Abstract
Background
Cardiac myocytes demonstrate significant swelling and associated reduced contractility in response to stress that is prevented by the adenosine triphosphate-sensitive potassium (KATP) channel opener, diazoxide (DZX) via an unknown mechanism. One proposed mechanism of cardioprotection is mitochondrial matrix swelling. To establish the relationship between mitochondrial and cellular volume during stress, this study examined the effect of DZX on mitochondrial volume.
Methods and Results
Isolated mouse mitochondria were exposed to the following solutions: Tyrode’s, Isolation Buffer (IB), cardioplegia (CPG) +/− DZX +/− KATP channel inhibitor, 5-hydroxydecanoate (5-HD), and metabolic inhibition (MI) +/− DZX +/− 5-HD. Mitochondrial volume was measured. DZX resulted in significant mitochondrial swelling (p<0.0001 vs Tyrode’s). MI and CPG resulted in significant mitochondrial swelling compared to baseline volume. The addition of DZX did not alter mitochondrial volume response to CPG (p=0.912) but increased swelling in response to MI (p=0.036).The addition of 5-HD to MI+DZX or CPG+DZX significantly reduced mitochondrial swelling (p<0.003 MI + DZX vs. MI + DZX + 5HD; p<0.001 CPG + DZX vs. CPG + DZX + 5HD).
Conclusions
Both cellular and mitochondrial volume increased during exposure to MI and CPG. DZX did not alter mitochondrial volume during CPG, however, it was associated with an increase in mitochondrial volume during MI. 5-HD reduced mitochondrial volume during exposure to both stresses with DZX supporting a role for a mitochondrial KATP channel in DZX’s mechanism of cardioprotection.
Keywords: cardiomyocyte, cardioplegia, myocardial ischemia, myocardial stunning, potassium channels
Introduction
In the presence of 3 different stresses (hyperkalemic cardioplegia, hypoosmotic stress, metabolic inhibition), cardiac myocytes demonstrate significant cell swelling and associated reduced contractility and may reflect one mechanism of myocardial stunning 1–5. These structural and functional derangements are prevented by adenosine triphosphate sensitive potassium (KATP) channel opener, diazoxide (DZX) 1–5. However, DZX’s exact mechanism of action remains undefined. It is widely believed that DZX works through a mechanism independent of the sarcolemmal KATP (sKATP) channel but through the purported mitochondrial KATP channel (mKATP) 6–9. One proposed hypothesis for DZX’s cardioprotective mechanism of action is through the mKATP channel regulation of the mitochondrial matrix volume 10–12.
Mitochondria are dynamic structures and the volume of the myocardial cell occupied by the mitochondria is correlated with the rate of energy utilization 13. Mitochondrial matrix volume comprises up to 35% of cellular volume and represents a large potential space 13–14. Under resting conditions, mitochondrial matrix is expanded with a narrow average intermembrane distance between the inner and outer mitochondrial membranes 14–15. Under stress conditions, mitochondrial matrix contracts, increasing the intermembrane distance 10. This contraction can be reversed and returned to near-normal state with the opening of mKATP channel 7. The primary regulator of mitochondrial matrix volume homeostasis is potassium (K+). K+ influx into the mitochondria carries with it inorganic phosphates (Pi), hydrogen, and water 7, 16. This is counterbalanced by K+ efflux via the K+/H+ antiporter. The maintenance of mitochondrial matrix volume homeostasis preserves vesicular integrity in the face of high inner mitochondrial membrane traffic of ions and water.
Mitochondria are vital to oxidative phosphorylation and the electron transport chain. Most enzymes that participate in oxidative phosphorylation are bound to the mitochondrial membrane in highly ordered structures that facilitate enzyme-membrane and enzyme-enzyme interactions 13. Within the electron transport chain, the generation of ATP utilizes the creation of a hydrogen gradient across the intermembrane space. ATP generated is then exported into the cytosol via a series of complexes (ATP/ADP translocator (ANT), mitochondrial creatinine kinase (mi-CK), and voltage-dependent anion channel (VDAC)) 17. Mi-CK bridges the space between the inner and outer mitochondrial membranes and is in close contact with ANT and VDAC, allowing for low conductance transfer of high energy phosphates. For this low conductance transfer to occur, there must be a narrow intermembrane space distance 6,10,14.
In the setting of ischemia, there is decreased mitochondrial K+ uptake resulting in an imbalance between K+ influx and efflux resulting in mitochondrial matrix contraction 6–7,14. This results in an increase in the intermembrane space resulting in increased distance among the intermembrane enzymes necessary for cellular function therefore compromising efficient energy transfer.
An increase in mitochondrial matrix volume has been demonstrated following exposure to mitochondrial KATP channel openers by multiple investigators 6,14,18–19. The primary function of mKATP channels is proposed to be regulation of mitochondrial volume. Open mKATP channels result in mitochondrial uptake of potassium and associated Pi, anions, and water and cells achieve a 15–20% increase in steady-state matrix volume in the mitochondria 10. This effect is inhibited by 5-hydroxydecanoate (5-HD), an inhibitor of the mKATP channel 7. The ratio of matrix water to cytosol water is approximately 1:4 and the influx of water into the mitochondria could cause depletion in cytosolic Pi, leading to an increased rate of reactive oxygen species (ROS) production, thereby benefiting the mycoyte 14. Therefore, most changes in the mitochondrial volume (as a result of mKATP channel opening) are reflected in a reciprocal change in the intermembrane space volume 7. The spatial relationship of enzymes and membranes in the mitochondria is not only reflective of cellular activity but is also vital to cellular function.
The relationship between cellular and mitochondrial matrix volume regulation has not been previously investigated. We hypothesize that DZX’s mechanism of action involves the mKATP channel and may involve mitochondrial matrix volume expansion as a compensatory mechanism following total myocyte volume increase during exposure to stress. Thus, by utilizing the huge potential space provided by the mitochondria, the cell maintains volume homeostasis. We have documented significant myocyte swelling following exposure to 3 stresses in 3 species, and therefore propose that adding a KATP channel opener would be associated with a subsequent increase in mitochondrial volume. This study was designed to investigate the relationship between myocyte and mitochondrial volume in response to stress (hyperkalemic cardioplegia and metabolic inhibition) with and without DZX and/or 5-HD.
Methods
All animal procedures were approved by the Animal Studies Committee at Washington University School of Medicine and all animals received humane care in compliance with the National Institute of Health’s Guide to Care and Use of Laboratory Animals 20.
Mitochondrial Isolation
Mitochondria were isolated from hearts of C57BL/6J mice. Mice (both sexes, 6–16 weeks old, average 24.8 grams) were anesthetized with 3% Avertin (0.3 grams 2,2,2-tribromoethanol, 0.186mL 2-methyl-2-butanol, 9.814mL sterile water) intraperitoneally and rapid cardiectomy was performed. Whole heart tissue was rapidly minced in cold buffer (in mM/L: 10 HEPES (N-[2-hydroxyethyl] piperazine-N-[4-butanesulfonic acid]), 1 EDTA-K2 (ethylene diamine tetraacetic acid potassium), 250 Sucrose, adjusted to a pH of 7.1 with 20% KOH and transferred to a 10mL glass tube with a teflon pestle (Glas-Col Homogenizer, Terre Haute, IN) and volume adjusted to 7mL with buffer. The tissue was mechanically homogenized at 120 rpm with 14–16 strokes/min for 1 minute on ice. The homogenate was transferred to 6 microcentrifuge tubes and centrifuged at 900 × g for 10 minutes at 4°C. Supernatant was combined into a clean test tube and mixed to get a homogeneous solution which was divided equally between 6 clean microcentrifuge tubes and centrifuged at 5000 × g for 15 minutes. One pellet was resuspended in 100µL buffer and 10µL were taken in duplicate for a Bradford protein assay (Thermo Scientific; Rockford, IL) to determine total protein. Each pellet was maintained on ice and was resuspended in test media volume to equal 0.3µg/µL.
Experimental Protocol
Isolation Buffer (IB) (in mM/L:10 HEPES, 1 EDTA-K2, and 250 sucrose, buffered to pH 7.1 with 20% KOH) was utilized as a control solution. Solutions of IB with 100µM DZX (Sigma; St. Louis, MO), 50µM Pinacidil (nonspecific KATP channel opener) (Sigma; St. Louis, MO), and 100µM 5-HD (Sigma; St. Louis, MO) were utilized to evaluate individual effects on mitochondrial matrix volume.
Additionally, mitochondria were perfused with 37°C physiologic Tyrode’s solution (in mM/L: 130 NaCl; 5 KCl; 2.5CaCl2; 1.2 MgSO4; 24 NaHCO3; 1.75 Na2HPO4; and 10 glucose) buffered to a pH of 7.4 using 95% O2-5% CO2 for 20 minutes. Tyrode’s was utilized as a control solution and more closely represents the extracellular environment. Additionally, 100µM DZX was added to Tyrode’s to evaluate the effect of DZX alone on mitochondrial matrix volume.
Mitochondria were exposed to stress solutions including hyperkalemic cardioplegia (CPG) and metabolic inhibition (MI) solution as these have been previously utilized to induce cellular stress 2,3,5. CPG is composed of Plegisol (Abbott Laboratories; North Chicago, IL) which contains (in mM/L): 110 NaCl, 16 KCl, 16 MgCl2, 1.2 CaCl2, equilibrated with 95% O2 and 5% CO2 and titrated to pH 7.3 with 8.4% NaHCO3. MI consisted of (in mM/L): 130 NaCl, 5 KCl, 2.5 CaCl2, 1.2 MgSO4, 24 NaHCO3, 1.75 Na2HPO4, 5 2-deoxyglucose, bubbled with 95% O2 - 5% CO2 and adjusted pH to 7.4, and 5 sodium cyanide (NaCN). NaCN is prepared as a stock solution of 250 mM/L in 670 mM/L HEPES. Experimental groups included: IB, CPG, MI, Tyrode’s, Tyrode’s+DZX, CPG+DZX, CPG+DZX+5-HD, MI+DZX, MI+DZX+5-HD, IB+DZX, IB+Pinacidil, and IB+5-HD.
Mitochondrial Matrix Volume Measurements
Mitochondrial matrix volume measurements were obtained using a light-scattering technique where the absorbance, at 520 nm, of a solution of isolated mitochondria was obtained every 2 minutes over a 20 minute time frame using UV Probe 2.33 (Shimadzu Scientific Instruments; Columbia, MD) and a spectrophotometer (UV-1700 Spectrophotometer, Shimadzu Scientific Instruments; Columbia, MD). This method is a well-established method for the estimation of mitochondrial matrix volume changes 21.
Each reaction was plotted as a change in absorbance (Y axis) versus time elapsed in minutes (X axis).
Statistical Analysis
Data were analyzed using SYSTAT 13 (SYSTAT Software Inc.; Point Richmond, CA). All data are presented as mean ± SEM, with n equal to the number of measurements in each group. A repeated-measures ANOVA with one factor design was used to compare values of change of absorbance over time (repeated measure) and between experimental solutions (factor). Post hoc multiple comparisons between different test groups were made separately for each test solution using contrast with a Dunn-Sidak correction when overall p values were significant for the ANOVA. P values are for the 20 min time point for the comparisons between experimental groups. P<0.05 was considered significant.
Results
Changes in mitochondrial matrix volume are represented as percent change in absorbance over time. Mitochondrial matrix swelling is inversely related to absorbance measured at 520 nm. Mitochondrial volume changes are summarized with previous findings in Figure 1.
Figure 1.
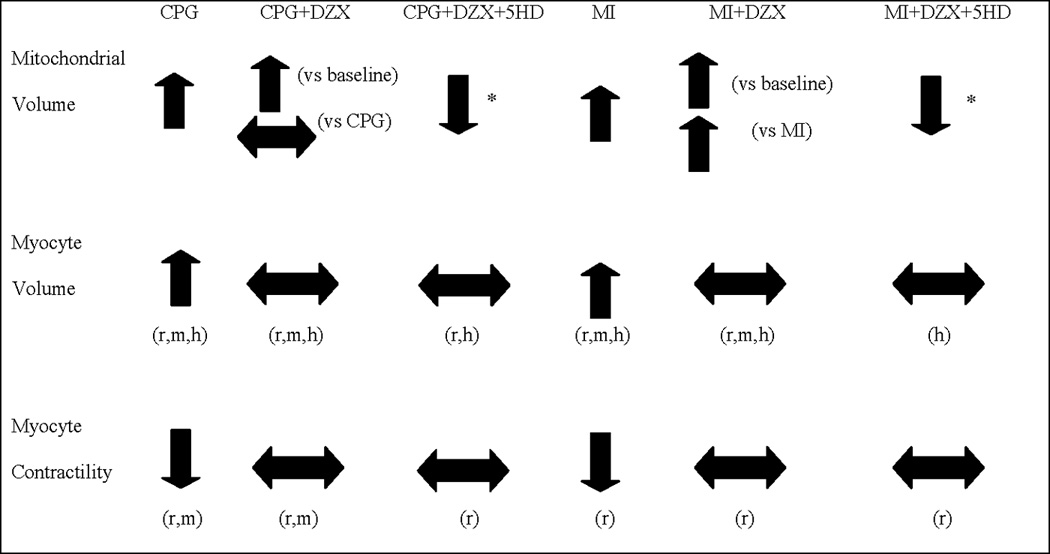
Cellular and mitochondrial volume derangements due to stress (CPG, MI) and the effect of DZX and 5-HD1–5, 9,24. Up arrow indicates increase from baseline, down arrow indicates decrease from baseline, and flat arrow indicates return to baseline. *Divergence from myocyte volume effect. Abbreviations: m=mouse r =rabbit h=human. Abbreviations: 5-HD=5-hydroxydecanoate, CPG= cardioplegia, DZX=diazoxide, MI=metabolic inhibition.
Mitochondria exposed to extracellular Tyrode’s solution demonstrated an average 15% change in absorbance from baseline, resulting in mitochondrial matrix swelling (Figure 2). The addition of DZX to Tyrode’s solution resulted in an 18% change in absorbance and increased matrix swelling compared to Tyrode's alone (p<0.001).
Figure 2.
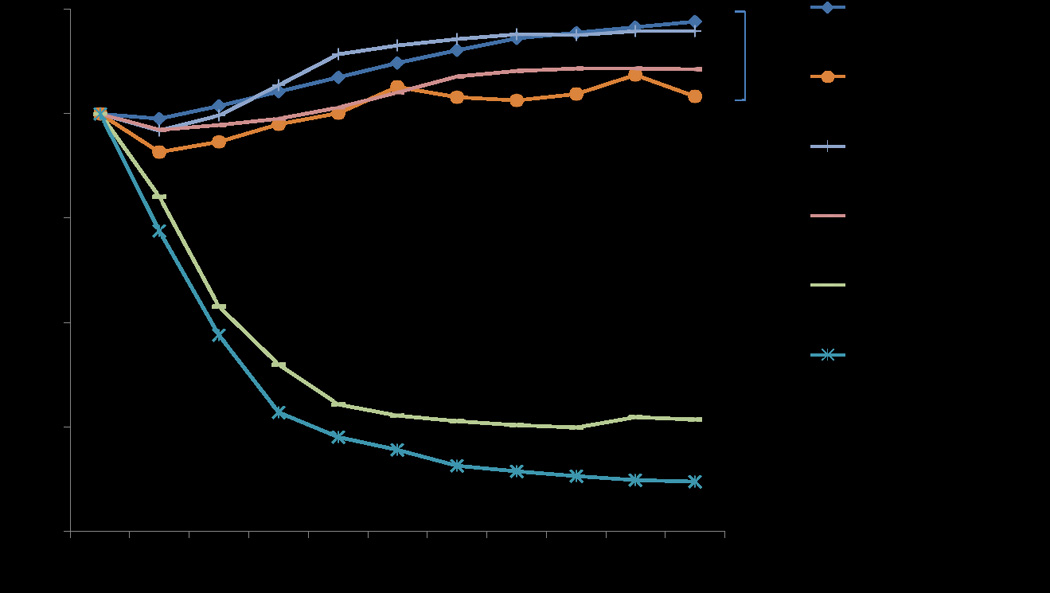
The effect of diazoxide and pinacidil on mitochondrial volume. The addition of DZX to Tyrode’s resulted in a significant increase in mitochondrial volume vs Tyrode’s alone. DZX did not result in a significant change in mitochondrial volume when added to IB. However, the addition of DZX to IB resulted in greater mitochondrial volume swelling compared to the addition of Pinacidil. Abbreviations: DZX=diazoxide, IB=isolation buffer.
Mitochondria exposed to isolation buffer (IB) demonstrated small changes in absorbance (average 4% change from baseline) representing non-significant changes in mitochondrial volume (Figure 2). The addition of DZX or Pinacidil produced a non-significant change in mitochondrial volume (p=0.077 and p=0.058, respectively vs IB). There was significantly greater mitochondrial swelling in IB+DZX compared to IB+ Pinacidil (p<0.001). The addition of 5-HD resulted in an increase in matrix volume compared to IB alone (p=0.016) and IB+Pinacidil (p<0.001). There was no significant difference between IB+DZX and IB+5-HD (p=0.085).
Mitochondria exposed to CPG resulted in an increase in mitochondrial volume (average percent change in absorbance of 17.2 % from baseline) (Figure 3). The addition of DZX did not result in a significant change in mitochondrial volume compared to CPG alone (p=0.912). The addition of 5-HD to CPG+DZX, however, did result in significant decrease in mitochondrial matrix swelling compared to CPG alone (p<0.001) and CPG+DZX (p<0.001).
Figure 3.
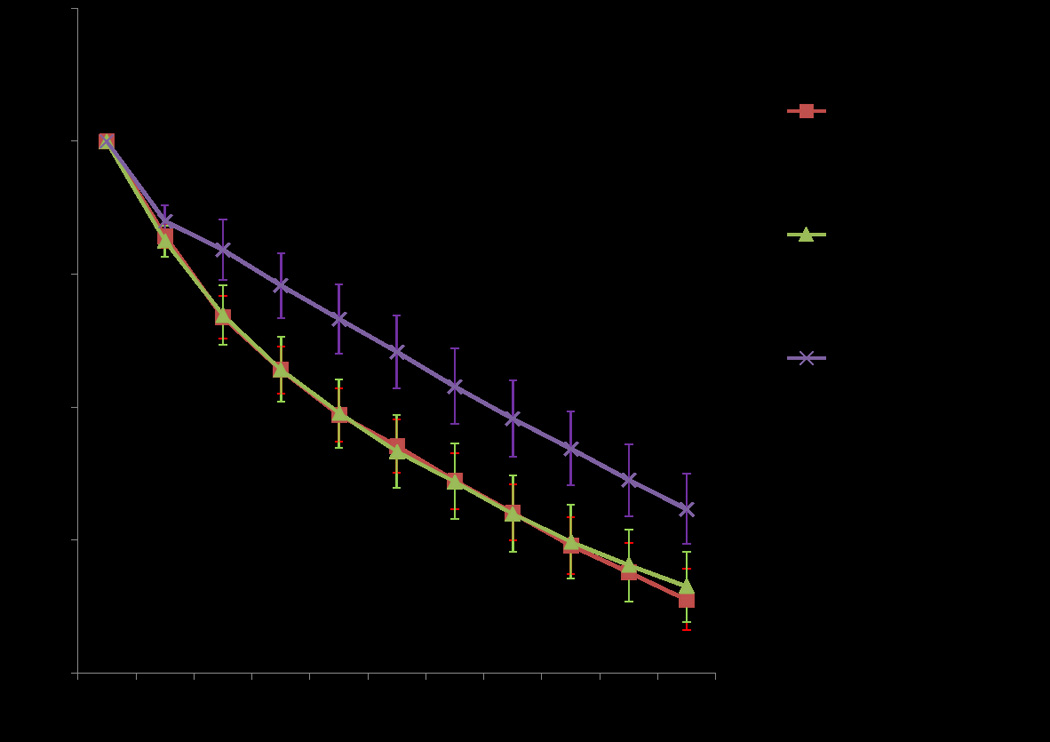
The effect of hyperkalemic cardioplegia on mitochondrial volume. CPG resulted in an increase in mitochondrial volume. The addition of DZX did not result in a significant change in mitochondrial volume. However, the addition of 5-HD to CPG+DZX resulted in a significant decrease in mitochondrial volume swelling compared to CPG alone. Abbreviations: 5-HD=5-hydroxydecanoate, CPG= cardioplegia, DZX=diazoxide.
All cardioplegia groups (CPG, CPG+DZX, and CPG+DZX+5-HD) resulted in significant mitochondrial swelling compared to IB (p< 0.001) and less swelling compared to Tyrodes+ DZX (p<0.0001), but no significant difference versus Tyrodes alone (p=0.275) (Figure 2, 3).
Mitochondria exposed to MI resulted in an increase in mitochondrial matrix volume with an average percent change of 21% from baseline (Figure 4). The addition of DZX did result in a statistically significant increase in mitochondrial volume compared to MI alone (p=0.036). The addition of 5HD to MI+DZX resulted in a statistically significant decrease in matrix volume compared to MI+DZX (p=0.003) and MI alone (p=0.026).
Figure 4.
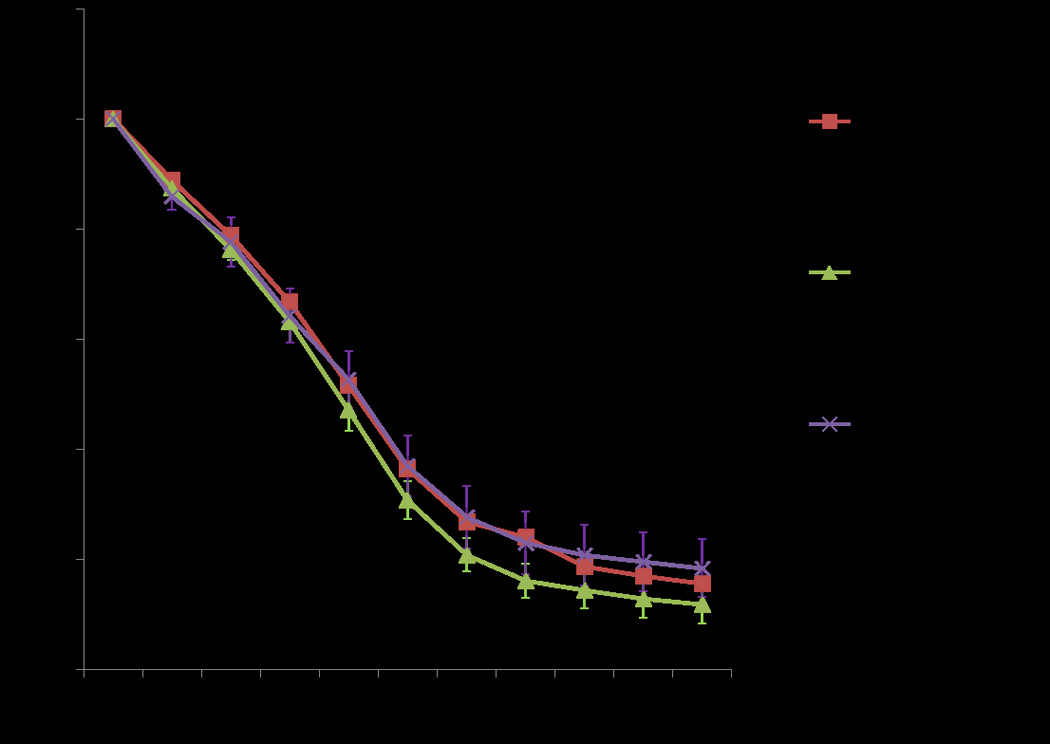
The effect of metabolic inhibition on mitochondrial volume. MI resulted in an increase in mitochondrial volume. The addition of DZX resulted in an additional significant increase in mitochondrial volume. The addition of 5-HD to MI+DZX resulted in a significant decrease in mitochondrial volume compared to MI alone and MI+DZX. Abbreviations: 5-HD=5-hydroxydecanoate, CPG= cardioplegia, DZX=diazoxide, IB=isolation buffer, MI=metabolic inhibition, Tyr =Tyrode’s.
All MI groups (MI, MI+DZX, MI+DZX+5-HD) demonstrated a statistically significant increase in mitochondrial matrix volume compared to IB (p<0.001) and Tyrode’s alone (p<0.001). Only the group MI+DZX resulted in significantly more mitochondrial volume swelling compared to the Tyrode’s+DZX group (p<0.001).
Discussion
We have documented derangements in myocyte volume and contractility secondary to exposure to stress (exposure to hyperkalemic cardioplegia, osmotic stress, and metabolic inhibition) as well as the ability to ameliorate these derangements with the addition of KATP channel opener DZX 1–5. These beneficial effects require the SUR1 subunit of a proposed mKATP channel 9 and recent work suggests the Kir pore-forming subunit may be the ROMK (renal outer medullary K+) inward rectifying K+ channel 8. A proposed mechanism of cardioprotection following opening of a purported mKATP channel is that of mitochondrial volume expansion.
The ability of mitochondria to compensate for volume change provides the cell with a potential reservoir or alternate location for the handling of excess water. The mitochondria are known to make up approximately 29–36% of cardiac myocyte volume 23 and a large volume change at the mitochondrial level may be sufficient to result in a change in total cellular volume. The relationship between myocyte and mitochondrial volume is unknown. Other investigators have proposed relationships between the two volumes and have suggested that mitochondrial swelling can comprise a significant portion of cell volume change and that the mitochondrial fraction increases to 40% of cytosolic volume in swollen cells 13,24. Ganote proposed 3 methods of how mitochondrial and cellular volume are related, noted an increase in both mitochondrial and cellular volume following ischemia, and documented that a reduction in either cytosolic or mitochondrial swelling would mediate decreased cell death 24.
The present study evaluated the relationship between cellular and mitochondrial volume in response to stress. Mitochondrial matrix volume remained unchanged during exposure to IB. The addition of DZX and Pinacidil to IB did not result in a significant change in mitochondrial matrix volume compared to IB alone. Physiologic Tyrode’s has been used previously as a control solution in myocyte volume experiments and demonstrated no significant change in myocyte volume 1–2, 4–5,22. Exposure of mitochondria to Tyrode’s resulted in significant mitochondrial volume swelling. The addition of DZX to Tyrode’s resulted in a significant increase in mitochondrial volume. Other investigators have documented significant mitochondrial volume expansion with exposure of KATP channel openers and have utilized mitochondrial volume change as a mKATP activity assay 7–8,19–20.
Tyrode’s likely resulted in tremendous mitochondrial swelling as it is a physiologic extracellular and not intracellular solution. The addition of DZX to both control solutions (Tyrodes and IB) resulted in an increase in mitochondrial volume (p<0.002 for Tyrode’s and p=0.077 vs IB) similar to work of others.
In response to stress, myocyte volume increases and an inverse relationship with contractility is observed 2–4. In the present study, the greatest increase in mitochondrial volume during stress was observed with MI (MI>Tyrodes>CPG). Thus, in response to stress, both cellular and mitochondrial volume significantly increase, which supports a hypothesis of a relationship between cellular volume homeostasis and mitochondrial volume.
The volume increase noted with MI was increased by the addition of DZX and decreased by the addition of 5-HD. In contrast, the mitochondrial volume increase observed with CPG was not increased by the addition of DZX; however, it was decreased by the addition of 5-HD. The differences observed in mitochondrial volume change in the present study may be due to the differences in the stress imposed upon the mitochondria. CPG results in a hypo-osmotic extracellular environment and depolarizes the cellular membrane; however upon exposure to isolated mitochondria it provides a lower potassium (16mM KCl) environment compared to the normal intracellular milieu (104–180mM potassium). MI results in a hyperosmotic intracellular environment 9 and inhibits oxidative phosphorylation and electron transport and provides a much lower environment for isolated mitochondria (5 mM KCl). These solutions may therefore illicit different responses at the mitochondrial level.
The observed effects of 5-HD (decreased swelling) suggest the mechanism behind mitochondrial matrix volume regulation involves a purported mKATP channel. Interestingly, previous work evaluating 5-HD at the cellular level did not alter observed myocyte swelling due to stress or its amelioration by DZX 2,22. These findings lend support to a mitochondrial location of action for DZX.
Ideally, the cardioprotection provided by diazoxide could be exploited during the ischemic stress (compounded by the stress of exposure to hyperkalemic cardioplegia) imposed upon the myocardium during cardiac surgery. We have shown that in the in vivo setting, the observed cardioprotection (maintenance of volume homeostasis and preserved contractility) requires KATP channel subunit SUR1 and involves the inhibition of succinate dehydrogenase. The present study is consistent with a location of action at the mitochondrial level. The clarification of the exact mechanism of diazoxide will significantly aid in its acceptance and translation in the clinical setting.
Limitations
It is unknown whether observed responses of isolated mitochondria to stress are similar to the responses of mitochondria within intact cells when exposed to the same stress. Ideally, mitochondria would be observed after exposing isolated cells to stress to correlate observations. Unfortunately, adequate amounts of viable mitochondria cannot be obtained utilizing this sequence.
Although the light scattering technique is the most commonly used technique for evaluating mitochondrial matrix volume changes, there is debate over its accuracy and reproducibility 7. Other investigators have reported changes in mitochondrial volume over a 3-minute period and the present study encompasses up to 20 minutes which may be responsible for the different observations 8.
Conclusion
The complex relationship between cellular and mitochondrial volume regulation is unknown. Both MI and CPG result in myocyte and mitochondrial volume swelling. There is divergence in DZX’s effect on myocyte and mitochondria volume during stress. On a cellular level, DZX exerts a cardioprotective effect on both MI and CPG exposed myocytes by providing volume homeostasis. However, at the subcellular level, DZX did not exert an additional effect on mitochondria exposed to CPG. The addition of 5-HD resulted in a decrease in observed mitochondrial volume supporting involvement of a purported mKATP channel in mitochondrial volume regulation.
Acknowledgments
Funding Sources: Thoracic Surgery Foundation for Research and Education Nina Starr Braunwald Career Development Award (JSL). American Heart Association Grant in Aid 09GRNT202045 (JSL). National Institutes of Health R01HL098182-01A1 (JSL). National Institutes of Health T325T32HL007776 (MMA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the American Heart Association Scientific Session, Los Angeles, CA, November 5, 2012
Conflict of Interest Disclosures: None
References
- 1.Mizutani S, Prasad SM, Sellitto AD, Schuessler RB, Damiano RJ, Lawton JS. Myocyte volume and function in response to osmotic stress: observations in the presence of an adenosine triphosphate-sensitive potassium channel opener. Circulation. 2005;112:219–223. doi: 10.1161/CIRCULATIONAHA.104.523746. [DOI] [PubMed] [Google Scholar]
- 2.Mizutani S, Al-Dadah AS, Bloch JB, Prasad SM, Diodato MD, Schuessler RB, Damiano RJ, Lawton JS. Hyperkalemic cardioplegia-induced myocyte swelling and contractile dysfunction: prevention by diazoxide. Ann Thorac Surg. 2006;81:154–159. doi: 10.1016/j.athoracsur.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 3.Prasad SM, Al-Dadah AS, Byrd GD, Flagg TP, Gomes J, Damiano RJ, Nichols CG, Lawton JS. Role of the sarcolemmal adenosine triphosphate sensitive potassium channel in hyperkalemic cardioplegia-induced myocyte swelling and reduced contractility. Ann Thorac Surg. 2006;81:148–153. doi: 10.1016/j.athoracsur.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 4.Al-Dadah AS, Voeller RK, Schuessler RB, Damiano RJ, Lawton JS. Maintenance of myocyte volume homeostasis during stress by diazoxide is cardioprotective. Ann Thorac Surg. 2007;84:857–863. doi: 10.1016/j.athoracsur.2007.04.103. [DOI] [PubMed] [Google Scholar]
- 5.Maffit SK, Sellitto AD, Al-Dadah AS, Schuessler RB, Damiano R, Jr, Lawton JS. Diazoxide maintains human myocyte volume homeostasis during stress. J Am Heart Assoc. 2012;1:1–8. doi: 10.1161/JAHA.112.000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HM, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover CJ. Cardioprotective effect of diazoxide and its interactions with mitochondrial ATP-sensitive K+ channels: possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 7.Garlid KD, Dos Santos P, Xie Z, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K+ channel in cardiac function and cardioprotection. Biochim Biophys Acta. 2003;1606:1–21. doi: 10.1016/s0005-2728(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 8.Foster DB, Jo AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, O’Rourke B. Mitochondrial ROMK channel is a molecular component of mitoKATP. Circ Res. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellitto AD, Maffit SK, Al-Dadah AS, Zhang H, Schuessler RB, Nichols CG, Lawton JS. Diazoxide maintenance of myocyte volume and contractility during stress: evidence for a non-sarcolemmal KATP channel location. J Thorac Cardiovasc Surgery. 2010;140:1153–1159. doi: 10.1016/j.jtcvs.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariossse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP-Sensitive K+ channel protects the ischemic heart. Am J Physiol Heart Circ Physiol. 2001;283:H284–H295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- 11.Costa ADT, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoKATP opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–H415. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 12.Kaasik A, Safiulina D, Zharkovsky A, Veksler V. Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol. 2007;292:C157–C163. doi: 10.1152/ajpcell.00272.2006. [DOI] [PubMed] [Google Scholar]
- 13.Katz AM. Physiology of the heart. 2nd edition. New York: Raven Press; 1992. pp. 23–28.pp. 98–118. [Google Scholar]
- 14.Bakeeva LE, Chentsov YuS, Skulachev VP. Mitochondrial framework in rat diaphragm muscle. Biochim Biophys Acta. 1978;501:349–369. doi: 10.1016/0005-2728(78)90104-4. [DOI] [PubMed] [Google Scholar]
- 15.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 16.Garlid KD, Paucek P. Mitochondrial potassium transport: the K+ cycle. Biochim Biophys Acta. 2003;1606:23–41. doi: 10.1016/s0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 17.Cross RL. Turning the ATP motor. Nature. 2004;427:407–408. doi: 10.1038/427407b. [DOI] [PubMed] [Google Scholar]
- 18.Lim KHH, Javadov SA, Das M, Clarke SJ, Suleiman M, Halestrap AP. The effects of ischaemic preconditioning, diazoxide, and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Opening of mitochondrial KATP channels enhances cardioprotection through the modulation of mitochondrial matrix volume, calcium accumulation, and respiration. Am J Physiol Heart Circ Physiol. 2004;287:H1967–H1976. doi: 10.1152/ajpheart.00338.2004. [DOI] [PubMed] [Google Scholar]
- 20.Institutes for Laboratory Animal Research, Division on Earth and Life Studies. 8th Edition. National Research Council, NIH; 2010. Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Guide to Care and Use of Laboratory Animals. [Google Scholar]
- 21.Beavis AD, Branna RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. J Biol Chem. 1985;260:13424–13433. [PubMed] [Google Scholar]
- 22.Sellitto AD, Al-Dadah AS, Schuessler RB, Nichols CG, Lawton JS. An open sarcolemmal adenosine triphosphate-sensitive potassium channel is necessary for detrimental myocyte swelling secondary to stress. Circulation. 2011;124:S70–S74. doi: 10.1161/CIRCULATIONAHA.110.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przyklenk K. Pharmacologic treatment of the stunned myocardium: the concepts and challenges. Coron Artery Dis. 2001;12:363–369. doi: 10.1097/00019501-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Ganote CE, Armstrong SC. Effects of CCCP-induced mitochondrial uncoupling and cyclosporine A on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J Mol Cell Cardiol. 2003;35:749–759. doi: 10.1016/s0022-2828(03)00114-7. [DOI] [PubMed] [Google Scholar]


