Abstract
Osteoporosis is overshadowed in an era of chronic illnesses, and a care gap exists between physicians and patients. The aim of this study was to determine the effectiveness of implementing an automated system for identifying and sending a letter to patients at high risk for osteoporosis. Patients 50 years of age and older were tagged with an International Classification of Diseases, Ninth Revision, diagnostic code upon initial visit to the emergency department (ED), identifying potential fragility fractures. Automatically generated letters were sent via our osteoporosis database system to each patient 3 months after the initial visit to the ED. The letter indicated that he or she was at risk for osteoporosis and suggested that the patient schedule a follow-up appointment with a physician. Patients were subsequently telephoned 3 months after receiving the letter and asked about their current plan for follow-up. The control group did not receive a letter after departure from the ED. In the control group, 84 (85.71%) individuals of the total 98 did not have any follow-up but the remaining 14 (14.29%) sought a follow-up. In the intervention group, 62 (60.19%) individuals of 103 did schedule a follow-up, while the remaining 41 (39.81%) did not seek a follow-up. Thus, the patient follow-up response rate after fracture treatment improved with intervention (P < .0001). Current literature has demonstrated the low rate of follow-up care addressing osteoporosis in patients experiencing fragility fractures (1%-25% without intervention). Research has shown the effectiveness of various types of intervention programs for improving the continuum of care for these high-risk patients. Nonautomated intervention programs can have a multitude of human-related system failures in identifying these patients. Our study successfully implements an automated system that is able to be applied to most hospitals with minimal cost and resources.
Keywords: fragility fractures, geriatric trauma, osteoporosis, trauma surgery, geriatric medicine, economics of medicine, systems of care
Introduction
Osteoporosis is characterized by a deterioration in the microarchitecture of the bone tissue, and this progressive bone fragility leads to greater fracture susceptibility.1 A total of 10 million people in the United States are living with osteoporosis and a striking 34 million are at risk for the disease.2 Nationwide, osteoporosis is responsible for more than 2 million fractures per year, and subsequent fracture risk is significant.2 For example, one report documented 342 (45%) of 766 female patients with hip fracture sustained a subsequent fracture within 5 years.3 Another stated the average 50-year-old caucasian woman carries a 40% lifetime risk for a repeat fragility fracture.1 In the elderly people, the risk of future fracture can increase by up to 9.5-fold.4 One retrospective study of >30 000 nursing home residents noted that 23.9% of patients with a hip fracture and 15.1% of non-hip fracture patients experienced another fracture within 2 years of admission.5
There is clear evidence that initiating treatment with calcium, vitamin D, and a bisphosphonate helps prevent future fractures.4,6 However, there are reports that less than 30% of postmenopausal women and less than 10% of men with prior fragility fracture are treated for osteoporosis.7 Some of the major barriers to implementation of care include lack of knowledge and awareness of both the physician and the patient,4 the perception by the orthopedic surgeon that the diagnosis and treatment are not his or her responsibility,4 low rates of referral to an appropriate osteoporosis service,8 the cost of therapy, side effects of medications, and multiple medical comorbidities.4 As a result, not only does the quality of life of these patients suffer, but there is a high cost associated with long-term treatment and management. In 2005, the direct costs of fragility fractures alone were US$17 billion; a compilation of 2.5 million medical office visits, 430 000 hospital admissions, and 180 000 nursing home admissions.2 In fact, studies suggest that about 22% of patients move to nursing home care within 1 year of the fragility fracture.9 In 2050, when 1 in every 5 individuals will be over the age of 60, the World Health Organization (WHO) estimates that 6 million hip fractures will occur each year worldwide.10 This is a striking rise, considering that in 1992 there were 1.7 million hip fractures.11
We propose that an automated osteoporosis intervention program can address this care gap in an efficient, standardized fashion to reduce health care costs and improve patient quality of life. Over the last decade, much research has been devoted to establishing osteoporosis intervention programs, and while many of these studies have reported positive results,1,2,4,7,8,12–19 the majority lack automation or would be difficult to implement in the average hospital system.
The purpose of our study was to establish and evaluate an automated system based on simple letter intervention for ease of implementation in any US hospital system.
Patients and Methods
This was a prospective study that was reviewed by our international review board committee and qualified for exemption. Patients with fracture, 50 years of age and older, were identified upon arrival to the emergency department (ED) of Penn State Hershey Medical Center, from September 24, 2008, to January 08, 2010. In total, 1565 billing records were compiled, and patients were tagged with an International Classification of Diseases, Ninth Revision (ICD-9) diagnostic code at the time of discharge from the ED. The code was determined by the billing department based on the documentation in the electronic medical record. Study patients were then identified based on specific ICD-9 fracture codes that initially suggested fragility fracture potential. The following fracture codes were identified: (1) pathologic fracture-vertebrae 733.13; (2) pathologic fracture-femoral neck 733.14; (3) orbital floor 802.6; (4) C7 vertebra 805.07; (5) dorsal vertebra 805.2; (6) lumbar vertebra 805.4; (7) sacrum/coccyx 805.6; (8) vertebral fracture NOS 805.8; (9) proximal humerus 812; (10) surgical neck of humerus 812.01; (11) anatomical neck of humerus 812.02; (12) Colles’ fracture 813.41; (13) distal radius 813.42; (14) distal radius with ulna 813.44; (15) femur, intracapsular NOS 820; (16) femur, mid-cervical neck 820.02; (17) femur, transcervical 820.09; (18) trochanteric NOS 820.2; (19) intertrochanteric 820.21; (20) subtrochanteric 820.22; (21) femoral neck NOS 820.8; and (22) polytrauma/multiple injury sites 959.8/959.9 (Table 1).
Table 1.
ICD-9 Fragility fracture Codes Assigned to Study Patients and Controls.
| ICD-9 Code | Fracture | N (Patients/Controls) |
|---|---|---|
| 733.13 | Pathologic Fx-vertebrae | 10/14 |
| 733.14 | Pathologic Fx-femur | 0/2 |
| 802.6 | Orbital floor | 1/0 |
| 805.07 | C7 vertebra | 1/0 |
| 805.2 | Dorsal vertebra | 12/7 |
| 805.4 | Lumbar vertebra | 11/7 |
| 805.6 | Sacrum/coccyx | 0/1 |
| 805.8 | Vertebral Fx NOS | 3/1 |
| 812 | Proximal humerus NOS | 13/12 |
| 812.01 | Surgical neck of humerus | 2/0 |
| 812.02 | Anatomical neck of humerus | 0/1 |
| 813.41 | Colles’ fracture | 10/11 |
| 813.42 | Unspecified distal radius | 22/8 |
| 813.44 | Distal radius with ulna | 4/2 |
| 820 | Femur, intracapsular NOS | 0/1 |
| 820.02 | Femur, mid-cervical | 1/1 |
| 820.09 | Femur, transcervical | 2/4 |
| 820.2 | Trochanteric Fx NOS | 1/3 |
| 820.21 | Intertrochanteric | 3/8 |
| 820.22 | Subtrochanteric | 0/3 |
| 820.8 | Femoral neck NOS | 5/12 |
| 959.8, 959.9 | Polytraumaa, Mlt injury sitesa | 2/0 |
| Total | 103/98 | |
Abbreviations: ICD-9, International Classification of Diseases, Ninth Revision; Mlt, manual ligament therapy.
a Although these cases typically do not qualify as fragility fractures, these were determined to be from low-energy traumas.
Hospital encounter screening identified the patients linked to these codes and patient information was downloaded in monthly increments by an individual in the hospital’s financial department. The data were autopopulated into our “Orthopaedic Osteoporosis Registry/Database.” We used Filemaker Pro for our database application. Patients were then prescreened from the database to exclude cases that would not be consistent with the definition of a fragility fracture: high-energy traumas, high-impact falls, and motor vehicle accidents (MVAs). Note that polytrauma exceptions were given, if the injuries sustained were from low-energy forces. Patients were also excluded if they were dead, are a repeat in the database (ie, multiple ED visits for fractures), or had fracture treatment at another institution. Finally, patients already being treated for osteoporosis or individuals unable to be contacted for various reasons (phone disconnected/out of service, patient admitted to nursing home, or having dementia) were also excluded.
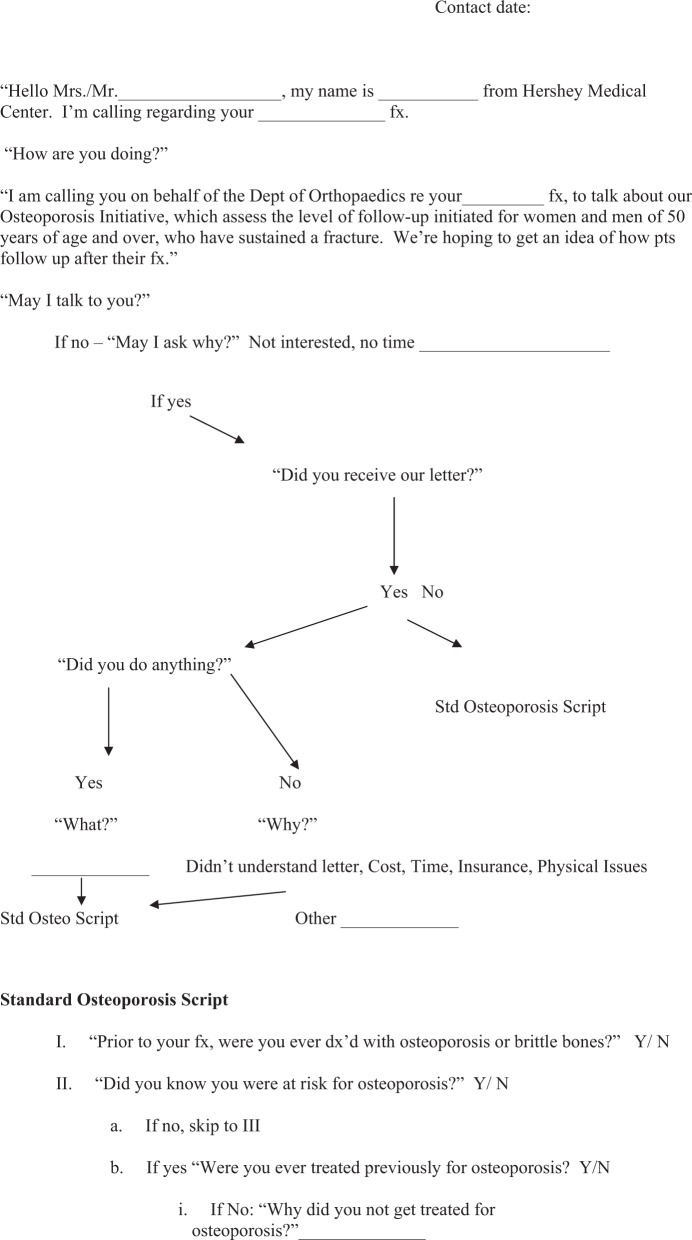
One hundred and three patients comprised the final intervention group. Computer-generated letters were sent to each patient up to 3 months after the initial visit to the ED (Appendix A: form 1 and Figure 1). The letter indicated that the patient was at risk for osteoporosis, and he or she was advised to schedule an appointment with his or her primary care physician (PCP) or with the bone health clinic at Hershey Medical Center. A developed phone script was used in this process, and patients were subsequently telephoned by a research associate 3 months after receiving the letter, and each was asked about his or her response to the letter and current plan for follow-up (Appendix A: form 2).
Figure 1.
Automated letter generated from the osteoporosis database and sent to each patient of the intervention group 3 months after discharge from the hospital.
For the control group, 645 billing records spanning 275 days (July 18, 2010-April 19, 2011) were reviewed. In all, 234 candidates were identified, and the final control group after screening was comprised of 98 patients. In order to keep the time to phone call consistent between the 2 groups, these individuals were contacted via telephone approximately 6 months after their departure from the ED to determine whether or not they had any current or future follow-up planned after being treated for their fracture (Appendix A: form 2).
We defined follow-up as a patient actively scheduling an appointment with one of his or her health care providers to address the risk of osteoporosis. The percentage response for both the control group and intervention group was calculated, and we employed the chi-square test in order to assess the effectiveness of our automated intervention program.
Results
In the control group, 84 (85.71%) individuals of the total 98 did not have any follow-up evaluation after being treated for their fracture, but the remaining 14 (14.29%) had some sort of follow-up. Similarly, in the intervention group, 41 (39.81%) of 103 did not schedule a follow-up, while the remaining 62 (60.19%) did seek a follow-up. Thus, the patient follow-up response rate after fracture treatment improved with intervention (P < .0001).
Discussion
Osteoporosis impacts millions of individuals each year, creating a large burden on society and the health care system.1,2,4,7–9,11–20 Despite this burden, current literature demonstrates the low rate of follow-up care received by patients experiencing fragility fractures.1,2,4,7,8,12–19 We chose our patient population based on recommendations from the National Osteoporosis Foundation, which states that all individuals ≥50 years of age experiencing fragility fractures should be evaluated and treated for osteoporosis.21 Using various types of intervention programs has demonstrated success, with the end goal of improving osteoporosis follow-up in high-risk patients.1,2,4,7,8,12–19 Our study builds on this current trend. However, most of these programs would be difficult to implement at an average hospital system, given the lack of standardization and automation. Furthermore, nonautomated systems are subject to human error and could result in failure to identify certain high-risk patients. Our protocol negates these barriers and has yielded promising results such that any hospital system could adopt this program at minimal cost and resources.
The notion that more automated programs are needed is highlighted in the current literature. Most intervention programs have not directly addressed the main barriers currently hindering the care gap. For example, Rozental et al proposed 2 different modes of intervention following distal radius fractures: one involved an orthopedic surgeon directly ordering the DEXA scan and the other route involved the orthopedic surgeon contacting the patient’s PCP for follow-up evaluation.18 Although both methods improved on the standard of care, the methods lack automation. Similarly, Ekman proposed a method utilizing the orthopedic surgeon as the coordinator in establishing continuity of care following fracture treatment.2 In this study, patients hospitalized for fragility fractures were simply instructed to follow-up with the PCP.2
Some programs highlight the importance of utilizing a dedicated osteoporosis care coordinator. Bogoch et al reported the successful implementation of an Osteoporosis Exemplary Care Program for the education, investigation, and treatment of high-risk patients. A central coordinator was hired to integrate the outpatient fracture clinic, the inpatient orthopedic unit, the metabolic bone disease clinic, and the nuclear medicine unit for the evaluation and management of patients sustaining fragility fractures. In total, 359 patients were identified as high risk and >95% were appropriately diagnosed, treated, and referred for care.4 Vaile et al created a first fracture project with a dedicated osteoporosis nurse (ON). The ON attended the fracture clinic daily and ensured that high-risk patients were educated and up to date on blood work and bone mineral density (BMD) testing. The ON also coordinated follow-up with the family physician. Prior to the intervention, less than 12% of patients were taking calcium, vitamin D, or other antiosteoporosis medications, and new treatment had been commenced in a very small percentage. Only 9% of patients were taking calcium, 11% vitamin D, and 11% a bisphosphonate. After 6 months of intervention, one-third of patients were taking calcium and/or a bisphosphonate and one-fourth were taking vitamin D.19 Although a dedicated ON seems like a feasible solution that directly addresses the current barriers, many institutions lack the personnel, resources, and funding for a dedicated position.
The ability to easily implement these programs in any hospital system remains the goal for long-term success. Simple letter intervention has already been shown to be an effective method. Leslie et al conducted a randomized-controlled trial (RCT) and found that patients more than 50 years of age sustaining a major fracture without prior BMD testing or treatment for osteoporosis experienced an improved follow-up rate via letter notification to the patient and/or his or her physician. The reported absolute increase for the combined end point of bone mineral density testing or pharmacologic treatment was 14.9%, and the number needed to notify to change patient care was 7.17 Additionally, a study by Sugi et al demonstrated improvement in follow-up rates via basic telephone intervention following automated fragility fracture identification using ICD-9 codes.7
While our protocol is easy to implement, one could reasonably conclude that if we established an osteoporosis care coordinator, as in the aforementioned studies,4,19 our sample size would be much larger. This is a pilot study with limited patients, given that phone calls can be time consuming. In reality, once the program is established, the process of sending out letters to the appropriate patients does not take much time nor does it require a dedicated, funded position. Although future studies may examine the effectiveness of utilizing both a phone call and a letter to further improve follow-up rates, we have already demonstrated success utilizing solely the latter.
As mentioned earlier, there is a demonstrated benefit in future fracture risk for patients started on calcium supplements.6 However, several studies have recently called into question the routine use of calcium supplementation across the general population. A thorough and detailed review of the available data is beyond the scope of this article, but the topic is worth addressing, given that some intervention programs define success based on the number of patients started on calcium supplements at follow-up.19 For example, it is well known that calcium supplements accelerate vascular calcification and mortality in patients with renal failure, including both dialysis and predialysis individuals.22–24 Furthermore, Bolland et al conducted a meta-analysis of randomized, placebo-controlled trials of calcium supplement usage and risk of cardiovascular outcomes. Although the included studies did not have cardiovascular outcomes as the primary end points, self-reports, hospital admissions, and death certificates were used to gather appropriate data. Eleven RCTs were analyzed, documenting a 30% increase in the incidence of myocardial infarction and a smaller, nonsignificant increase in the risk of stroke and mortality. The authors reported that such a modest increase must be taken into context with the widespread usage of calcium supplements and the marginal benefit demonstrated in future fracture risk prevention.25
It is also worth highlighting that given the nature of our intervention protocol, some individuals with dementia were unable to be contacted to determine their response to the letter. In some cases, family members or individuals familiar with that specific patient’s care were able to respond on his or her behalf. Patients with dementia pose additional issues such as polypharmacy, medication noncompliance, multiple medical comorbidities, increased risk of side effects, and increased fall risk; these factors hinder follow-up rates and increase the morbidity, mortality, and financial burden for the patient as well as the entire health care system. The need to reduce the incidence of repeat fragility fractures in patients with dementia26 must be balanced with the dangers of placing these older patients on osteoporosis medications.27
We did experience some limitations in our study. There was a slight time lag in patient contact (ie, telephone intervention) due to a research associate leaving the department. However, the time frame for screening and contacting patients did not exceed 3 months after receiving the computer-generated letter. Thus, all patients in the intervention group received the letter up to 3 months after fracture treatment, and all patients in the intervention group were contacted up to 3 months after receiving the letter (ie, approximately 6 months after fracture treatment). This was consistent with our control group telephone contact period, which was set at approximately 6 months after fracture treatment.
In an era of chronic illnesses, osteoporosis remains overshadowed, and the barriers to narrowing the care gap must be addressed to ensure long-term success. The automated orthopedic osteoporosis initiative we have proposed is the initial step in the goal toward establishing a better continuum of care. A reduction in the incidence of repeat fragility fractures will decrease the burden of osteoporosis on society. Our program builds on the current successful trends and adds a further benefit by negating the potential for human error that would result in failure to guide the high-risk population to follow-up osteoporosis care. The automated nature of our program requires very minimal technologic resources. A simple Filemaker Pro program allows for input of all data fields and parameters. Patient information can be obtained from the hospital financial/billing department, and a basic template letter allotting for individual name and address can be created in a Microsoft Word document.
Appendix A
Form 1. Phone script used in contacting each patient in the intervention group 3 months after receiving the intervention letter (6 months after discharge from the hospital).
Form 2. Phone script used in contacting patients in the control group 6 months after discharge from the hospital.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bessette L, Ste-Marie LG, Jean S, et al. Recognizing osteoporosis and its consequences in Quebec (ROCQ): background, rationale, and methods of an anti-fracture patient health-management programme. Contemp Clin Trials. 2008;29(2):194–211 [DOI] [PubMed] [Google Scholar]
- 2. Ekman E. The role of the orthopaedic surgeon in minimizing mortality and morbidity associated with fragility fractures. J Am Acad Orthop Surg. 2010;18(5):278–285 [DOI] [PubMed] [Google Scholar]
- 3. von Friesendorff M, Besjakov J, Akesson K. Long-term survival and fracture risk after hip fracture: a 22-year follow-up in women. J Bone Miner Res. 2008;23(11):1832–1841 [DOI] [PubMed] [Google Scholar]
- 4. Bogoch ER, Elliot-Gibson V, Beaton DE, Jamal SA, Josse RG, Murray TM. Effective initiation of osteoporosis diagnosis and treatment for patients with a fragility fracture in an orthopaedic environment. J Bone Joint Surg Am. 2006;88(1):25–34 [DOI] [PubMed] [Google Scholar]
- 5. Lyles KW, Schenck AP, Colon-Emeric CS. Hip and other osteoporotic fractures increase the risk of subsequent fractures in nursing home residents. Osteop Int. 2008;19(8):1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res. 1996;11(12):1961–1966 [DOI] [PubMed] [Google Scholar]
- 7. Sugi MT, Sheridan K, Lewis L, et al. Active referral intervention following fragility fractures leads to enhanced osteoporosis follow-up care. J Osteoporos. 2012;2012: 234381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marsh D, Akesson K, Beaton DE, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int. 2011;22(7):2051–2065 [DOI] [PubMed] [Google Scholar]
- 9. Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320(7231):341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anonymous Chronic diseases and health promotion. 2012. World Health Organization. http://www.who.int/chp/topics/rheumatic/en/ Accessed September 1, 2012.
- 11. Cumming RG, Nevitt MC, Cummings SR. Epidemiology of hip fractures. Epidemiol Rev. 1997;19(2):244–257 [DOI] [PubMed] [Google Scholar]
- 12. Bogoch ER, Elliot-Gibson V, Wang RY, Josse RG. Secondary causes of osteoporosis in fracture patients. J Orthop Trauma. 2012;26(9):e145–e152 [DOI] [PubMed] [Google Scholar]
- 13. Byszewski A, Lemay G, Molnar F, Azad N, McMartin SE. Closing the osteoporosis care gap in hip fracture patients: an opportunity to decrease recurrent fractures and hospital admissions. J Osteoporos. 2011;2011: 404969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edwards BJ, Bunta AD, Anderson J, et al. Development of an electronic medical record based intervention to improve medical care of osteoporosis. Osteoporos Int. 2012;23(10):2489–2498 [DOI] [PubMed] [Google Scholar]
- 15. Harrington T, Lease J. Osteoporosis disease management for fragility fracture patients: new understanding base on three years’ experience with an osteoporosis care service. Arthritis Rheum. 2007;57(8):1502–1506 [DOI] [PubMed] [Google Scholar]
- 16. Khan SA, de Geus C, Holroyd B, Russell AS. Osteoporosis follow-up after wrist fractures following minor trauma. Arch Intern Med. 2001;161(10):1309–1312 [DOI] [PubMed] [Google Scholar]
- 17. Leslie WD, LaBine L, Klassen P, Dreilich D, Caetano PA. Closing the gap in postfracture care at the population level: a randomized controlled trial. CMAJ. 2012;184(3):290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. a prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953–961 [DOI] [PubMed] [Google Scholar]
- 19. Vaile J, Sullivan L, Bennett C, Bleasel J. First fracture project: addressing the osteoporosis care gap. Intern Med J. 2007;37(10):717–720 [DOI] [PubMed] [Google Scholar]
- 20. Johnell O, Kanis JA, Oden A, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15(3):175–179 [DOI] [PubMed] [Google Scholar]
- 21. National Osteoporosis Foundation Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013 [Google Scholar]
- 22. Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–1483 [DOI] [PubMed] [Google Scholar]
- 23. Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71(5):438–441 [DOI] [PubMed] [Google Scholar]
- 24. Russo D, Miranda I, Ruocco C, et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72(10):1255–1261 [DOI] [PubMed] [Google Scholar]
- 25. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Menzies IB, Mendelson DA, Kates SL, Friedman SM. Prevention and clinical management of hip fractures in patients with dementia. Geriatr Orthop Surg Rehabil. 2010;1(2):63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brauner DJ, Muir JC, Sachs GA. Treating nondementia illnesses in patients with dementia. JAMA. 2000;283(24):3230–3235 [DOI] [PubMed] [Google Scholar]