Abstract
Background
Because HIV-related neurocognitive impairment is usually mild and variable, clinical ratings (CR) and global deficit scores (GDS) are recommended for detecting HIV-associated neurocognitive disorders (HAND). The CR-approach requires impairment in at least two ability domains; GDS considers number and severity of impairments across all measures. We examined classification agreement and clinical correlates of the two methods.
Method
Neurocognitive functioning of 1574 HIV-infected participants was assessed via a comprehensive, seven-domain neuropsychological battery. Global neurocognitive impairment was defined for each participant independently by CR and GDS. Participants were classified into four categories (Dually-normal, impaired by CR-only, impaired by GDS-only, or Dually-impaired).
Results
There was 83% concordance between CR and GDS classifications; in total, 56% of participants were deemed impaired by CR and 41% were classified as impaired by GDS. Impairment by GDS virtually guaranteed CR impairment, but 16% of participants were additionally classified as impaired only by CR. As compared to Dually-normal participants, those classified as Dually and CR-only impaired were more likely to have AIDS, have more severe co-occurring conditions, have more severe depressive symptoms, be unemployed, and have more everyday functioning complaints (ps < 0.05).
Conclusion
Impairment classifications of the two methods were in high agreement; however, more people were classified as impaired using the CR-approach compared to the GDS approach, and those impaired according to CR-only showed fewer neurocognitive and functional deficits than the Dually-impaired participants. The CR approach may be most appropriate for detecting more subtle forms of neurocognitive impairment. Clinicians and researchers should recognize the strengths and weaknesses of each method when evaluating neurocognitive complications in HIV.
Keywords: Infectious Disease, Assessment, Cognition
Introduction
HIV-associated neurocognitive disorders (HAND) continue to be observed in one-third to one-half of individuals with HIV-infection (HIV+) despite dramatic improvements in medical morbidity and life expectancy due to combination antiretroviral therapy (Heaton et al., 2010). Importantly, although neuropsychological (NP) impairment persists, these deficits are typically mild and heterogeneous, presenting as a “spotty” cognitive profile that is not consistent across infected individuals (Butters et al., 1990; Heaton, Kirson, Velin & Grant, 1994; Heaton et al., 2010). As such, it is imperative to identify HAND classification methods that are sensitive to the relatively mild and variable patterns of neurocognitive deficits in individuals with HIV.
In 1990, a National Institute of Mental Health (NIMH) sponsored workgroup recommended clinical ratings by expert neuropsychologists as the best method for detecting neurocognitive impairment in individuals with HIV/AIDS (Butters et al., 1990). In particular, the workgroup emphasized the inability of group mean scores to accurately reflect the inconsistent NP profiles across HIV-infected individuals. For example, one individual’s above average score on a given task may mask an impaired performance by another individual on the same task; similarly, within individuals, good performance on some tests may obscure obvious deficits on others (Butters et al., 1990). By contrast, the NP clinician is able to recognize and classify HAND that is manifested as different patterns of strengths and deficits across patients. Heaton and colleagues (1994) proposed a detailed clinical ratings (CR) approach to classifying NP data in HIV; this approach of standardized CR has been supported and expanded in a subsequent publications showing good inter-rater reliability (e.g., Woods et al., 2004).
Briefly, in the clinical ratings approach, demographically corrected test scores (i.e., T-scores) from a comprehensive NP battery are categorized by domain of functioning (e.g., learning, processing speed). Clinical ratings for all domains are then assigned on a scale that ranges from one (above average) to nine (severely impaired), with a cutoff score of five indicating definite mild impairment and a score of four denoting borderline NP performance (see Table 1). In order for an individual to be classified as “impaired” overall, (s)he must evidence definite impairment (CR of 5 to 9) in at least two ability domains. This is consistent with recently published guidelines for HAND classification, which represented the consensus of a large group of international neuroAIDS experts that met in Frascati, Italy in 2005 (so-called “Frascati criteria;” Antinori et al., 2007). Importantly, CR is associated with everyday functioning abilities (e.g., medication adherence and management, activities of daily living, and employment; Heaton et al., 1994, 2004; Malaspina et al., 2011), HIV disease variables (e.g., immunosuppression, duration of infection; Heaton et al., 2011), and structural neural changes (i.e., synaptodendritic injury; Moore et al., 2006) among individuals with HIV, thus supporting the external validity for this approach.
Table 1.
Conversion table for transforming T scores into Clinical Ratings.
| T score | Clinical Rating | Impairment descriptor |
|---|---|---|
| ≥ 55 | 1 | Above average |
| 45–54 | 2 | Average |
| 40–44 | 3 | Low average |
| -- | 4 | Borderline* |
| 35–39 | 5 | Definite mild impairment |
| 30–34 | 6 | Mild-to-moderate impairment |
| 25–29 | 7 | Moderate impairment |
| 20–24 | 8 | Moderate-to-severe impairment |
| ≤ 19 | 9 | Severe impairment |
“Borderline” used only for Domain and Global summary ratings (not individual test scores).
The Global Deficit Score (GDS) approach is an alternative method employed to determine cognitive impairment among individuals living with HIV (Gonzalez et al., 2003; Hinkin et al., 2004; Levine et al., 2004). The GDS was originally created to be a “user friendly,” automated approach that also emphasizes deficits in performance. Specifically, it considers both the number and severity of deficits in performance throughout the test battery while assigning less weight to performances considered to be in the normal range (e.g., Heaton et al., 1994, 1995, 2004). In the GDS approach, individual test scores (i.e., T-scores) from a comprehensive NP battery are each converted to a deficit score ranging from zero (no impairment) to five (severe impairment) (see Table 2). The deficit scores are then simply averaged across all tests in the battery to create the GDS. Therefore, the GDS overcomes the same disadvantages as clinical ratings do compared to averaging absolute level of performance on a test battery (the latter giving equal weight to good and poor scores) and has been shown to be able to detect mild, HIV-associated cognitive impairment involving variable patterns of domains (Heaton, Kirson et al., 1994; Heaton et al., 1995; Carey et al., 2004). For example, Carey and colleagues (2004) found that the GDS discriminated between HIV+ and healthy comparison subjects, as well as accurately classified those HIV+ participants with NP impairment versus those without NP impairment defined by independent, blind clinical ratings. Additionally, the GDS has been found to be associated with biomarkers of HIV disease progression, including CD4 count and HIV RNA viral load in cerebrospinal fluid (Gonzalez et al., 2003), as well as aspects of everyday functioning, such as antiretroviral medication adherence (Hinkin et al., 2004; Levine et al., 2004).
Table 2.
Conversion table for transforming T scores into Deficit Scores.
| T score | Deficit score | Impairment descriptor |
|---|---|---|
| ≥ 40 | 0 | None (Normal) |
| 35–39 | 1 | Mild |
| 30–34 | 2 | Mild-to-Moderate |
| 25–29 | 3 | Moderate |
| 20–24 | 4 | Moderate-to-Severe |
| ≤ 19 | 5 | Severe |
Although research has supported the use of both CR and the GDS for detecting impairment across the “spotty” neurocognitive profiles in HIV, previous studies have not determined the degree to which the two methods agree or when use of each method may be most warranted. Therefore, the current study aims to determine how differences between the methods may affect outcomes and delineate under which circumstances each method is most appropriate. Specifically, we aim to 1) determine the overall level of agreement between CR and GDS classifications in a large sample of HIV-infected individuals (i.e., participants are classified as either cognitively impaired or intact on both the CR and GDS); and 2) examine which demographic, HIV-associated biomarkers, functional, and cognitive variables are associated with discrepant classifications in order to determine how these methods may differ.
Methods
Participants
The 1574 participants in this study were HIV+ and drawn from the CNS HIV Antiretroviral Therapy Research Effects (CHARTER) study, a prospective cohort study conducted in HIV clinics at six academic medical centers: Johns Hopkins University (Baltimore, MD); The Mt. Sinai School of Medicine (New York, NY); University of California at San Diego (San Diego, CA); University of Washington (Seattle, WA); University of Texas Medical Branch (Galveston, TX), and Washington University (St. Louis, MO; see Heaton et al., 2010). Participants were excluded only if they could not complete the assessment at the time of evaluation (i.e., presence of comorbid condition was not an exclusion criterion, because the intent was to recruit representative clinic samples in these settings). However, using detailed neuromedical history and examination data, all participants were classified with respect to three increasing levels of non-HIV related comorbidities, as defined in Antinori et al. (2007; for a detailed outline operationalizing specific conditions see supplement E-2): minimal (“incidental”), moderate (“contributing”), and severe (“confounded”) (see also Heaton et al., 2010, for reliability of these ratings). Briefly, a minimal comorbidity could have minor effects on NP test results, but it is unlikely to cause even mild global impairment and therefore does not preclude a HAND diagnosis. A moderate comorbidity is likely to have at least mild effects on NP test results but unlikely to cause clinically significant global NP impairment by itself and therefore also does not preclude a HAND diagnosis. Lastly, a severe comorbidity is likely to have major effects on NP test results, with significant neurocognitive impairment and functional disability, or NP results are invalid to due poor effort; therefore, classification of a severe comorbidity precludes HAND diagnosis at the time of assessment (see Table 2 in Heaton et al., 2010 for a more detailed description of the specific comorbid conditions within the CHARTER cohort). The demographic, psychiatric, and HIV disease and treatment characteristics of the study participants are summarized in Table 3.
Table 3.
Demographic and clinical characteristics of CHARTER participants (N = 1574); Mean (SD), Median (IQR), or Percent.
| Dually Normal1 (n=679) | Dually Impaired1 (n=626) | CR-only Impairment (n=254) | GDS-only Impairment (n=15) | p- value | |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 42.6 (8.7) | 43.2 (78.0) | 44.0 (9.0) | 45.5 (7.6) | 0.08 |
|
| |||||
| Gender (% M) | 81% | 73% | 75% | 53% | <0.001 |
|
| |||||
| Education (years) | 12.5 (2.5) | 12.5 (2.5) | 12.6 (2.7) | 12.8 (2.0) | 0.85 |
|
| |||||
| Ethnicity | <0.001 | ||||
| Caucasian | 40% | 40% | 39% | 40% | |
| African-American | 52% | 44% | 47% | 53% | |
| Hispanic | 5% | 13% | 11% | 0% | |
| Other | 3% | 3% | 3% | 7% | |
|
| |||||
| Current CD4 (cells/μL) | 420 (266,614) | 418 (251,607) | 425 (255,573) | 519 (381,704) | 0.57 |
|
| |||||
| Nadir CD4 (cells/μL) | 200 (58,338) | 150 (40,265) | 166.5 (49.3, 301.5) | 205 (63,362) | 0.006 |
|
| |||||
| AIDS | 56% | 68% | 66% | 40% | <0.001 |
|
| |||||
| HIV CSF viral load detect | 40% | 33% | 28% | 22% | 0.01 |
| Detect if on ART | 31% | 27% | 20% | 29% | 0.04 |
|
| |||||
| HIV plasma viral load detect | 62% | 59% | 55% | 40% | 0.09 |
| Detect if on ART | 56% | 54% | 49% | 27% | 0.10 |
|
| |||||
| On ART | 84% | 88% | 88% | 73% | 0.049 |
|
| |||||
| Comorbidity Ratings | <0.001 | ||||
| Mild (Incidental) | 69% | 37% | 61% | 47% | |
| Moderate (Contributing) | 27% | 35% | 27% | 47% | |
| Severe (Confounding) | 5% | 28% | 12% | 7% | |
|
| |||||
| Unemployed | 67% | 79% | 76% | 87% | <0.001 |
|
| |||||
| BDI-II | 13.1 (10.6) | 14.8 (10.9) | 14.1 (11.1) | 14.0 (11.1) | 0.06 |
|
| |||||
| WRAT-3 | 96.0 (13.7) | 86.1 (17.5) | 92.0 (16.7) | 87.5 (18.2) | <0.001 |
Both GDS and CR agree
Note: CR = Clinical Ratings; GDS = Global Deficit Score; CSF = cerebrospinal fluid; ART = antiretroviral therapy; WRAT-3 = Wide Range Achievement Test–version 3.
Procedures
Standard Protocol Approvals and Participant Consents
The Human Subjects Protection Committees of each participating institution approved the study procedures. Written informed consent was obtained from all study participants.
Laboratory Assessment
HIV infection was diagnosed by enzyme linked immunosorbent assay with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin test, and CD4+ T cells (flow cytometry) were performed at each site’s Clinical Laboratory Improvement Amendments (CLIA)-certified, or CLIA equivalent, medical center laboratory. HIV RNA levels were measured in plasma and CSF by reverse transcriptase-polymerase chain reaction (Roche Amplicor, v. 1.5, lower limit of quantitation 50 copies/mL). AIDS was diagnosed using available clinical and immunologic data (defined as has having a nadir CD4 cell count < 200 cells/μL or any history of an AIDS-defining clinical condition utilizing the CDC AIDS classification system; CDC, 1992).
Neurobehavioral Examination
Participants completed a comprehensive neurocognitive test battery at baseline, covering seven ability domains commonly affected by HIV (see Heaton et al., 2010 for listing of specific tests). Raw test scores were converted to demographically-corrected standard scores (T-scores). The most comprehensive normative standards available were used, which correct for effects of age, education, sex and ethnicity, as appropriate (Heaton, Taylor, Manly & Tulsky, 2003; Heaton et al., 2004; Norman et al., 2011).
Clinical Ratings
For in-depth discussion of the standardized guidelines for clinical ratings, which operationalize the Frascati criteria for classifying HAND, see Woods et al. (2004). Clinical ratings of NP function were assigned by a computerized algorithm for each of the seven major ability areas using a nine-point scale (1 = above average functioning to 9 = severe impairment) with a global rating of five or above indicating abnormal NP functioning (i.e., for the current study a global rating of five or above was denoted as “neurocognitive impairment”) (see Table 2; Antinori et al., 2007; Woods et al., 2004). Consistent with the DSM-IV (APA, 1994) and the Frascati criteria (Antinori et al., 2007) guidelines, two domains must be in the impaired range in order to assign a global rating of five or greater. If two or more domains are impaired at the same level, that is the global rating as well (e.g., Learning = 5, Executive Function = 5, Global Clinical Rating = 5); if the two are impaired at different levels, then the global rating reflects the highest level of impairment minus one (e.g., Verbal = 5 and Executive Function = 7, then global rating = 6). The global rating does not reflect an average of the domain ratings, but rather affords impaired domains greater importance in that the global rating equals the level of the two worst domain scores (if the same) or the worst domain score minus one (if impaired at different levels). Importantly, there are some caveats built into the CR approach that are not a part of the GDS approach. For example, if learning and memory (delayed recall) are the only two domains impaired, the participant must evidence “forgetting” as measured by percent retained on the learning measures (i.e., Hopkins Verbal Learning Test-Revised and Brief Visual Memory Test-Revised) in order to receive a rating of impaired in the memory domain. This is to avoid double penalizing a person with poor learning as also having a memory impairment. Similarly, if a participant is not impaired on the “Perseverative Responses” score of the Wisconsin Card Sorting Task-64 (WCST-64), then their “Categories Completed” score is examined for impairment. The WCST-64 is assigned a rating of “mildly impaired” in the event that Perseverative Responses falls within normal limits, but Categories Competed is impaired. A rating of mild impairment is always assigned to the WCST-64 under these circumstances, even if Categories Completed is more than mildly impaired, because the latter score is considered secondary to the Perseveration score mildly, moderately, or severity impaired.
Deficit Scores
Global Deficit Scores were calculated by converting the demographically-corrected T-scores on each individual NP measure into a deficit score using a five-point scale (see Table 3; 0 = no impairment to 5 = severe impairment; Carey et al., 2004; Heaton et al., 2004). The individual test deficit scores were then averaged to create a Global Deficit Score (GDS) for each individual. Previous studies have found that a GDS cutoff of ≥ 0.5 to indicate abnormal NP functioning yields the most optimal balance between sensitivity and specificity (Heaton et al., 2004), so this cutoff was utilized in the current study.
Mood Assessment
Current degree of depressive symptomology was assessed via the Beck Depression Inventory-II (Beck, 1996). The BDI-II is a 21-item questionnaire reflecting the affective/cognitive as well as the somatic symptoms of depression. Responses are summed to derive a total score, higher scores reflect greater severity of depression: 0–13 = Minimal; 14–19 = Mild; 20–28 = Moderate; 29–63 = Severe.
Functional Outcomes
The Patient’s Assessment of Own Functioning Inventory was administered as an indicator of participant’s ability complaints in everyday life (PAOFI; Chelune, 1986). The PAOFI is a 41-item questionnaire in which the participant reports the frequency with which (s)he has difficulties with memory, language and communication, use of his/her hands, sensory-perception or higher level cognitive and intellectual functions in his/her everyday functioning (e.g., “how often do you forget people whom you have met in the last day or two?”).
To assess dependence in performing instrumental activities of daily living (IADLs), a modified version of the Lawton and Brody scale was utilized (Woods et al., 2006). Eleven items from this scale were included detailing the degree to which individuals independently function in the areas of Financial Management, Home Repair, Medication Management, Laundry, Transportation, Grocery Shopping, Shopping, Housekeeping (Cleaning), Cooking, Work, and Telephone Use. For each activity the participant separately rates his/her current level of independence and highest previous level of independence. The total score is the total number of activities for which there is currently a need for increased assistance (i.e., dependence), with a range of zero (no change) to 11 (increased dependence in all activities).
Statistical analyses
Agreement in overall neurocognitive impairment classifications by the GDS and CR algorithm was examined at baseline by creating a “discrepancy variable” with four classification groups: 1) Dually-normal: Normal by GDS and CR; 2) Dually-impaired: Impaired by GDS and CR; 3) Discrepant: Impaired by CR-only (but not via GDS); 4) Discrepant: Impaired by GDS-only (but not impaired via CR). These classification groups were then compared across HIV disease, functional, and neurocognitive variables utilizing chi-square and analysis of variance (ANOVAs) techniques, where appropriate. As there were too few participants to conduct meaningful statistics on in the Discrepant: Impaired by GDS-only group (n = 15; 1.0%), our analyses focused on the impaired by CR-only group (n = 254; 16%).
In order to determine which clinical variables were most associated with “true” impaired and unimpaired classifications (those in which both methods agreed), a series of chi-square and ANOVA analyses were conducted examining group differences across disease, functional, and cognitive domains between the Dually-normal and Dually-impaired groups. In this manner a pattern of expected associations was established from which the discrepant CR impairment group associations could be examined. Chi-square and ANOVAs were again conducted to compare the impaired by CR-only classification group to the Dually-normal and Dually-impaired groups across the same clinical variables. We were thus able to determine how alike and dissimilar the impaired by CR-only individuals were compared to the agreed-upon cognitively normal and impaired groups. Additionally, ANOVAs were conducted to compare the impaired by CR-only group to the Dually-impaired group across the number of impaired cognitive domains and severity of global neurocognitive impairment (i.e., average CR for each group).
Results
Agreement between GDS and CR
As shown in Figure 1, at baseline there was 83% (1305/1574) concordance between the CR and GDS NP impairment classifications. Although less than 1% (15/1574) of participants were classified as impaired by GDS-only, 16% (254/1574) were classified as impaired by CR-only.
Figure 1.
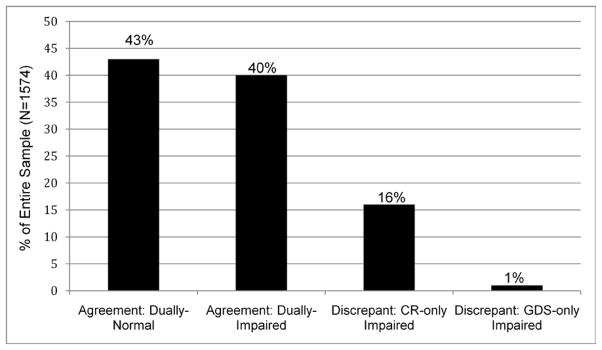
Frequency of classifications using the Clinical Ratings (CR) and Global Deficit Score (GDS) approaches.
Proportion of agreement between the Clinical Ratings (CR) and Global Deficit Score (GDS); “Dually Normal” indicates those participants classified as cognitively normal by both methods; “Dually Impaired” indicates those participants classified as cognitively impaired by both methods. Dually Normal and Dually Impaired indicate the participants on which the CR and GDS agreed (83% of the sample). “Impaired by CR only” indicates those participants classified as cognitively impaired only by CR (and not GDS); “Impaired by GDS only” indicates those participants classified as cognitively impaired only by the GDS (and not CR).
Dually-normal versus Impaired by CR-only
Participants classified as impaired only by CR (but not GDS) were more likely to be classified as having AIDS compared to participants classified as Dually-normal (66% vs. 56%; χ2 = 6.6, p = 0.01). For those participants on ART therapy, rates of detectable HIV RNA in plasma or CSF were not different between the CR-only impaired participants and the cognitively intact participants (p’s > 0.05). Participants who were impaired by CR-only did not differ from the cognitively intact participants on any other of the HIV disease related variables (i.e., current CD4 and nadir CD4 counts). CR impaired participants were more likely to have a severe co-occurring condition (i.e., a non-HIV related comorbidities) than the Dually-normal participants (12% vs. 5%; χ2 = 16.5, p < 0.001).
Participants with CR-only impairment were more likely to be unemployed (76% vs. 67%; χ2 = 6.8, p = 0.009) and had more ability complaints in everyday life (F(1,931) = 15.2, p < 0.001) than dually normal participants. The CR-only impaired participants did not differ from Dually-normal participants on reported independence on IADLs or depressive symptoms (all p’s > 0.05).
Dually-impaired versus Dually-normal/Impaired by CR-only
Those individuals classified as being Dually-impaired (i.e., impaired on both the GDS and CR) had lower nadir CD4 counts (F(1,1301) = 12.7, p < 0.001) and were more likely to be classified as AIDS (68% vs. 56%; χ2 = 18.1, p < 0.001) than participants classified as being Dually-normal. Impaired and unimpaired participants did not differ on percent receiving ART, current CD4 count or HIV RNA concentration in plasma or CSF (ps > 0.05; see Tables 3 and 4). Impaired participants were also more likely to have a severe co-morbid condition (28% vs. 5%; χ2 = 183.86, p < 0.001)
Table 4.
Comparison of neurocognitive impairment classification groups across variables of interest.
| Dually-impaired > Dually-normal | CR-only impaired > Dually-normal | Dually-impaired > CR-only impaired | |
|---|---|---|---|
| HIV disease | |||
| Current CD4 | |||
| ↓ Nadir CD4 | X | ||
| ↑ AIDS | X | X | |
| Detectable virus in plasma | |||
| Detectable virus in CSF | |||
| ↑ Severe co-occurring conditions | X | X | X |
| Functional | |||
| ↑ PAOFI | X | X | X |
| ↑ IADL dependence | X | ||
| ↓ Employed | X | X | |
| ↑ BDI-II | X | X | |
Note: CSF = cerebrospinal fluid; PAOFI = Patient’s Assessent of Own Functioning; IADL = Instrumental Activities of Daily Living; BDI-II = Beck Depression Inventory-II
Compared to Dually-normal participants, those with Dual-impairment were more likely to be unemployed (79% vs. 67%, χ2 = 21.9, p < 0.001), reported more ability complaints in everyday life (F(1,1302) = 66.49, p < 0.001), and were more likely to be dependent on IADLs (22% vs. 17%; χ2 = 4.5, p = 0.03). Participants with Dual-impairment classifications also reported more depressive symptoms (F(1, 1292) = 7.18, p = 0.008) than those without impairment.
Participants classified as impaired by CR-only did not differ from those with Dual-impairment on any of the HIV-related disease variables (i.e., AIDS classification, current and nadir CD4 counts, detectable HIV RNA in plasma and CSF; p’s > 0.05). The impaired by CR-only participants reported fewer ability complaints in everyday life than Dually-impaired participants (F(1,877) = 5.72, p < 0.02). However, Dually-impaired participants were more likely to have a severe co-occurring condition (28% vs. 12%; χ2 = 48.1, p < 0.001). The CR-only impaired participants did not differ from the Dually-impaired participants on the other functional and mood variables (i.e., employment, IADL independence, and BDI; p’s > 0.05).
Number of Neurocognitive Domains Impaired and Impairment Severity
The Dually-impaired participants had almost twice as many domains impaired on average (i.e., domain clinical rating ≥ 5) compared to the CR-only impaired participants (4.0 vs. 2.4 domains impaired; F(1,878)=330.77, p < 0.001). Additionally, the Dually-impaired participants showed an increased severity of global impairment compared to the CR-impaired participants (average global CR: 6.3 vs. 5.3; F(1,878)=280.62, p < 0.001).
Impairment classifications excluding “severe” comorbidities
When those individuals with “severe” comorbidities were excluded from our analyses, again the CR and GDS methods agreed 83% of the time (49% Dually-normal and 34% Dually-impaired). Similarly, 17% (224/1334) were classified as impaired by CR-only while 1% (14/1334) were classified as impaired by GDS-only.
The CR-only impaired participants showed a similar pattern of relationships to the relevant HIV-related and functional variables. CR-only participants had a higher incidence of AIDS (χ2 = 4.48, p =0.03), were less likely to be employed (χ2 =5.0, p = 0.025), and reported more ability complaints in everyday life (F(1,870)=11.1, p<0.001) than Dually-normal participants. Additionally the participants impaired by CR-only had more “minimal” co-occurring conditions (χ2 = 19.46, p <0.001) and a showed a trend toward more ability complaints in everyday life (F(1,670)=3.80, p = 0.052) compared to Dually-impaired participants. There were no other significant differences between the CR-only and Dually-normal or -impaired participants.
Discussion
Overall, the GDS and CR approaches were largely similar in their classification of NP impairment in persons living with HIV infection (83% agreement). Using the GDS approach virtually guarantees CR impairment (i.e., impairment in ≥ 2 ability domains), but the CR method also classified 16% more as impaired (i.e., meeting criteria for a potential HAND diagnosis). On average, however, these CR-only impaired participants were less severely NP impaired, had fewer cognitive domains affected, and tended to show less impairment-related problems with everyday functioning compared to Dually-normal participants. Thus, despite agreement in the large majority of cases, the GDS method was less likely to render an impairment classification overall. The question is whether the CR method may slightly “overclassify” HAND (since more individuals were identified as impaired with the CR method) or the GDS may slightly “underclassify” (since fewer individuals were classified as impaired with the GDS method) such conditions. Unfortunately there is no independent “gold standard” that can be used to settle this question, and the best one can do is consider similarities and differences in the subgroups impaired by GDS (virtually the same as the Dually-impaired) and the CR-only impaired participants compared to the subgroup that is clearly normal on the test battery (i.e., Dually-normal).
Consistent with results from previous NP studies of HIV infection, individuals who were classified as neurocognitively impaired by either GDS and CR-only approaches displayed an expected pattern of disease and functional deficits (e.g., lower nadir CD4, higher incidence of AIDS, increased functional complaints and depression, and decreased rates of employment) compared to individuals classified as neurocognitively intact by both methods (Heaton et al., 2010; Heaton, Velin, et al., 1994; Thames et al., 2010). This finding not only delineates those variables that are expected to differ between individuals who are cognitively intact versus impaired, but it also supports the construct validity of these classification methods by illustrating associations with variables that are conceptually related to cognitive impairment.
When examining the discrepant CR classifications, those individuals identified as CR-only impaired showed a similar pattern of associated variables to the Dually-impaired (essentially the GDS-impaired) participants in that they were more likely to be classified as having AIDS, had more serious co-occurring conditions, more functional complaints and depressive symptoms, and decreased rates of employment compared to the Dually-normal participants. Importantly, however, the CR-only impaired participants differed from the Dually-impaired participants in their breadth and severity of cognitive deficits, as well as severity of disease and functional deficits. Regarding the latter, the Dually-impaired participants were even more likely than the CR-only impaired participants to have worse co-occurring conditions, and report more ability complaints in everyday life compared to the Dually-normal participants. Therefore, although the CR-only impaired participants had a pattern of deficits that closely mirrored that of the Dually-impaired participants, the overall burden of cognitive impairment was not as severe. Consequently, the CR-only impaired participants may be best viewed as individuals who are not as impaired as the Dually-impaired participants, but are also not equivalent to the Dually-normal participants. Additionally, the stronger association between the Dually-impaired (essentially GDS-impaired) participants and variables of functional decline compared to the CR-only participants suggests that use of the GDS would more likely identify more participants who meet criteria for a symptomatic HAND diagnosis (i.e., reach the “symptomatic” threshold for functional complaints), while use of the CR would identify an additional subset of participants who might be more likely to be diagnosed with asymptomatic HAND.
When examining CR and GDS impairment classifications among the participants with definite HIV-related impairment (i.e., when excluding the HIV+ participants with “severe” comorbidities), a similar pattern of results emerged. Specifically, the two methods showed the same agreement rate (83%) in those with and without severe comorbidities. The participants impaired by CR-only maintained very similar associations with the Dually-normal and Dually-impaired participants before and after excluding individuals with severe comorbidities; these findings again illustrate that the CR-only participants had more impairment-related problems (e.g., higher incidence of AIDS, unemployment, and ability complaints in everyday life) than the Dually-normal participants but fewer impairment-related problems (e.g., less severe co-occurring conditions) than the Dually-impaired participants. These results suggest that the GDS and CR perform the same whether the severely confounded participants are included or not (i.e., in both a heterogeneous HIV population and one with HIV-specific impairment) and neither approach gives differential sensitivity to “pure” HIV-related effects. Therefore, these methods may be appropriate to detect cognitive impairment regardless of the etiology.
In sum, although both the GDS and CR methods are in high agreement, the CR approach will identify more individuals with potentially less severe levels of HIV-associated NP impairment in which individuals are not fully comparable to Dually-impaired (essentially GDS-impaired). Therefore, the CR-only approach would be most useful for clinicians and researchers aiming to include individuals with more mild forms of neurocognitive impairment; whereas the GDS approach should be utilized when more severe levels of cognitive impairment identification are desired. Both approaches are related to theoretically important variables supporting their construct validity; however, understanding the nuances of each approach has important implications for HIV-associated NP impairment classifications. Both researchers and clinicians can benefit from utilizing each method in the most appropriate context particularly since each of these approaches meet the recently published international guidelines for HAND classifications (Antinori et al., 2007).
Although the current results focus on NP impairment within the context of neuroAIDS, future studies examining the performance of both of these approaches in other neurologic conditions could help to further inform when utilization of each method is most appropriate as well as inform how each method performs across neurocognitive disorders.
Acknowledgments
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) is supported by award N01 MH22005 from the National Institutes of Health.
* The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: J. Allen McCutchan, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.,.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Terry Alexander, R.N.; Laboratory, Pharmacology and Immunology Component: Scott Letendre, M.D. (P.I.), Edmund Capparelli, Pharm.D.,; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Matthew Dawson; Virology Component: Joseph K. Wong, M.D. (P.I.); Imaging Component: Terry Jernigan, Ph.D. (Co-P.I.), Michael J. Taylor, Ph.D. (Co-P.I.), Rebecca Theilmann, Ph.D.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman,; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Christopher Ake, Ph.D., Florin Vaida, Ph.D.; Protocol Coordinating Component: Thomas D. Marcotte, Ph.D. (P.I.), Rodney von Jaeger, M.P.H.; Johns Hopkins University Site: Justin McArthur (P.I.), Gilbert Mbeo, MBChB; Mount Sinai School of Medicine Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Susan Ueland, R.N.; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Trudy Jones, M.N., A.R.N.P.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Heckendorn, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award MH 62512 from NIMH.
*The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H., Shannon LeBlanc; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Steven Paul Woods, Psy.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D. (Consultant); Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S., Tanya Wolfson, M.A.
This work was also supported by a T32 grant DA031098 from NIDA.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Authors report no conflicts of interest affecting this article.
References
- Butters N, Grant I, Haxby J, Judd LL, Martin A, Stover E. Assessment of Aids-related cognitive changes: Recommendations of NIMH workshop on neuropsychological assessment approaches. Journal of Clinical and Experimental Neuropsychology. 1990;12(6):963–978. doi: 10.1080/01688639008401035. [DOI] [PubMed] [Google Scholar]
- Carey C, Woods SP, Gonzalez R, Conover E, Marcotte TD, Heaton RK. Predictive validity of Global Deficit Scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbidity and Mortality Weekly Report. 1992;41(Suppl 44-17):1–19. [PubMed] [Google Scholar]
- Gonzalez R, Heaton RK, Moore DJ, Letendre S, Elliott R, Grant I for the HNRC Group. Computerizes reaction time battery versus a traditional neuropsychological battery: Detecting HIV-related impairments. Journal of the International Neuropsychological Society. 2003;9:64–71. doi: 10.1017/s1355617703910071. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Kirson D, Velin RA, Grant I. the HNRC Group. The utility of clinical ratings for detecting cognitive change in HIV infection. In: Grant I, Markin A, editors. psychology of HIV infection. New York: Oxford University Press; 1994. pp. 188–206. [Google Scholar]
- Heaton RK, Velin RA, McCutchan JA, Cgulevich SJ, Atkinson JH, Grant I for the HNRC Group. Neuropsychological Impairment in Human Immunodeficiency Virus-Infection: Implications for Employment. Psychosomatic Medicine. 1994;56:8–17. doi: 10.1097/00006842-199401000-00001. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Abramson I for the HNRC Group. The HNRC 500–Neuropsychology of HIV infection at different disease stages. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically adjusted for neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Grant I for the CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Lentendre SL. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient care, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Hinkin CH, Castellon SA, Mason KI, Lam MN, Hardy DJ. Variations in patterns of highly active antiretroviral therapy (HAART) adherence. AIDS and Behavior. 2004;9(3):355–362. doi: 10.1007/s10461-005-9009-y. [DOI] [PubMed] [Google Scholar]
- Malaspina L, Woods SP, Moore DJ, Depp C, Letendre SL, Grant I the HNRC. Successful cognitive aging in persons living with HIV infection. Journal of Neurovirology. 2011;17(1):110–119. doi: 10.1007/s13365-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Ellis RJ, Mallory M, Heaton RK, Marcotte TD, McCutchan JA. Dendritic injury is a pathological substrate for human immunodeficiency virus- related cognitive disorders. Annals of Neurology. 1997;42(6):963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Heaton RK. Sensitivity and specificity of WAIS-III/WMS-III demographically corrected factor scores in neuropsychological assessment. Journal of the International Neuropsychological Society. 2001;7:867–874. [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Hinkin CH. Medication and finance management among HIV-infected adults: The impact of age and cognition. Journal of Clinical and Experimental Neuropsychology, iFirst. 2010:1–10. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Dawson M, Scott JC, Grant I The HNRC Group. Action (verb) fluency predicts dependence in instrumental activities of daily living in persons infected with HIV-1. Journal of Clinical and Experimental Neuropsychology. 2006;28:1030–1042. doi: 10.1080/13803390500350985. [DOI] [PubMed] [Google Scholar]


