Abstract
Little information is currently available regarding the pharmacokinetics of busulfan in infants and small children to help guide decisions for safe and efficacious drug therapy. The objective of this study was to develop an algorithm for individualized dosing of intravenous busulfan in infants and children weighing less than or equal to 12kg, that would achieve targeted exposure with the first dose of busulfan. Population pharmacokinetic modeling was conducted using intensive time-concentration data collected through the routine therapeutic drug monitoring of busulfan in 149 patients from 8 centers. Busulfan pharmacokinetics were well described by a 1-compartment base model with linear elimination. The important clinical covariates impacting busulfan pharmacokinetics were actual body weight and age. Based on our model, the predicted clearance of busulfan increases approximately 1.7-fold between 6 weeks to 2 years of life. For infants less than 5 months of age, the model-predicted doses (mg/kg) required to achieve the therapeutic Css range of 600–900 ng/mL (AUC range = 900–1350 uM·min) were much lower compared to standard busulfan doses of 1.1mg/kg. These results could help guide clinicians and inform better dosing decisions for busulfan in young infants and small children undergoing hematopoietic cell transplantation.
INTRODUCTION
The pharmacokinetics (PK) and pharmacodynamics of drugs in infants can differ widely between children and adults.(1–3) Within the first year of life, age-related developmental changes in physiologic and metabolic processes can significantly lead to altered drug disposition.(1, 4) Additionally, the relationship between dose, plasma concentration and pharmacodynamic effect may be highly variable across different age groups and disease states. The value of understanding therapeutic differences in drug response because of developmental factors is dependent on the ability to define a dose-concentration relationship.(5) Unfortunately, barriers unique to the pediatric population can make performing PK studies difficult, particularly in infants. Clinical therapeutic trials are often limited by ethical considerations, low study consent rates, limitations on blood volumes, and inadequate assay sensitivity.(6)
Busulfan (Busulfex®) is a bifunctional alkylating agent routinely used in conditioning regimens prior to hematopoietic cell transplantation (HCT) for the treatment of a variety of childhood malignant and nonmalignant disorders.(7) Despite the widespread use of busulfan, PK studies in infants remain inadequate to ensure safe and efficacious drug therapy. As defined by manufacturer’s guidelines, initial doses of busulfan are based upon actual body weight, aiming to achieve an area-under-the-curve (AUC) of 900–1350 uM·min (equivalent to a concentration-at-steady-state (Css) between 600 – 900 ng/mL)(Table 1).(7) This dose however, was based upon the results of a single clinical trial of only 24 children undergoing HCT who received busulfan in combination with cyclophosphamide.(7, 8) Patients ranged in age from 3 months to 16 years (mean 6.3 years) and included only 14 children less than or equal to 4 years of age.(7) More recently, several population PK studies in children have shown that individualized (e.g.personalized) model-based algorithms for busulfan clearance which incorporate body size and/or age provide improved targeted therapy when compared to stratified weight or age-based regimens alone.(9–13) Unfortunately, most published covariate models have included only a limited amount of data in infants and small children less than 12kg. Hence, the appropriateness of the extrapolation of these dosing algorithms to infants or very young children remains unclear.
Table 1.
Different PK Parameters Used in the TDM of Busulfan Expressed in Unit Equivalents
| PK parameter (units) | Equivalent value of the therapeutic range1 |
|---|---|
| Css (ng/mL) | 600 – 900 |
| AUC (uM·min) | 900 – 1350 |
| AUC (mg*hr/L) | 3.6 – 5.4 |
Equivalent values reflect the therapeutic range for a 6-hour dosing interval
Intervention with HCT very early in life is often considered critical to effectively treat several childhood diseases including many immunodeficiencies and genetic metabolic disorders.(14–17) Children with Severe Combined Immunodeficiency Disease (SCID), for instance, usually require definitive therapy with HCT soon after diagnosis. These children typically present early in life (<6 months of age). With newborn screening for SCID becoming increasingly available, children are now being diagnosed in the first 4 weeks of life, allowing HCT to be offered at a young age when outcomes are superior.(18) For children with Hurler’s Disease, a mucopolysaccharidosis, the younger a child is treated with HCT (the only effective treatment currently available), the better the overall outcome.(17) The inclusion of busulfan in the conditioning regimens of the very young is often desirable to promote stem cell engraftment, correct B cell functionality, and avoid long-term consequences of total body irradiation (TBI) including growth and developmental delay, poor jaw and tooth development, cataracts and increased risk of malignancy later in life.(19) Unfortunately, limited busulfan PK data are available in infants and very small children to guide dosing and ensure optimal drug therapy. The objective of this study was therefore to develop an algorithm for individualized dosing of busulfan in children less than 12kg that would achieve targeted exposure with the first dose of busulfan.
PATIENTS AND METHODS
Study Population
This retrospective study utilized PK data available from routine therapeutic drug monitoring of busulfan levels in 149 pediatric patients treated with HCT from multiple study centers. Eligibility criteria for busulfan PK analysis in this study included (1) an actual body weight less than or equal to 12kg, (2) related or unrelated HCT that included intravenous busulfan therapy, and (3) busulfan plasma time-concentration data available for analysis. Busulfan PK data were provided by three different study groups throughout the United States, Canada, Europe, and the United Kingdom (Table 2). Specific centers (n=8) within the three study groups contributing data for analysis included: The University of California San Francisco Benioff Children’s Hospital, Boston Children’s Hospital, University Medical Center Utrecht, Leiden University Medical Center, Royal Manchester Children’s Hospital, University of Manitoba, and Children’s Hospital of Westmead, Sydney. Local institutional review boards approved this study and written informed consent to undergo therapy and pharmacokinetic studies was obtained in all patients and guardians.
Table 2.
Patient Demographics and Baseline Characteristics By Study Group
| Median (range) / Number | |||
|---|---|---|---|
| Study Group A1 | Study Group B2 | Study Group C3 | |
| Number of subjects | 24 | 24 | 101 |
| Age (years) | 0.8 (0.1–3.0) | 0.7 (0.08–1.8) | 1 (0.1–3.3) |
| ≤ 6 months of age | 4 | 6 | 10 |
| > 6 months of age | 20 | 18 | 91 |
| Weight (kg) | 8.3 (3–12) | 6.9 (3.3–10.3) | 9.2(3.5–12) |
| ≤ 6 kg | 1 | 6 | 14 |
| > 6–12 kg | 23 | 18 | 87 |
| Male/Female4 | 11/13 | 16/8 | 43/41 |
| Height (cm) | 70 (51–88) | 65 (51–78) | 74 (51–123) |
| BSA (m2) | 0.4 (0.2–0.6) | 0.4 (0.2–0.5) | 0.4 (0.2–0.6) |
| Dose (mg) | 8.4 (2–17) | 7.35 (3.3–11) | 20(3.5–70) |
| Dose (mg/kg) | 0.97 (0.67–1.5) | 1.1 (0.6–1.1) | 4.1(0.8–7.5) |
| Dosing interval | Q6hours | Q6hours | Q6hours or Q24h |
University of San Francisco Benioff Children’s Hospital
Boston Children’s Hospital
Combined data from six collaborative centers (reference 9): University Medical Center Utrecht, Leiden University Medical Center, Royal Manchester Children’s Hospital, Newcastle upon Tyne Hospital, University of Manitoba, and the Children’s Hospital at Westmead, Sydney
Gender was not reported in 17 subjects
Detailed information regarding the preparative regimen, diagnoses, timing of pharmacokinetic sampling, and bioanalytical analysis has been previously described.(9, 20–22) Briefly, patients underwent HCT for a wide variety of malignant and nonmalignant pediatric disorders. HCT preparative regimens included busulfan along with different chemotherapeutic agents according to different study sites and diagnoses. Busulfan was administered intravenously in all subjects. In the majority of patients, busulfan was administered every 6 hours or every 24 hours (once-daily) over a period of 3 to 4 days. As part of routine clinical care, busulfan plasma concentrations were therapeutically monitored and dose adjustments made to achieve individual protocol specific targets.
Population PK Analysis
Pharmacokinetic model development using busulfan plasma concentration-time data was performed with the nonlinear mixed effects modeling program NONMEM (version 7, ICON Development Solutions, Ellicott City, MD). Diagnostic graphics and post-processing of NONMEM output and simulations were performed using the statistical software R and Xpose. The first order conditional estimation method with interaction (FOCE-I) was used throughout the model building process to estimate PK parameters and variability. Model development was guided by exploratory analysis of the data, changes in the NONMEM objective function value (OFV), diagnostic plots, and the potential biological plausibility of a relationship between clinical covariates and pharmacokinetic parameters. Because many subjects had intensive sampling on more than one occasion, inter-occasion variability was investigated. Residual unexplained variability was characterized by an additive and proportional error model. Using standard principles of allometric scaling, weight was built into the base model a prior and scaled to a reference patient having a median weight of 8kg.(23) The model was parameterized in terms of clearance (CL) and volume of distribution in the central compartment (Vc).
Patient specific factors considered for covariate testing included age, height, body surface area, and sex. Different covariate relationships on PK parameters were investigated and included power, linear, and exponential functions. Correlations between covariates were also investigated. The final PK model was built through the stepwise covariate model building process of forward selection and backward elimination of clinical covariates. The likelihood ratio test was used to assess the significance of all covariates in the final model. During forward selection, covariates were univariately tested and deemed significant if the OFV decreased by at least 3.84 (χ2, P ≤ 0.05, df = 1) with its inclusion in the model. During backward elimination, significance of the covariates were confirmed by removing one at a time from the full model and required an increase in the OFV of at least 5.99 (χ2, P ≤ 0.01, df = 1) to remain in the model.
Model Evaluation
To evaluate the precision of the final model parameter estimates, a nonparametric bootstrap was performed. A total of 1000 bootstrap datasets were generated by repeated sampling with replacement from the original data and the final PK model fitted to each of the bootstrap datasets. The median, 5th and 95th percentiles were then obtained for each PK parameter and compared with the final model PK estimates. For the visual predictive check (VPC), 500 datasets using the covariate distributions from the original dataset were simulated using the parameter estimates from the final model and the median, 5th and 95th percentiles compared with observed concentrations. Individual VPCs for every 6-hour dosing and once-daily administration were performed and are presented.
Determination of Targeted Dose
Based on our final model, simulations were performed to achieve the conventional therapeutic targets for busulfan exposure. Additionally, simulated Css values based on conventional dosing were compared to the model-based strategy for achieving conventional exposure. The model-based equation used for the simulations was:
Model-based doses were calculated to achieve the midpoint AUC corresponding to a targeted Css range for conventional exposure. Expressed in terms of mg, conventional exposure as proposed by the manufacturer’s guidelines was defined as an AUCtarget of 4.5 mg*hr/L (range, 3.6–5.4 mg*hr/L) over a 6-hour dosing interval. This target is equivalent to a Css of 750ng/mL (range, 600–900ng/mL) and AUC of 1098 uM·min (range, 900–1350uM·min) or 4.5 mg*h/L (range, 3.6 – 5.4mg*h/L). The equation AUC /dose was used to calculate Css.
RESULTS
Demographics of Patients
Patient demographics and baseline characteristics by study group are presented in Table 2. Among the 149 study subjects, the overall median age of patients was 0.94 years (11 months, range 0.1–3.3 years), with 14% of subjects younger than 6 months of age. Overall median actual body weight was 8 kg (range 3–12) and included 14% of children weighing less than or equal to 6 kg. Doses normalized to body weight were variable with higher doses reflective of once-daily versus every 6 hour administration.
Population PK Model Building
A total of 1247 quantifiable concentrations were available for population PK modeling and were best described with a 1-compartment base model with linear elimination. The range of observations was 25–8778 ng/mL. Irrespective of each individual center specific assay, <1% of busulfan plasma concentrations were below the level of quantification and were included in the analysis. A one-compartment model provided an adequate fit to the data. Addition of the second disposition compartment and/or non-linear elimination did not offer further improvement. Inter-occasion variability improved the model markedly (ΔOFV>222, p<10−50). Separate models were developed for different dosing schedules to examine potential differences between once daily and every 6-hour dosing, however none were detected. Different residual error models were allowed for different centers, to accommodate potential between-center differences in assay errors.
Effects of Covariates on Busulfan CL
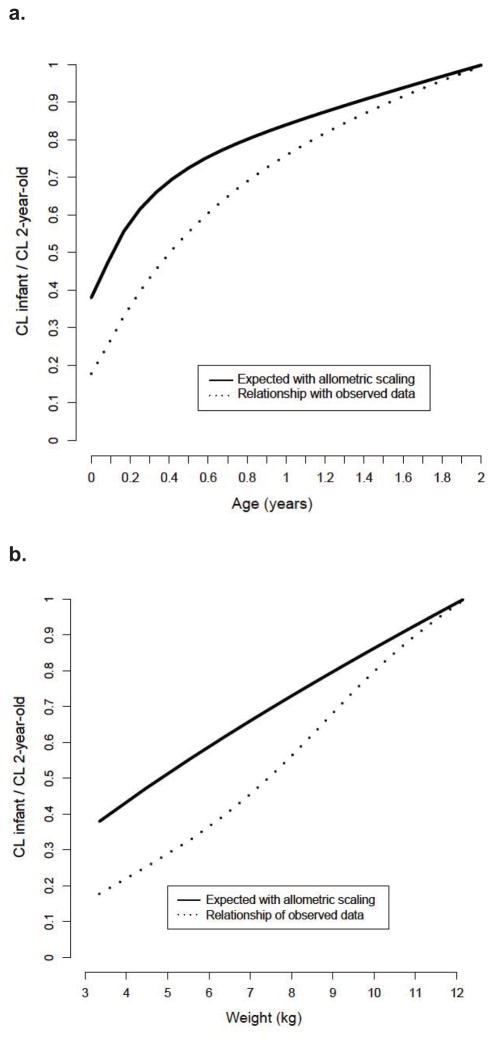
Important patient-specific covariates found to significantly impact busulfan CL were actual body weight and age. All covariates identified were supported by individual Bayesian PK parameter estimates versus covariate plots. Implementing allometric scaling of PK parameters greatly improved the model, however, it was not sufficient to describe the observed growth dependent changes in clearance in very young children (Figure I). Estimated CL values suggested that younger children have lower CL compared to the value anticipated by allometric scaling only. The addition of a nonlinear function of clearance versus age was implemented to describe maturation of clearance and this function was significant (p<0.001) and beneficial for the model. No significant impact of other covariates on busulfan CL was identified.
Figure I.
Fraction of busulfan clearance for infants compared to an average 2-year-old child by (a) change in busulfan clearance verses weight and (b) change in busulfan clearance verses age.
Final Population PK Model
The population PK parameters estimates and their relative standard errors (%) from the final model are presented in Table 3. The final model for busulfan CL incorporating both a weight and maturation effect were as follows:
where 2.3 L/h is the typical value of busulfan CL, Matmag is the estimated maturation magnitude effect for age on clearance and Kmat is the maturation rate constant for the effect of age on clearance. The goodness of fit plots for the base and final model showed clear improvement with good distribution of population-predicted concentration around the line of unity indicating the data were adequately described by the final model (data not shown). Ninety-five percent of conditional weighted residuals fell within 2 standard deviations demonstrating good predictability of the model. No trend in the residuals was observed.
Table 3.
Final population PK model parameter estimates and bootstrap results
| Final Model Results | Bootstrap Results | ||||
|---|---|---|---|---|---|
| Population PK Parameters | Units | Typical Value Estimates1 | RSE(%)2 | Median Value | 95% Confidence Interval |
| CL, clearance | L/hr | 2.3 | 6 | 2.3 | 2.1–2.6 |
| Exponent for effect of weight on CL | 0.75 (fixed) | - | 0.75 (fixed) | - | |
| Exponent for effect of weight on Vc | 1 (fixed) | - | 1 (fixed) | - | |
| Vc, volume of central compartment | L/kg | 6.4 | 2 | 6.4 | 6.1–6.7 |
| Maturation magnitude (Matmag) | fraction | 0.46 | 9 | 0.45 | 0.36–0.55 |
| Maturation rate constant (kmat) | 1/year | 1.4 | 27 | 1.4 | 0.8–2.3 |
| Inter-individual variability (IIV)3 | |||||
| IIV for CL, %CV | 25 | 14 | 26 | 23–29 | |
| IIV for Vc, %CV | 25 | 20 | 24 | 18–29 | |
| Correlation CL-V | 0.74 | 20 | 0.64 | 0.58–0.69 | |
| Residual variability, %CV3 | |||||
| Proportional, study group A | 8 | 20 | 8 | 5–12 | |
| Proportional, study group B | 12 | 17 | 11 | 7–16 | |
| Proportional, study group C | 16 | 12 | 16 | 14–18 | |
| Additive, study group A | ng/mL | 46 | 9 | 61 | 33–84 |
| Additive, study group B | ng/mL | 63 | 15 | 62 | 39–87 |
| Additive, study group C | ng/mL | 17 | 7 | 17 | 11–23 |
Mean typical value of the PK parameter in the final model,
Relative standard error (%),
Presented as CV % (the ratio of the standard deviation to the mean).
Model Evaluation
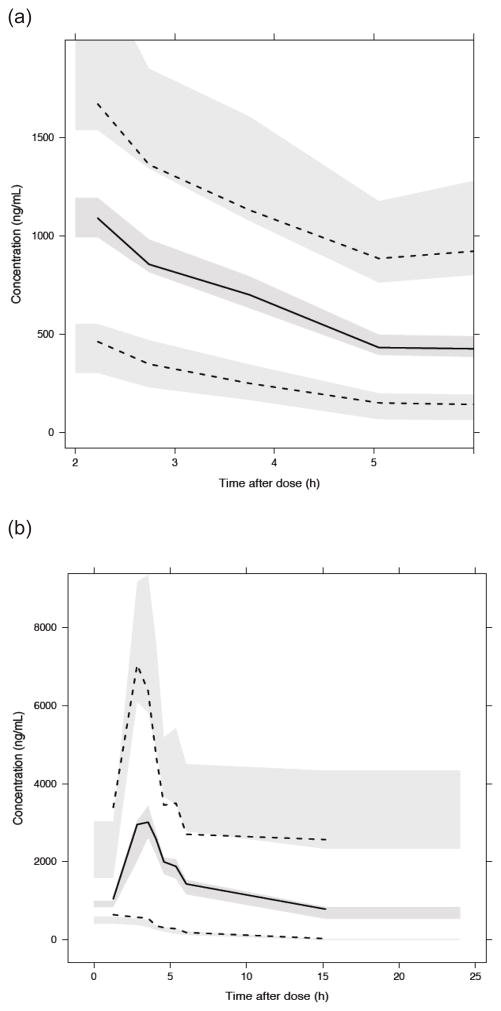
The median PK parameter estimates and 95% confidence intervals from the bootstrap analysis are presented in Table 3. Median estimates of PK parameters, inter-patient variability, and residual unexplained variability derived from the bootstrap analysis were comparable with the typical values derived from the original population PK analysis. The visual predictive check showed the median and percentiles of 500 simulated datasets captured the median and percentiles of the original observed PK data well for both dosing schedules, every 6-hours and once-daily (Figure II).
Figure II.
Visual Predictive Check for (a) every 6-hour dosing and (b) once-daily administration. Tick solid line is data median. Upper and lower dashed lines are 97.5th and 2.5th data percentile. Upper, middle and lower shaded areas are simulated 97.5th percentile, median and 2.5 the percentile with uncertainty, respectively. Appropriate model fit is indicated if lines are contained within shaded areas.
Model-Based Dosing to Achieve Conventional Therapeutic Target
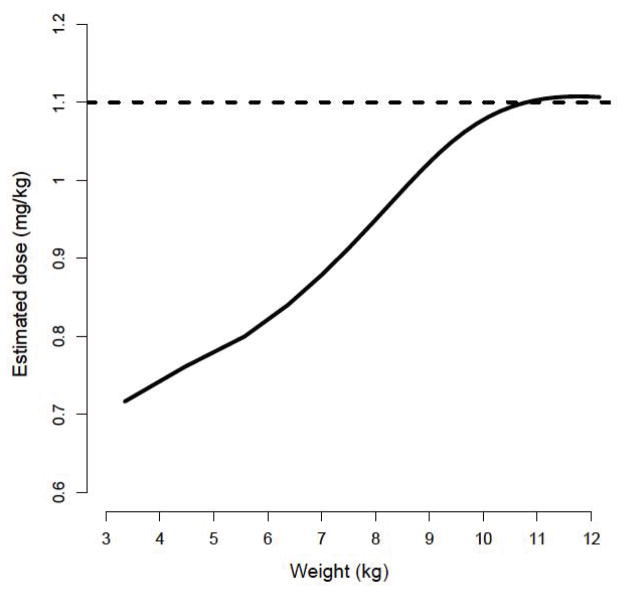
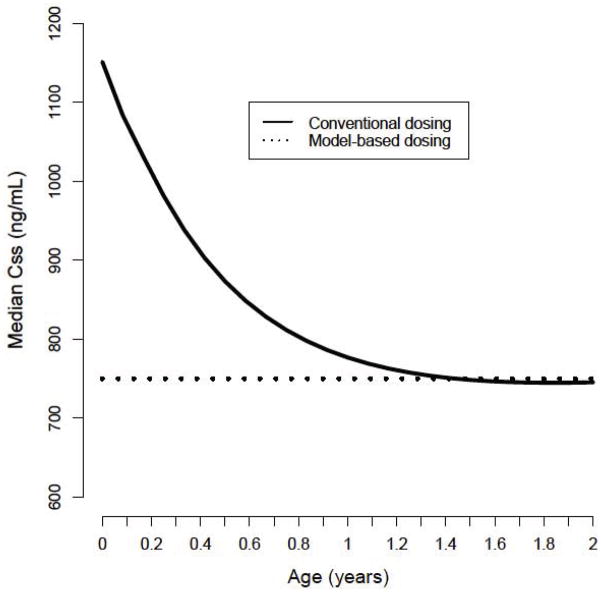
Doses (per actual body weight) for achieving conventional exposure (600–900ng/mL) were simulated and estimated using the model-based algorithm. In particular, children less than 10kg required lower doses with the model-based algorithm when compared to conventional dosing recommending 1.1mg/kg (Figure III). Table 4 provides the estimated doses (mg/kg) required for a typical 6-week, 3-month and 6-month individual to achieve therapeutic exposure at steady-state using the model-based algorithm. For example, the model-predicted dose needed to achieve a Css of 750ng/mL for 6-week old infant weighing 4.5 kg would be 3.6mg (0.79mg/kg), representing a decrease of approximately 28% compared to conventional dosing. Figure IV demonstrates conventional dosing vs the model-based algorithm for achieving a Css of 600–900ng/mL by age. With the conventional dosing algorithm, children less than approximately 5 months of age are more likely to achieve Css values above the recommended threshold of toxicity (900ng/mL) verses model-based dosing.
Figure III.
Model-based estimated dose (mg/kg) required to achieve a therapeutic Css of 600–900ng/mL (solid line). For comparison, the dashed line represents conventional dosing of 1.1mg/kg in children less than 12kg.
Table 4.
Estimated doses for a typical 6-week, 3-month and 6-month individual to achieve therapeutic exposure at steady-state using the model-based algorithim1
| Age of individual | |||
|---|---|---|---|
| Therapeutic target (Css 600–900 ng/mL)2 | 6 weeks | 3 months | 6 months |
| Weight (kg) | 4.5 | 6.4 | 8.4 |
| Dose (mg/kg) | 0.79 | 0.84 | 0.97 |
| Dose (mg) | 3.6 | 5.4 | 8.1 |
Based on an “average” estimate of weight per age; provided by the WHO growth standards for infants and children 0–2 years of age.
Doses are based on achievement of the desired therapeutic Css over a 6-hour dosing interval. An estimated Css of 750ng/ml (range 600–900ng/mL is equivalent to an AUC of 1098 uM·min (range 900–1350uM·min) or 4.5 mg*h/L (range, 3.6–5.4 mg*h/L).
Figure IV.
Plot demonstrating estimated Css with conventional dosing vs the model-based algorithm for achieving a therapeutic target of 600–900ng/mL by age. Currently recommended dose (1.1mg/kg) is shown for reference (horizontal dashed line)
DISCUSSION
To our knowledge, this is the largest PK analysis comprised exclusively of children weighing less than or equal to 12kg and receiving busulfan as part of their conditioning regimen for HCT. Prior to these results, very little information was available regarding the PK of busulfan in young infants and small children to help determine initial doses for drug therapy. In this study, we developed a population PK model for busulfan CL using time-concentration data collected through routine therapeutic drug monitoring from multiple study centers. Plasma concentrations of busulfan were well described by a one-compartment model with linear elimination. In our covariate analysis we found both weight and age to be significant patient-specific factors impacting busulfan CL.
Investigating age-related changes that occur as a result of physiological and enzymatic processes can make the PK modeling of drugs in infants and small children particularly challenging.(5) Precise quantification of maturation processes requires a large sample size of children with a wide range of weight and age combinations. The allometric scaling of weight provides a mechanistic and physiologic-based approach that if used a priori, allows for the delineation of the effect of size from other covariates that show a high degree of correlation.(24) By choosing weight as our primary covariate and by assuming the exponent of ¾, we were able to identify the impact of maturation on busulfan CL. Other authors may chose to estimate the exponent for the effect of weight on CL instead of fixing it to the physiological value of 0.75.(8, 9, 13, 25) Even though this approach may provide a good fit to the model and may result in the most parsimonious model, mechanistic interpretation of such parameters is limited. Having both weight and age as part of our model, we account not only changes in drug clearance due to body size and liver blood flow, but also for the maturation of enzymes, which is best described as a function of age. Additionally, the use of body surface area has previously been suggested as a predictor of busulfan clearance.(10, 26) Body surface area is a derived parameter from height and weight and as such, limits physiological interpretation of such models.
Based on our model, the predicted CL of busulfan increases approximately 1.7-fold between 6 weeks to 2 years of life. Busulfan undergoes extensive metabolic conversion in the liver through conjugation with glutathione by glutathione s-transferases (GST) enzymes, predominantly via GSTA1, and minor contributions from GSTM1 and GSTP1.(27, 28) Expression of GST enzymes involved in busulfan metabolism can undergo significant changes in activity and/or expression, increasing gradually over the first 2 years of life.(1, 27, 29) It is plausible that the variability in PK demostrated between infants, children and adolescents/adults is due to differences in GST activity and/or expression with age. To date, no formal studies investigating the relationship between busulfan drug levels and the ontogeny of hepatic GSTs in the very young have been reported. Additionally, given that busulfan undergoes extensive metabolic conversion in the liver it is plausible that prematurity may significantly impact busulfan exposure. Unfortunately, we were unable to obtain individual information on gestational age, and therefore, the appropriateness of the application of our model to preterm infants is unknown and should be used with caution.
Strategies for the TDM of busulfan differ among treatment centers, such as Css or AUC accumulative exposure over the entire course of therapy.(7, 30) The incorporation of age and weight relationships into our model for busulfan CL enables all individuals to have the same likelihood of reaching the desired therapeutic exposure, irrespective of the selected targeted goal. Particularly for children < 5 months of age, the model-based algorithm demonstrates significant improvement over conventional dosing, given these children are more likely to experience drug concentrations above the therapeutic threshold for toxicity with standard dosing. The model-predicted doses required to achieve the therapeutic range of 600–900 ng/mL in children <5 months of age are much lower when compared to standard doses of 1.1mg/kg. Improved dosing strategies for busulfan in infants would be expected to reduce morbidity and mortality through improved rates of stem cell engraftment and less drug-related toxicity (e.g. hepatic sinusoidal obstructive syndrome). Furthermore, infant survivors of HCT can experience significant life-long consequences associated with high-dose chemotherapy, including impaired pulmonary function, hypothyroidism, metabolic syndrome and cognitive impairment.(31) Individualized busulfan therapy has the potential to minimize risk for severe long-term complications attributable to chemotherapy in survivors of pediatric HCT and improve overall quality of life. This is particularly true in patients with non-malignant disorders in which, depending on the disease and goal for degree of stem cell chimerism, lower levels of busulfan may be possible.
Although the PK parameters were well estimated with our final model, the between-subject variability remained modest, at approximately 25%. This suggests other covariates not evaluated in this analysis may be important determinants of busulfan CL. Physiological changes induced by specific disease states, including inborn errors of metabolism and thalassemia have been demonstrated to alter busulfan CL.(32–34) Given the heterogeneity of diseases included in our study population, covariate analysis of different disease groups was not feasible. Similarly, the impact of medications previously shown to alter busulfan PK through the induction or inhibition of GSTs could not adequately be investigated. Information regarding the co-administration of the known enzyme inducer phenytoin was not available for a majority of subjects. Drug-drug interactions between busulfan, azole anti-fungals and metronidazole have been shown to alter busulfan CL through the inhibition of metabolic enzymes but were also unable to be evaluated.(35, 36) Other potential factors unexplored in this analysis include genetic variants of genes involved in busulfan metabolism and disposition. In vitro studies have shown variants in GSTP1 result in functional alterations in activity leading to decreased enzymatic activity.(37) Clinically, the impact of several GST genetic variants on busulfan exposure has been investigated, reporting variable results.(38–41) Furthermore, genomic studies in children can prove difficult to conduct given the effects of ontogeny on drug metabolizing enzymes and require careful consideration.(42)
Model validation, including prospective evaluation, is critical to ensure the predictability of any model. To adequately investigate the effects of maturation on metabolic pathways a large number of subjects spanning a wide range of ages are required. Therefore, for this analysis we elected to build the model using all data available (vs data splitting) and model evaluation performed using a nonparametric bootstrap. Currently we are collecting additional retrospective data, which will serve as an external validation data set. Additionally, the authors are in the early stages of planning a formal prospective evaluation of the busulfan algorithm in very young children undergoing transplantation early in life for the treatment of SCID. This work will be presented in subsequent publications in the future.
In summary, we developed a model for busulfan CL based on age and weight that can be used to determine initial doses in infants and young children less than or equal to 12kg. When compared to the conventional dosing guidelines, our individualized model-based algorithm may provide an improved dosing strategy for achieving targeted busulfan exposure in infants.
Acknowledgments
We would like to thank the Primary Immune Deficiency Treatment Consortium and its members for their contributions to the dataset.
Footnotes
CONFLICT OF INTEREST
Peter J Shaw (Expert for Orphan Australia Pty, to assist in registration of IV Busulfex in Australia, June 2006; member of Busulfex Global Steering Committee, Otsuka Pharmaceutical Development and Commercialization, Inc, 2009, 2010)
FINANCIAL DISCLOSURE STATEMENTS
This work was supported in part by NIH-NIAID grant U54 AI082973 (MJC and CCD) and NIH-NIAID grant R13 AI094943 (MJC).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. doi: 10.1056/NEJMra035092. Epub 2003/09/19. [DOI] [PubMed] [Google Scholar]
- 2.van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. Handbook of experimental pharmacology. 2011;205:51–75. doi: 10.1007/978-3-642-20195-0_2. Epub 2011/09/02. [DOI] [PubMed] [Google Scholar]
- 3.Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45(11):1077–97. doi: 10.2165/00003088-200645110-00003. Epub 2006/10/20. [DOI] [PubMed] [Google Scholar]
- 4.Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2):250–67. doi: 10.1016/j.pharmthera.2008.02.005. Epub 2008/04/15. [DOI] [PubMed] [Google Scholar]
- 5.Barrett JS, Della Casa Alberighi O, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clinical pharmacology and therapeutics. 2012;92(1):40–9. doi: 10.1038/clpt.2012.64. Epub 2012/06/07. [DOI] [PubMed] [Google Scholar]
- 6.Meibohm B, Laer S, Panetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7(2):E475–87. doi: 10.1208/aapsj070248. Epub 2005/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anonymous. IV Busulfex (Busulfan) Package Insert. Tokyo, Japan: Otsuka America Pharmaceutical, Inc; 2009. [Google Scholar]
- 8.Booth BP, Rahman A, Dagher R, Griebel D, Lennon S, Fuller D, et al. Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol. 2007;47(1):101–11. doi: 10.1177/0091270006295789. Epub 2006/12/29. [DOI] [PubMed] [Google Scholar]
- 9.Bartelink IH, Boelens JJ, Bredius RG, Egberts AC, Wang C, Bierings MB, et al. Body weight-dependent pharmacokinetics of busulfan in paediatric haematopoietic stem cell transplantation patients: towards individualized dosing. Clin Pharmacokinet. 2012;51(5):331–45. doi: 10.2165/11598180-000000000-00000. Epub 2012/03/30. [DOI] [PubMed] [Google Scholar]
- 10.Trame MN, Bergstrand M, Karlsson MO, Boos J, Hempel G. Population pharmacokinetics of busulfan in children: increased evidence for body surface area and allometric body weight dosing of busulfan in children. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(21):6867–77. doi: 10.1158/1078-0432.CCR-11-0074. Epub 2011/09/16. [DOI] [PubMed] [Google Scholar]
- 11.Bleyzac N, Souillet G, Magron P, Janoly A, Martin P, Bertrand Y, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28(8):743–51. doi: 10.1038/sj.bmt.1703207. Epub 2002/01/10. [DOI] [PubMed] [Google Scholar]
- 12.Tse WT, Duerst R, Schneiderman J, Chaudhury S, Jacobsohn D, Kletzel M. Age-dependent pharmacokinetic profile of single daily dose i.v. busulfan in children undergoing reduced-intensity conditioning stem cell transplant. Bone Marrow Transplant. 2009;44(3):145–56. doi: 10.1038/bmt.2008.437. Epub 2009/02/03. [DOI] [PubMed] [Google Scholar]
- 13.Paci A, Vassal G, Moshous D, Dalle JH, Bleyzac N, Neven B, et al. Pharmacokinetic behavior and appraisal of intravenous busulfan dosing in infants and older children: the results of a population pharmacokinetic study from a large pediatric cohort undergoing hematopoietic stem-cell transplantation. Therapeutic drug monitoring. 2012;34(2):198–208. doi: 10.1097/FTD.0b013e31824c2f60. Epub 2012/03/13. [DOI] [PubMed] [Google Scholar]
- 14.Lipstein EA, Vorono S, Browning MF, Green NS, Kemper AR, Knapp AA, et al. Systematic evidence review of newborn screening and treatment of severe combined immunodeficiency. Pediatrics. 2010;125(5):e1226–35. doi: 10.1542/peds.2009-1567. Epub 2010/04/21. [DOI] [PubMed] [Google Scholar]
- 15.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8. doi: 10.1182/blood.v99.3.872. Epub 2002/01/25. [DOI] [PubMed] [Google Scholar]
- 16.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. The Journal of allergy and clinical immunology. 2005;115(2):391–8. doi: 10.1016/j.jaci.2004.10.012. Epub 2005/02/08. [DOI] [PubMed] [Google Scholar]
- 17.Boelens JJ, Aldenhoven M, Purtill D, Ruggeri A, Defor T, Wynn R, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121(19):3981–7. doi: 10.1182/blood-2012-09-455238. Epub 2013/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddad E, Leroy S, Buckley RH. B-cell reconstitution for SCID: Should a conditioning regimen be used in SCID treatment? The Journal of allergy and clinical immunology. 2013;131(4):994–1000. doi: 10.1016/j.jaci.2013.01.047. Epub 2013/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulcahy Levy JM, Tello T, Giller R, Wilkening G, Quinones R, Keating AK, et al. Late effects of total body irradiation and hematopoietic stem cell transplant in children under 3 years of age. Pediatr Blood Cancer. 2013;60(4):700–4. doi: 10.1002/pbc.24252. Epub 2012/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law J, Cowan MJ, Dvorak CC, Musick L, Long-Boyle JR, Baxter-Lowe LA, et al. Busulfan, Fludarabine, and Alemtuzumab As a Reduced Toxicity Regimen for Children with Malignant and Nonmalignant Diseases Improves Engraftment and Graft-versus-Host Disease without Delaying Immune Reconstitution. Biol Blood Marrow Transplant. 2012;18(11):1656–63. doi: 10.1016/j.bbmt.2012.05.006. Epub 2012/05/23. [DOI] [PubMed] [Google Scholar]
- 21.Lai WK, Pang CP, Law LK, Wong R, Li CK, Yuen PM. Routine analysis of plasma busulfan by gas chromatography-mass fragmentography. Clinical chemistry. 1998;44(12):2506–10. Epub 1998/12/04. [PubMed] [Google Scholar]
- 22.Kellogg MD, Law T, Sakamoto M, Rifai N. Tandem mass spectrometry method for the quantification of serum busulfan. Therapeutic drug monitoring. 2005;27(5):625–9. doi: 10.1097/01.ftd.0000173372.04945.7b. Epub 2005/09/22. [DOI] [PubMed] [Google Scholar]
- 23.Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30(5):329–32. doi: 10.2165/00003088-199630050-00001. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 24.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug metabolism and pharmacokinetics. 2009;24(1):25–36. doi: 10.2133/dmpk.24.25. Epub 2009/03/03. [DOI] [PubMed] [Google Scholar]
- 25.Bartelink IH, van Kesteren C, Boelens JJ, Egberts TC, Bierings MB, Cuvelier GD, et al. Predictive performance of a busulfan pharmacokinetic model in children and young adults. Therapeutic drug monitoring. 2012;34(5):574–83. doi: 10.1097/FTD.0b013e31826051bb. Epub 2012/09/14. [DOI] [PubMed] [Google Scholar]
- 26.McCune JS, Baker KS, Blough DK, Gamis A, Bemer MJ, Kelton-Rehkopf MC, et al. Variation in prescribing patterns and therapeutic drug monitoring of intravenous busulfan in pediatric hematopoietic cell transplant recipients. J Clin Pharmacol. 2013;53(3):264–75. doi: 10.1177/0091270012447196. Epub 2013/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbs JP, Liacouras CA, Baldassano RN, Slattery JT. Up-regulation of glutathione S-transferase activity in enterocytes of young children. Drug metabolism and disposition: the biological fate of chemicals. 1999;27(12):1466–9. Epub 1999/11/26. [PubMed] [Google Scholar]
- 28.Hassan M, Ljungman P, Bolme P, Ringden O, Syruckova Z, Bekassy A, et al. Busulfan bioavailability. Blood. 1994;84(7):2144–50. Epub 1994/10/01. [PubMed] [Google Scholar]
- 29.Gibbs JP, Murray G, Risler L, Chien JY, Dev R, Slattery JT. Age-dependent tetrahydrothiophenium ion formation in young children and adults receiving high-dose busulfan. Cancer Res. 1997;57(24):5509–16. Epub 1998/01/04. [PubMed] [Google Scholar]
- 30.Eurpopean Group For Blood and Marrow Transplantation. EBMT/ESID guidelines for haematopoietic stem cell transplantation for primary immunodeficiencies. [cited 2013 June 24, 2013]; Available from: http://www.ebmt.org/Contents/About-EBMT/Who-WeAre/ScientificCouncil/Documents/EBMT_ESIDGUIDELINESFORINBORNERRORSFINAL2011.pdf.
- 31.Baker KS, Bresters D, Sande JE. The burden of cure: long-term side effects following hematopoietic stem cell transplantation (HSCT) in children. Pediatric clinics of North America. 2010;57(1):323–42. doi: 10.1016/j.pcl.2009.11.008. Epub 2010/03/24. [DOI] [PubMed] [Google Scholar]
- 32.Vassal G, Fischer A, Challine D, Boland I, Ledheist F, Lemerle S, et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. Blood. 1993;82(3):1030–4. Epub 1993/08/01. [PubMed] [Google Scholar]
- 33.Bertholle-Bonnet V, Bleyzac N, Galambrun C, Mialou V, Bertrand Y, Souillet G, et al. Influence of underlying disease on busulfan disposition in pediatric bone marrow transplant recipients: a nonparametric population pharmacokinetic study. Therapeutic drug monitoring. 2007;29(2):177–84. doi: 10.1097/FTD.0b013e318039b478. Epub 2007/04/10. [DOI] [PubMed] [Google Scholar]
- 34.Gaziev J, Nguyen L, Puozzo C, Mozzi AF, Casella M, Perrone Donnorso M, et al. Novel pharmacokinetic behavior of intravenous busulfan in children with thalassemia undergoing hematopoietic stem cell transplantation: a prospective evaluation of pharmacokinetic and pharmacodynamic profile with therapeutic drug monitoring. Blood. 2010;115(22):4597–604. doi: 10.1182/blood-2010-01-265405. Epub 2010/03/20. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson C, Aschan J, Hentschke P, Ringden O, Ljungman P, Hassan M. The effect of metronidazole on busulfan pharmacokinetics in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31(6):429–35. doi: 10.1038/sj.bmt.1703896. Epub 2003/04/01. [DOI] [PubMed] [Google Scholar]
- 36.Buggia I, Zecca M, Alessandrino EP, Locatelli F, Rosti G, Bosi A, et al. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo) Anticancer research. 1996;16(4A):2083–8. Epub 1996/07/01. [PubMed] [Google Scholar]
- 37.Hu X, Pal A, Krzeminski J, Amin S, Awasthi YC, Zimniak P, et al. Specificities of human glutathione S-transferase isozymes toward anti-diol epoxides of methylchrysenes. Carcinogenesis. 1998;19(9):1685–9. doi: 10.1093/carcin/19.9.1685. Epub 1998/10/15. [DOI] [PubMed] [Google Scholar]
- 38.Johnson L, Orchard PJ, Baker KS, Brundage R, Cao Q, Wang X, et al. Glutathione S-transferase A1 genetic variants reduce busulfan clearance in children undergoing hematopoietic cell transplantation. J Clin Pharmacol. 2008;48(9):1052–62. doi: 10.1177/0091270008321940. Epub 2008/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbasi N, Vadnais B, Knutson JA, Blough DK, Kelly EJ, O’Donnell PV, et al. Pharmacogenetics of intravenous and oral busulfan in hematopoietic cell transplant recipients. J Clin Pharmacol. 2011;51(10):1429–38. doi: 10.1177/0091270010382915. Epub 2010/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansari M, Krajinovic M. Can the pharmacogenetics of GST gene polymorphisms predict the dose of busulfan in pediatric hematopoietic stem cell transplantation? Pharmacogenomics. 2009;10(11):1729–32. doi: 10.2217/pgs.09.135. Epub 2009/11/07. [DOI] [PubMed] [Google Scholar]
- 41.Zwaveling J, Press RR, Bredius RG, van Derstraaten TR, den Hartigh J, Bartelink IH, et al. Glutathione S-transferase polymorphisms are not associated with population pharmacokinetic parameters of busulfan in pediatric patients. Therapeutic drug monitoring. 2008;30(4):504–10. doi: 10.1097/FTD.0b013e3181817428. Epub 2008/07/22. [DOI] [PubMed] [Google Scholar]
- 42.Leeder JS, Kearns GL, Spielberg SP, van den Anker J. Understanding the relative roles of pharmacogenetics and ontogeny in pediatric drug development and regulatory science. J Clin Pharmacol. 2010;50(12):1377–87. doi: 10.1177/0091270009360533. Epub 2010/02/13. [DOI] [PubMed] [Google Scholar]