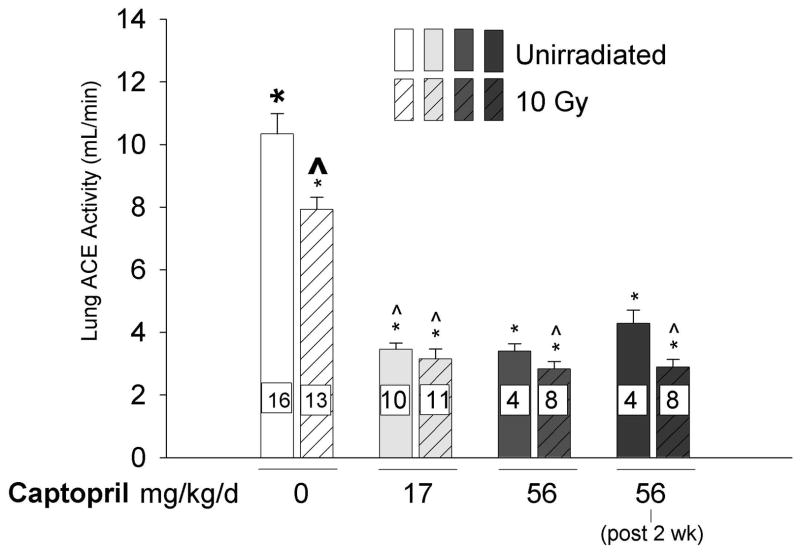
Irradiation (10 Gy) to the thorax resulted in a fall in total ACE activity (bars 1 and 2 in Fig. 3). Captopril exacerbated this effect, even at the low dose of 17 mg/kg/day (Fig. 3) that did not alter SBP or PRA.
Figure 3.
Effect of thoracic irradiation and captopril therapy on total lung ACE activity. Lung ACE activity was measured using a spectrophotometric assay in isolated perfused lungs from rats studied 2-months after irradiation. Values (mean±sem) represent mean ACE activity in relative units of available ACE binding sites. Drug therapy groups received captopril (17 or 56 mg/kg/d) immediately or delayed 2-weeks after irradiation. Irradiation alone decreased lung ACE activity. Captopril therapy further reduced lung ACE activity. (Number of rats in individual groups indicated on the bars, * p ≤ 0.006, ^ p ≤ 0.001, difference from bold marker).
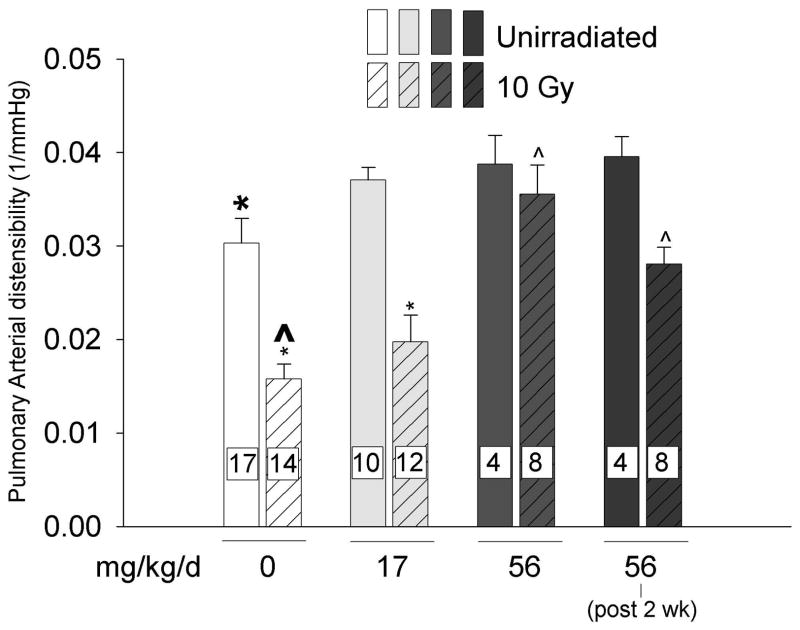
Mitigation of radiation induced reduction in PAD by captopril
Irradiation (10 Gy) to the thorax resulted in reduced PAD (bars 1 and 2 in Fig. 4). Captopril treatment did not affect PAD in unirradiated rats. Irradiated rats that were treated with 56 mg/kg (immediately or after 2 weeks) but not 17 mg/kg of captopril per day had increased PAD as compared to irradiated rats only, as shown in (Fig. 4).
Figure 4.
Effect of thoracic irradiation and captopril therapy on pulmonary arterial distensibility (PAD). Values (mean±sem) measured at 2-months after exposure using microfocal computed tomography of intact, contrast filled arterial trees in isolated lung preparations. Drug therapy groups received captopril (17 or 56 mg/kg/d) immediately or delayed 2-weeks after irradiation. The vasoactive dose of captopril (56 mg/kg/d) mitigated radiation-induced decrease in PAD. (Number of rats in individual groups indicated on the bars, * p ≤ 0.047, ^ p ≤ 0.001, difference from bold marker).
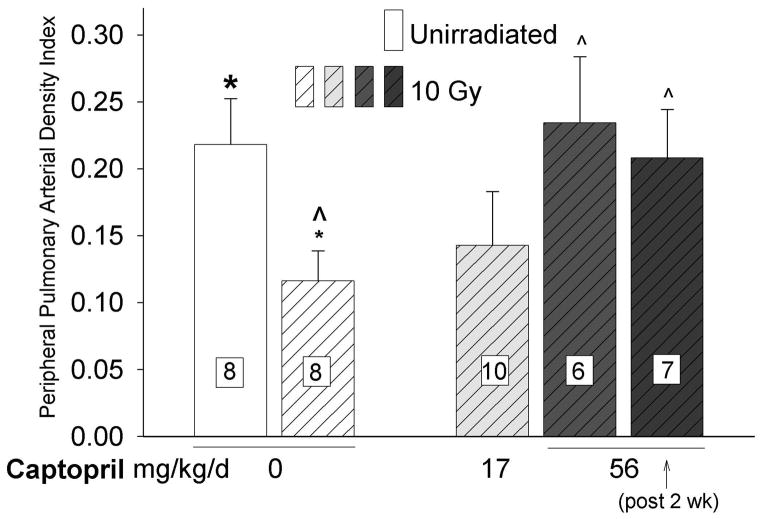
Mitigation of radiation induced reduction in distal pulmonary arterial density by captopril
Irradiation (10 Gy) to the thorax resulted in reduction in arterial density in the lung (bars 1 and 2 in Fig. 5). Irradiated rats that were treated with 56 mg/kg (immediately or after 2 weeks) but not 17 mg/kg of captopril per day had increased pulmonary vascular density as compared to irradiated rats only. However, the pulmonary arterial density in irradiated rats given 17 mg/kg/day of captopril was also not different from that observed in unirradiated rats.
Figure 5.
Effect of thoracic irradiation and captopril therapy on peripheral pulmonary arterial density index. Values (mean±sem) measured at 2-months after radiation exposure using high-magnification planar angiography of distal portions of the intact, contrast filled arterial tree in isolated lung preparations. Drug therapy groups received captopril (17 or 56 mg/kg/d) immediately or delayed 2 weeks after irradiation. Irradiation caused a decrease in peripheral arterial density. The vasoactive dose of captopril (56mg/kg/d) increased peripheral arterial density compared to irradiated, untreated rats. Vessel densities after irradiation and therapy with a vasoactive dose of captopril (immediate or delayed) were no different from values in unirradiated controls. (Number of rats in individual groups indicated on the bars, * p ≤ 0.026, ^ p ≤ 0.046, difference from bold marker).
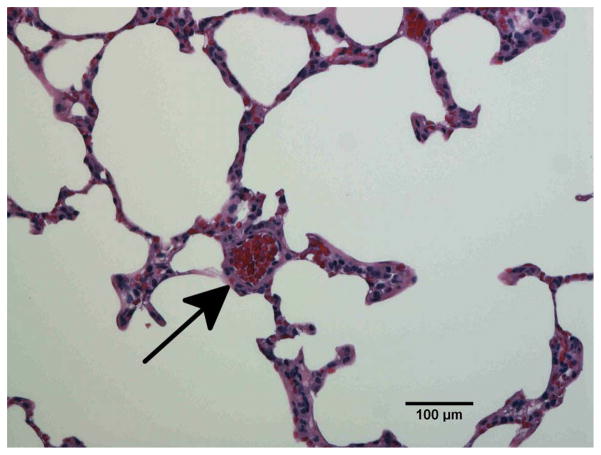
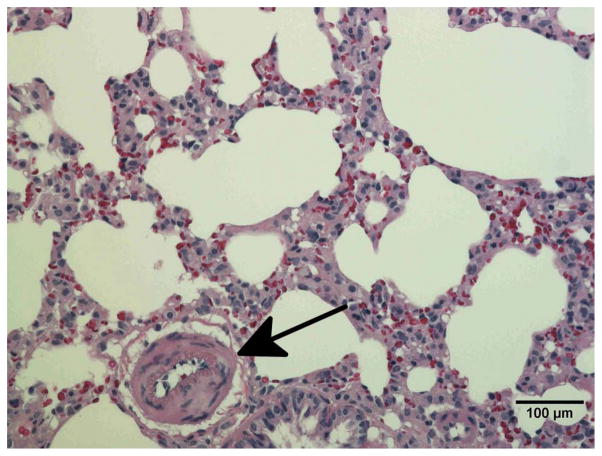
Radiation induced vessel injury in the lung by histology
To confirm vascular injury the lungs of unirradiated rats (Fig. 6a) and rats receiving 10 Gy with or without captopril (17–56 mg/kg/d) (Figs. 6b–e) were examined by histology at 2 months after irradiation. Blood vessels (marked by arrows in Fig. 6) including some with vascular injury, such as increase in vessel wall thickness and partial occlusion of the lumen, were identified in irradiated lung sections.
Figure 6.
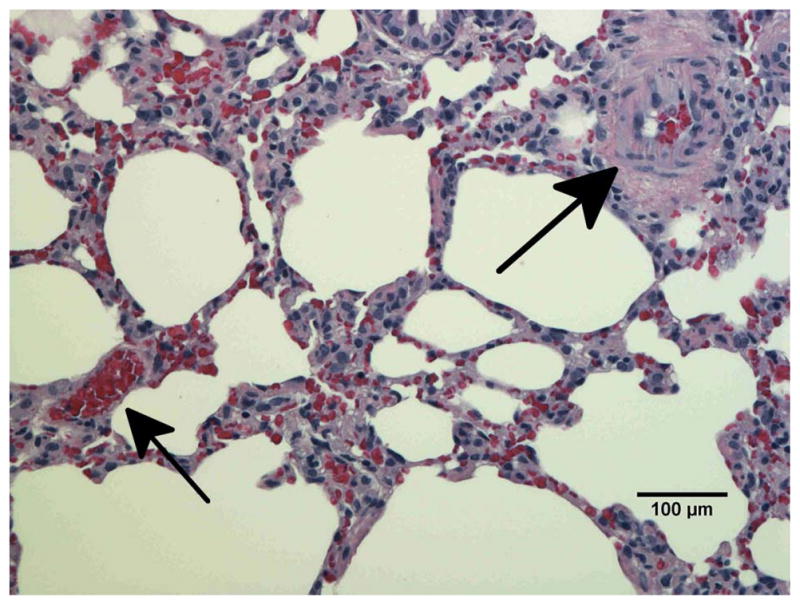
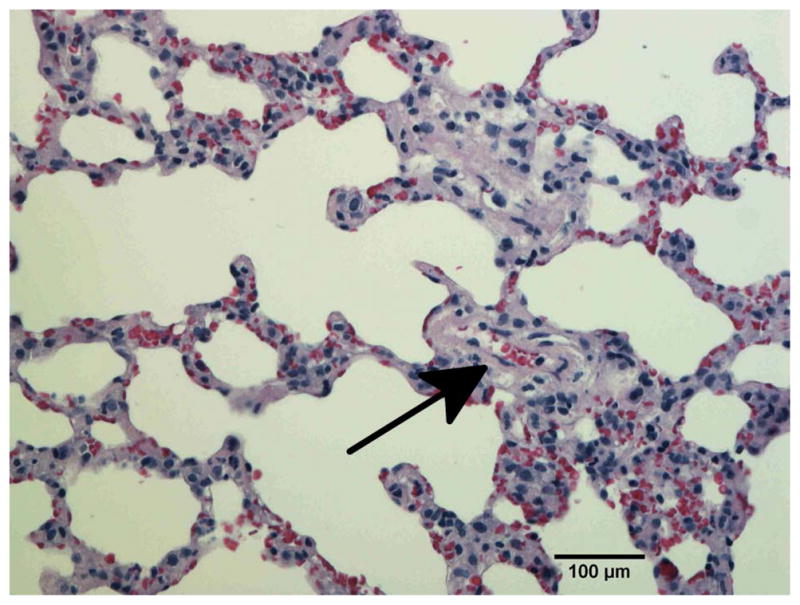
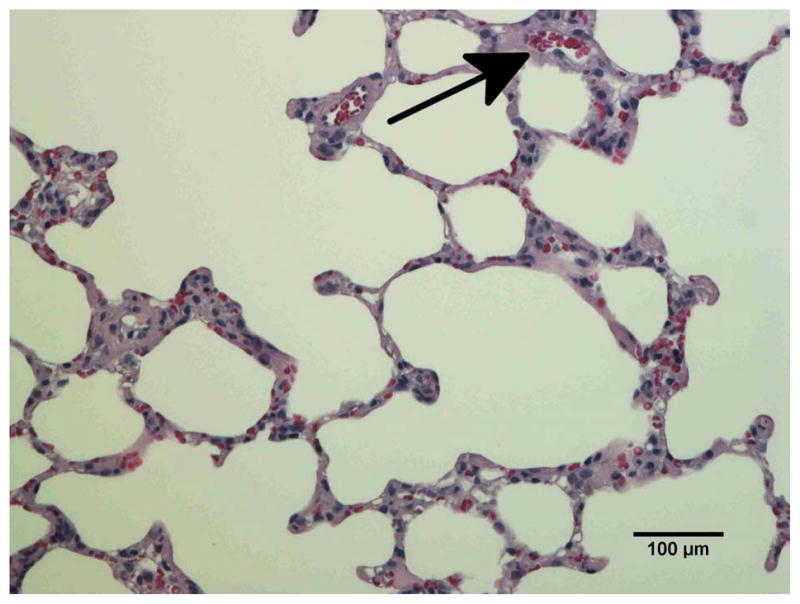
Histological sections of lung stained with hematoxylin-eosin demonstrating vascular abnormality at 2-months after radiation. a) Unirradiated lung b) 10 Gy c) 10 Gy with captopril (17 mg/kg/d) immediately d) 10 Gy with captopril (56 mg/kg/d) immediately e) 10 Gy with captopril (56 mg/kg/d) delayed by 2 weeks. Arrows point to blood vessels. Scale bars represent 100 μm.
In summary, we observed that the effect of captopril depended on the dose of the drug given. Rats given a vasoactive dose (56 mg/kg/day of captopril) had improved pulmonary vascular indices compared to irradiated, untreated rats. The higher dose of captopril also preserved the PAD and distal pulmonary vessel density to levels no different from unirradiated controls even with a substantial delay of 2 weeks before the start of therapy. A lower dose of captopril (17 mg/kg/day), that did not affect SBP or PRA, provided no improvement in the indices we measured.
DISCUSSION
Most work in the field of radiation-induced injury has focused on prophylactic schedules started before or immediately after irradiation. This study demonstrates that captopril may be an effective mitigator of radiation-induced pulmonary inflammation and vascular injuries, even if treatment is delayed. Results with captopril (56 mg/kg/day), show not only improvements in PVR, an index that recovers after pneumonitis from 10 Gy, but also PAD and peripheral vessel density, injuries that do not recover even up to 1 year post-exposure in the same rat model (4, 20). Mitigation by doses of captopril that result in a modest drop in SBP, presents a compelling argument that it could be used as a countermeasure against lung injury from radiation exposure. Although a small fraction (1.6–7%) of patients will suffer a cough from ACE inhibitors (25), cough associated with ACE inhibitors is time limited and will resolve in 3–5 days after the ACE inhibitor is discontinued (26).
Mechanisms within the renin-angiotensin system have been shown to be involved in modulating radiation-induced lung injury (10, 11, 13, 27). Ward et al. reported that thiol (such as captopril) and non-thiol (such as CGS 13945) ACE inhibitors reduced radiation-induced pulmonary endothelial radioresponsiveness and dysfunction (11, 28). Molteni et al. described protective effects of ACE inhibitors and an angiotensin II type 1 receptor blocker (non-thiol) in a model of right hemithorax radiation-induced lung injury (10). ACE inhibitors spared morphological damage (vasculitis and inflammation), but only the thiol containing ACE inhibitors had strong effect against collagen deposition. These studies involved high doses of fractionated or partial lung exposures lethal, with or without intervention, if given in a single dose to the whole lung (29). Our results indicate that captopril is effective in models more relevant to an accident or terrorism related injury.
ACE activity measured in the isolated lung was independent of the dose of captopril, as seen in Figure 3, suggesting that a high level of the lung ACE activity is inhibited by a non-vasoactive dose of captopril and not inhibited further by increasing the dose. Therefore, mitigation could, at least in part, be from pluripotent or indirect actions of the drug, such as induction of angiotensin-(1–7), which upregulates the release of kinins and/or other non-humoral brief acting local autacoids functioning similar to hormones (30, 31). It should be noted that the pseudo-substrate used to measure ACE activity in our study is not identical to angiotensin I. Interestingly, Molteni et al. reported an increase in ACE activity in homogenized lung extracts from rats given captopril (10).
We examined the effects of ACE inhibition on the mitigation of lung injury in rats during pneumonitis at 8 weeks after irradiation because our previous studies demonstrated increases in PVR, RVH, and dry lung weight accompanied by a decrease in PAD (4, 17) at the same time, in the same model of irradiation. Some components of pathology start to resolve spontaneously without drug treatment up to 1 year (4, 17). Radiation induced inflammation has been observed in untreated rats at the 8 week time point and as early as 2 weeks. Inflammation is mitigated by captopril, although more effectively when treatment begins immediately after irradiation (13). Vascular injury and its mitigation by captopril may be independent of surrounding pulmonary inflammation, since captopril mitigated radiation-induced loss of vasoconstriction as well as endothelium-dependent vasodilation in isolated pulmonary arteries ex vivo (13). Doses of captopril (34 mg/kg/day) were effective at one, but not 2 weeks after thoracic irradiation (13).
In addition to potent antimitotic (32–34) and anti-inflammatory mechanisms, our data support the concept a vasoactive dose of captopril mitigates distal vessel loss, consistent with evidence it increases peripheral vascular density (35–37). The prevention of a functional deficit in conductive luminal space could be the result of a reduction in active remodeling (decreasing PAD), a reduction in congestive inflammation (increasing distal perfusion) or both. he decreased PAD measured in our studies is likely due mostly to the increase in wall muscularization and extracellular matrix, common components of vascular remodeling observed in models of pulmonary hypertension (38, 39). These components limit distal luminal filling at equivalent intravascular pressures and decrease the total effective vascular cross section, in agreement with previous findings (40). In vivo, a reduction in PA pressure (less vessel distention) causes the vascular biomechanics to fall on a more compliant portion of the operating curve, resulting in the increased active distensibility of the entire pulmonary arterial tree. Therefore, the reduction in PA pressure has an additive effect and reduces shear stress and pressure related forces and strains on the endothelial surfaces that would otherwise result in further remodeling and cellular proliferation. Pulmonary vascular impedance, which is highly dependent on PAD, is clearly recognized as a key factor in cardiopulmonary health, right ventricle to pulmonary artery performance (ventricular-vascular coupling), and prognosis in pulmonary vascular disease (41–44). Captopril at a dose of 56 mg/kg/day completely eliminated the decrease in passive, or fixed, PAD caused by radiation injury. ACE inhibitors have been reported to decrease pulmonary arterial wall thickening and other pulmonary damage induced by various models of pulmonary injury (10, 11, 13, 35, 45–48), consistent with decreased PVR, PAD, and peripheral vessel drop out. The increase in effective peripheral luminal area and decrease in PAD are important findings, since previous reports indicate the beneficial action of ACE inhibition may primarily be from the prevention of vasoconstriction (48).
In conclusion, our data suggest ACE inhibitor therapy can mitigate radiation injury and benefit pulmonary vascular status. Our findings address important pulmonary vascular indices that have not been measured in other reports of ACE inhibition in radiation-induced lung injury (10, 11, 13). More importantly, we are the first to propose a dose of captopril (56 mg/kg/day) that is effective for vascular end points even when started 2 weeks after irradiation in rats. Proper dosing and scheduling of drugs are likely to be important. With the diverse range of approved ACE inhibitors available, each displaying unique chemical characteristics and capacities, there is optimism that a range of mitigators will be identified to increase effectiveness and decrease side effects.
Summary at a glance.
A single large dose of radiation to the lung induces pneumonitis after approximately two months. Our results demonstrate that a commonly used ACE inhibitor, captopril, attenuates radiation induced pulmonary vascular remodeling during pneumonitis in rats. These results indicate that captopril acts by mitigating injury to the vasculature in the lung.
Acknowledgments
The authors would like to acknowledge funding from National Institute of Health, National Institute of Allergy and Infectious Diseases, U19 AI067734-01, RC-1 AI 81294, and the Department of Veterans Affairs. The authors would like to thank Camille Torres for conducting the plasma renin assays, Vladimir Semenenko, X. Allen Li, Ying Gao, Feng Gao, Stephanie Gruenloh, Dena Hall, Tarrant Csida, Marylou Mader, and Yvonne Morauski for help with the animals, conducting irradiation, dosimetry, administration, and their dedication to the goals of the Center for Radiation Injury Intervention. Jayashree Narayanan assisted with preparation of the manuscript.
References
- 1.Augustine AD, Gondre-Lewis T, McBride W, et al. Animal models for radiation injury, protection and therapy. Radiat Res. 2005;164(1):100–9. doi: 10.1667/rr3388. [DOI] [PubMed] [Google Scholar]
- 2.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162(6):711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 3.Fleckenstein K, Zgonjanin L, Chen L, et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68(1):196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh SN, Wu Q, Mader M, et al. Vascular injury after whole thoracic x-ray irradiation in the rat. Int J Radiat Oncol Biol Phys. 2009;74(1):192–9. doi: 10.1016/j.ijrobp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63(1):5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Hill RP. Radiation effects on the respiratory system. BJR Suppl. 2005;27:75–81. [Google Scholar]
- 7.Marks LB, Yu X, Vujaskovic Z, et al. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13(3):333–45. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 8.Robbins ME, Diz DI. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64(1):6–12. doi: 10.1016/j.ijrobp.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 9.Calveley VL, Khan MA, Yeung IW, et al. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005;81(12):887–99. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- 10.Molteni A, Moulder JE, Cohen EF, et al. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76(4):523–32. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 11.Ward WF, Molteni A, Kim YT, et al. Structure-function analysis of angiotensin-converting enzyme inhibitors as modifiers of radiation-induced pulmonary endothelial dysfunction in rats. Br J Radiol. 1989;62(736):348–54. doi: 10.1259/0007-1285-62-736-348. [DOI] [PubMed] [Google Scholar]
- 12.Travis EL. The sequence of histological changes in mouse lungs after single doses of x-rays. Int J Radiat Oncol Biol Phys. 1980;6(3):345–7. doi: 10.1016/0360-3016(80)90145-5. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh SN, Zhang R, Fish BL, et al. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75(5):1528–36. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward WF, Lin PJ, Wong PS, et al. Radiation pneumonitis in rats and its modification by the angiotensin-converting enzyme inhibitor captopril evaluated by high-resolution computed tomography. Radiat Res. 1993;135(1):81–7. [PubMed] [Google Scholar]
- 15.Molteni A, Wolfe LF, Ward WF, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13(13):1307–16. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 16.Gross NJ. The pathogenesis of radiation-induced lung damage. Lung. 1981;159(3):115–25. doi: 10.1007/BF02713907. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Ghosh SN, Zhu D, et al. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: injury and recovery. Int J Radiat Biol. 2008;84(6):487–97. doi: 10.1080/09553000802078396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sealey JE, Laragh JH. How to do a plasma renin assay. Cardiovascular Medicine. 1977;2:1079–92. [Google Scholar]
- 19.Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res. 2011;175(1):29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molthen RC, Wu Q, Krenz G, et al., editors. Imaging radiation pneumonitis in a rat model of a radiological terrorism incident; Medical Imaging: Biomedical Applications in Molecular, Structural, and Functional Imaging 2009; Feb. 8–12, 2009; Lake Buena Vista, FL. Bellingham, WA: SPIE; 2009. [Google Scholar]
- 21.Molthen RC, Karau KL, Dawson CA. Quantitative models of the rat pulmonary arterial tree morphometry applied to hypoxia-induced arterial remodeling. J Appl Physiol. 2004;97(6):2372–84. doi: 10.1152/japplphysiol.00454.2004. discussion 54. [DOI] [PubMed] [Google Scholar]
- 22.Linehan JH, Haworth ST, Nelin LD, et al. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol. 1992;73(3):987–94. doi: 10.1152/jappl.1992.73.3.987. [DOI] [PubMed] [Google Scholar]
- 23.Miska W, Croseck H, Schill WB. Proteins of the kallikrein-kinin system in human semen. Isolation and properties of kininase II. Andrologia. 1990;22 (Suppl 1):178–84. doi: 10.1111/j.1439-0272.1990.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 24.Shingrani R, Krenz G, Molthen R. Automation process for morphometric analysis of volumetric CT data from pulmonary vasculature in rats. Comput Methods Programs Biomed. 2010;97(1):62–77. doi: 10.1016/j.cmpb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yesil S, Yesil M, Bayata S, et al. ACE inhibitors and cough. Angiology. 1994;45(9):805–8. doi: 10.1177/000331979404500908. [DOI] [PubMed] [Google Scholar]
- 26.Coulter DM, Edwards IR. Cough and angiotensin converting enzyme inhibitors. Br Med J (Clin Res Ed) 1988;296(6625):863. doi: 10.1136/bmj.296.6625.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward WF, Solliday NH, Molteni A, et al. Radiation injury in rat lung. II. Angiotensin-converting enzyme activity. Radiat Res. 1983;96(2):294–300. [PubMed] [Google Scholar]
- 28.Ward WF, Kim YT, Molteni A, et al. Radiation-induced pulmonary endothelial dysfunction in rats: modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. 1988;15(1):135–40. doi: 10.1016/0360-3016(88)90357-4. [DOI] [PubMed] [Google Scholar]
- 29.Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173(4):557–78. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata M, Cowling RT, Gurantz D, et al. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol. 2005;289(6):H2356–63. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- 31.Liu YH, Yang XP, Sharov VG, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99(8):1926–35. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen L, Ward WF, Ts’ao CH, et al. Captopril inhibits proliferation of human lung fibroblasts in culture: a potential antifibrotic mechanism. Proc Soc Exp Biol Med. 1994;205(1):80–4. doi: 10.3181/00379727-205-43681. [DOI] [PubMed] [Google Scholar]
- 33.Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269 (Heart Circ Physiol 38):H1186–H94. doi: 10.1152/ajpheart.1995.269.4.H1186. [DOI] [PubMed] [Google Scholar]
- 34.Morrell NW, Atochina EN, Morris KG, et al. Angiotensin converting enzyme expression is increased in small pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. J Clin Invest. 1995;96:1823–33. doi: 10.1172/JCI118228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molthen R, Wu Q, Baumgardt S, Kohlhepp L, Shingrani R, Krenz G, editors. Arterial Morphology Responds Differently to Captopril than N-acetylcysteine in a Monocrotaline Rat Model of Pulmonary Hypertension; Medical Imaging: Biomedical Applications in Molecular, Structural, and Functional Imaging; February 13–18, 2010; San Diego, CA. Bellingham, WA. Bellingham, WA: SPIE; 2010. [Google Scholar]
- 36.McIff TE, Poisner AM, Herndon B, et al. Mitigating Effects of Captopril and Losartan on Lung Histopathology in a Rat Model of Fat Embolism. J Trauma. 2010 doi: 10.1097/TA.0b013e3181e50df6. [DOI] [PubMed] [Google Scholar]
- 37.Wang XM, Zhou TF, Liu B, et al. Changes of MMP-2,9 and TIMP-1 expressions in rats with pulmonary arterial hypertension after captopril and losartan interventions. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40(2):255–9. [PubMed] [Google Scholar]
- 38.Hislop A, Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1976;57(5):542–54. [PMC free article] [PubMed] [Google Scholar]
- 39.van Suylen RJ, Smits JF, Daemen MJ. Pulmonary artery remodeling differs in hypoxia- and monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1423–8. doi: 10.1164/ajrccm.157.5.9709050. [DOI] [PubMed] [Google Scholar]
- 40.Peterson LM, Evans ML, Graham MM, et al. Vascular response to radiation injury in the rat lung. Radiat Res. 1992;129(2):139–48. [PubMed] [Google Scholar]
- 41.Vanderpool RR, Kim AR, Molthen R, et al. Effects of acute Rho kinase inhibition on chronic hypoxia-induced changes in proximal and distal pulmonary arterial structure and function. J Appl Physiol. 2011;110(1):188–98. doi: 10.1152/japplphysiol.00533.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120(11):992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 43.Sniderman AD, Fitchett DH. Vasodilators and pulmonary arterial hypertension: the paradox of therapeutic success and clinical failure. Int J Cardiol. 1988;20(2):173–81. doi: 10.1016/0167-5273(88)90261-6. [DOI] [PubMed] [Google Scholar]
- 44.Arkles JS, Opotowsky AR, Ojeda J, et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183(2):268–76. doi: 10.1164/rccm.201004-0601OC. [DOI] [PubMed] [Google Scholar]
- 45.Okada K, Bernstein ML, Zhang W, et al. Angiotensin-converting enzyme inhibition delays pulmonary vascular neointimal formation. Am J Respir Crit Care Med. 1998;158(3):939–50. doi: 10.1164/ajrccm.158.3.9710007. [DOI] [PubMed] [Google Scholar]
- 46.Molteni A, Ward WF, Ts’ao C, et al. Monocrotaline-induced cardiopulmonary injury in rats: modification by the nonthiol ACE inhibitors CGS13945 and CGS16617. Arch Int Pharmacodyn Ther. 1988;291:21–40. [PubMed] [Google Scholar]
- 47.Jeffery TK, Wanstall JC. Perindopril, an angiotensin converting enzyme inhibitor, in pulmonary hypertensive rats: comparative effects on pulmonary vascular structure and function. Br J Pharmacol. 1999;128(7):1407–18. doi: 10.1038/sj.bjp.0702923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herget J, Pelouch V, Kolar F, et al. The inhibition of angiotensin converting enzyme attenuates the effects of chronic hypoxia on pulmonary blood vessels in the rat. Physiol Res. 1996;45(3):221–6. [PubMed] [Google Scholar]