Abstract
We evaluated changes in gene expression of mTOR, p21, caspase-3, ULK1, TNFα, matrix metalloproteinase (MMP)-9, and cathepsin K in the whole blood of rheumatoid arthritic (RA) patients treated with methotrexate (MTX) in relation to their rheumatoid factor status, clinical, immunological, and radiological parameters, and therapeutic response after a 24-month follow-up. The study group consisted of 35 control subjects and 33 RA patients without previous history of MTX treatment. Gene expression was measured using real-time RT-PCR. Decreased disease activity in patients at the end of the study was associated with significant downregulation of TNFα expression. Downregulation of mTOR was observed in seronegative patients, while no significant changes in the expression of p21, ULK1, or caspase-3 were noted in any RA patients at the end of the study. The increase in erosion numbers observed in the seropositive patients at the end of the follow-up was accompanied by upregulation of MMP-9 and cathepsin K, while seronegative patients demonstrated an absence of significant changes in MMP-9 and cathepsin K expression and no increase in the erosion score. Our results suggest that increased expression of MMP-9 and cathepsin K genes in the peripheral blood might indicate higher bone tissue destruction activity in RA patients treated with methotrexate. The clinical study registration number is 0120.0810610.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovial hyperplasia, mononuclear cell infiltration, bone erosion, and joint destruction. Early diagnosis and immediate aggressive treatment are required for the amelioration of progressive joint damage and patient disability [1, 2].
Methotrexate (MTX) is the most conventional disease-modifying antirheumatic drug (DMARD) for RA, with the best efficacy and the fewest adverse effects [3, 4]. However, only approximately 30% of patients respond to MTX treatment [5, 6]. The identification of patients who are less responsive to MTX could avoid delays in adjusting their treatment and prevent future irreversible joint damage [7].
Rheumatoid factor (RF) is a part of the 2010 American College of Rheumatology (ACR) classification criteria for RA [8]. RF is an autoantibody directed against the Fc portion of IgG and is associated with disease persistence and progressive joint destruction [9–11]. However, the data related to RF status in treatment response to MTX is inconsistent, as some studies reported no association between RF positivity and treatment efficacy [12–21], while others indicated that seropositive patients exhibited worse responses to MTX therapy in early rheumatoid arthritis [9, 22, 23].
Variations in disease manifestations assessed by clinical and laboratory tests produce a specific disease phenotype, which results in changes in gene expression in various affected tissues and immune effector cells [24]. Therefore, differentially expressed genes may serve as biomarkers for disease status and predictors of the response to therapy [25–28]. As the peripheral immune system is activated in RA patients [29], gene expression changes in the peripheral blood mononuclear cells (PBMCs) could provide informative biomarkers. Several studies involving DNA microarray technology have revealed differences in the expression of specific gene clusters observed in the PBMCs of early RA patients and in patients with established progressive disease versus normal subjects [30]. Higher expression of type I interferon-regulated genes was also observed in the peripheral blood cells of RA patients compared with healthy controls [31]. In addition, twin studies have shown that similar genes are highly overexpressed in both blood and synovial fluid of RA patients versus controls [32].
Of particular importance are ubiquitously expressed human genes that are required for the regulation of basic cellular processes [33]. Previous studies have revealed differential gene expression associated with apoptosis in the PBMCs of RA patients [25, 34]. In addition, low apoptotic activity has been reported in the synovial fluid leucocytes and synoviocytes of RA patients [35–37].
Mammalian target of rapamycin (mTOR) is considered a key regulator of cell growth and proliferation [38]. It has been shown recently that mTOR inhibition downregulated mitogen-induced T- and B-lymphocyte proliferation and IL-1 and TNFα production in vitro [39, 40]. Moreover, animal studies have shown that mTOR downregulation alleviated paw swelling in antigen-induced arthritis [41].
Autophagy occurs upon arrest of proliferation and is associated with production of cyclin-dependent kinases such as p21 [42]. As autophagy can also be induced by proinflammatory cytokines and autoantibodies, it could be an important factor in RA pathogenesis [43]. Indeed, it has been shown that autophagy induction in RA synovial fibroblasts promoted their survival [44].
Several studies have presented evidence of upregulated proteolytic activity in the PBMCs of RA patients versus healthy subjects [32], which might result from joint destruction in RA. Articular cartilage and bone degradation are associated with the upregulation of matrix metalloproteinases (MMPs) and osteolytic enzymes, such as MMP-9 and cathepsin K, respectively [45–47], in the serum and synovial fluid of RA patients [48, 49]. Moreover, serum concentrations of cathepsin K significantly correlated with radiological joint destruction in RA patients [50]. MMP-9 expression is activated by proinflammatory cytokines including TNFα [51] and has been shown to be both decreased [52, 53] and increased [54] in response to anti-TNF therapy.
Here, we evaluated changes in the expression of genes responsible for cell proliferation and growth (mTOR), regulation of cell cycle progression (p21), apoptosis (caspase-3), and autophagy (ULK1), as well as the proinflammatory cytokine TNFα and genes associated with bone and articular cartilage turnover (MMP-9 and cathepsin K) in the whole blood of rheumatoid arthritic patients treated with MTX in relation to their RF status, clinical, immunological, and radiological parameters, and their therapeutic response at a 24-month follow-up. Our results suggest that the higher radiographic joint destruction associated with RF positivity is accompanied by the upregulation of MMP-9 and cathepsin K gene expression in the PBMCs of RA patients treated with methotrexate.
2. Materials and Methods
2.1. Ethics
Our clinical study was in compliance with the Helsinki Declaration. The study protocol was approved by the Local Committee on the Ethics of Human Research, and informed consent was obtained from all subjects.
2.2. Patients
Inclusion criteria of the control subjects were as follows. The control group consisted of 35 subjects, 7 men and 28 women, (average age 46.4 ± 13.2 years; range 19–69 years) with no current chronic or acute infection and no family history of autoimmune diseases.
Inclusion criteria of the RA patients were as follow. The RA patient group consisted of 33 consecutive, unrelated rheumatoid arthritic patients, 5 men and 28 women (average age 47.2 ± 14.2 years; range 18–68 years), who visited the clinic of the Institute of Rheumatology, Russian Academy of Medical Sciences, between January and December 2008. Inclusion criteria involved a diagnosis of RA, as defined by the American College of Rheumatology (ACR) 1987 [55], age ≥ 18 years, and symptom duration of <2 years without previous history of MTX treatment. Exclusion criteria were previous treatment with DMARDS and/or systemic corticosteroids and DMARD intolerance.
All patients included in this study started treatment with oral MTX at a dosage of 10 mg per week; after two weeks, the dosage was increased to 15 mg. Out of 33 patients, 11 were given MTX in combination with methylprednisolone, 8 mg daily. Each patient was followed up by the same investigator at six months, one year, and two years after inclusion. Remission was defined according to ACR criteria for clinical remission by the disease activity score based on the simplified 28-joint score (DAS28) [56, 57].
2.3. Demographic, Clinical, and Immunologic Assessment
The evaluation data were collected at baseline and at 24 months. These data included age, gender, disease duration, Steinbrocker's radiographic stage [58], duration of morning stiffness (min), and the disease activity score (DAS) using a modified index involving 28 joints [56, 57]. Concentrations of serum C-reactive protein (cutoff value, 5 mg/L) and IgM class rheumatoid factor (RF) (a standard cutoff value of 15 mU/L was used) were measured by nephelometry using a BN-100 analyzer (Dade Bering, Germany). Anticitrullinated protein autoantibodies (ACPA) were detected by ELISA according to the manufacturer's recommendations (the cutoff level was set at 5 U/mL for antibody positivity) (Axis Shield Diagnostics Limited, UK).
2.4. Radiographic Assessment
Radiographs of hands and feet were obtained at months 0 and 24. The radiographs were evaluated blind and in chronological order by two independent observers and scored using Sharp's method as modified by van der Heijde et al. [59]. For each patient, an erosion and joint space narrowing score was registered for hands and feet, and the mean of the scores from two observers was used to determine the final radiographic scores for erosions and joint space narrowing.
2.5. Total RNA Isolation and Reverse Transcriptase (RT) Reaction
For detection of gene expression total RNA was isolated from 100 μL of whole blood immediately after withdrawal using Ribo-zol-A kit (InterLabService, Moscow, Russia) in accordance with the manufacturer's recommendations. Total RNA had an A260/290 > 1.9. The RT reaction was performed using a Reverta kit containing M-MLV reverse transcriptase, random hexanucleotide primers, and total RNA according to the manufacturer's recommendations (InterLabService, Moscow, Russia).
2.6. Real-Time Quantitative PCR
The following premade primers and probes were used for the TaqMan assay (Applied Biosystems, Foster City, CA, USA): mTOR (Hs00234522_m1), Unc-51-like kinase 1 (ULK1) (Hs00177504_m1), p21WAF1/Cip1 (p21) (Hs00355782_m1), caspase 3 (Hs00263337_m1), TNFα (Hs00174128_m1), MMP-9 (Hs00234579_m1), and cathepsin K (Hs00166165_m1). β-Actin was used as an endogenous control.
The quantification of gene expression was conducted using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) as described previously [60]. Briefly, 1 μL of RT product was subjected to real-time PCR in a 15 μL total reaction mixture containing 7.5 μL of TaqMan Universal PCR Master Mix (Applied Biosystems), 900 nM sense and antisense primers, 50 nM probe, and template cDNA. After a single step of 50°C for 2 min and an initial activation at 95°C for 10 min, the reaction mixtures were subjected to 40 amplification cycles (15 s at 95°C for denaturation and 1 min of annealing and extension at 60°C).
Relative mRNA expression was determined using the delta-delta CT method, as detailed by the manufacturer guidelines (Applied Biosystems) [61]. The delta CT value was calculated by subtracting the CT value for the housekeeping β-actin gene from the CT value for each sample. A delta-delta CT value was then calculated by subtracting the delta CT value of the control (each healthy patient) from the delta CT value of each RA patient. Each PCR was performed in duplicate. Three “no template” controls were consistently negative for each reaction.
2.7. Statistical Analysis
The variables did not have a Gaussian distribution; therefore, descriptive values were expressed as medians and interquartile ranges. The statistical comparison between the independent patient groups was performed using Mann-Whitney U test and Spearman's rank correlations. For the statistical comparison between the RA patient groups before and after treatment the Wilcoxon matched pairs test was applied. To compare percentages, a one-tailed Z-test for percentages was applied. The Statistica 6 Software (StatSoft, Tulsa, OK, USA) was used for all statistical analyses. P values ≤ 0.05 were considered significant.
3. Results
3.1. Whole Blood Gene Expression in Rheumatoid Arthritic Patients at Baseline and at 24 Months
All of examined genes, the regulator of cell growth and proliferation mTOR, the autophagy marker ULK1, the cyclin-dependent kinase inhibitor p21, the apoptosis indicator caspase-3, the proinflammatory cytokine TNFα, and the proteases MMP-9 and cathepsin K, were significantly upregulated at baseline in a sample of RA patients (n = 33) compared with healthy subjects (data not shown).
An analysis of bivariate correlations using Spearman's correlation coefficient for the expression of the examined genes at baseline showed positive correlations (P < 0.05) with each other in the RA patients examined (n = 33) (Table 1). However, no correlation was observed between the expression of mTOR and TNFα and that of MMP-9. A positive correlation was also noted between ULK1 and MMP-9 gene expression and serum C-reactive protein levels. In contrast, expression of the p21, caspase-3, and TNFα genes negatively correlated with serum RF amounts. As RF concentration correlated with the expression of the examined genes, the RA patients were divided into seronegative and seropositive subsets for further analyses.
Table 1.
Correlation coefficients (Spearman's) and their significance (P) are shown for the expression of the examined genes in relation to each other and the disease markers in a sample of RA patients (n = 33).
| mTOR | ULK1 | p21 | Caspase-3 | TNFα | MMP-9 | |
|---|---|---|---|---|---|---|
| mTOR | 0.399 P = 0.01 |
0.702 P < 0.001 |
0.765 P < 0.001 |
0.581 P < 0.001 |
||
| ULK1 | 0.531 P = 0.001 |
0.539 P < 0.001 |
0.383 P = 0.02 |
|||
| p21 | 0.915 P < 0.001 |
0.770 P < 0.001 |
||||
| Caspase-3 | 0.632 P < 0.001 |
|||||
| MMP-9 | 0.661 P < 0.001 |
0.367 P = 0.03 |
0.389 P = 0.02 |
|||
| Cathepsin K | 0.634 P < 0.001 |
0.628 P < 0.001 |
0.708 P < 0.001 |
0.708 P < 0.001 |
0.688 P < 0.001 |
0.499 P = 0.002 |
| Rheumatoid factor | −0.384 P = 0.02 |
−0.430 P = 0.009 |
−0.348 P = 0.04 |
|||
| C-reactive protein | 0.379 P = 0.02 |
0.303 P = 0.07 |
Examination of gene expression in the blood of 12 seronegative RA patients revealed that all of examined genes were significantly upregulated at baseline compared to healthy controls (Figure 1). At the end of the study, downregulation was observed only for the TNFα (P = 0.03 versus baseline) and mTOR genes, the expression of which became similar to that in the control subjects. Some decrease in the expression of ULK1, p21, caspase-3, and cathepsin K was also observed; however, these differences were not statistically significant, and the expression of these four genes exceeded that observed in the healthy controls.
Figure 1.
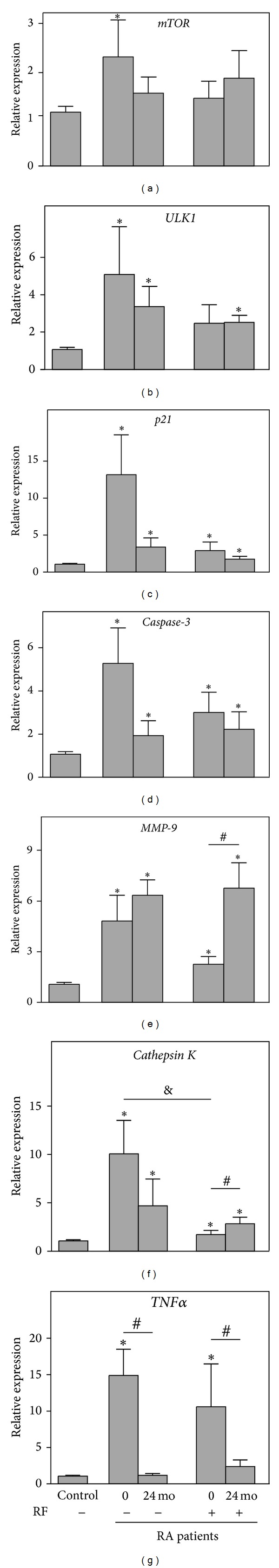
Relative expression of the genes mTOR (a), ULK1 (b), p21 (c), caspase-3 (d), MMP-9 (e), and cathepsin K (f), and TNFα (g) with reference to β-actin determined by real-time PCR analyses in the whole blood of seronegative (RF−) (n = 12) and seropositive (RF+) (n = 21) rheumatoid arthritic patients compared with healthy controls (Control) (n = 35) at baseline (0) and after 24 months of follow-up (24 mo). Control bar is shown as 1.0 as required for relative quantification with the real-time PCR protocol. Asterisks indicate significant differences from the control in pairwise comparisons (Mann-Whitney U test). Number sign (#) shows significant difference from the baseline value (Wilcoxon matched pairs test). & sign indicates significant difference between seronegative and seropositive RA patients (Mann-Whitney U test).
Assessment of gene expression in the blood of 21 seropositive RA patients showed that their mTOR and ULK1 levels were similar to those in healthy subjects at baseline, while the other examined genes, p21, caspase-3, TNFα, MMP-9, and cathepsin K, were significantly upregulated (Figure 1). At the end of the study, no significant changes were observed in the expression of ULK1, p21, and caspase-3, as these genes remained upregulated compared with the controls. In contrast, the expression of MMP-9 and cathepsin K was significantly upregulated versus that at baseline (P = 0.02 and P = 0.05, resp.) and compared with the controls, while TNFα gene expression was significantly decreased compared with baseline (P = 0.05).
Direct comparison of gene expression between RF-positive and RF-negative RA patients showed that, at baseline, the seronegative subjects exhibited significantly higher cathepsin K gene expression compared with seropositive RA patients (P = 0.02), while the expression of the remaining genes was not significantly different (Figure 1(f)). Additionally, no significant differences in gene expression were observed between RF-positive and RF-negative patients at the end of the follow-up.
3.2. Clinical, Immunological, and Radiological Parameters at Baseline and 24 Months and Therapeutic Response in Rheumatoid Arthritic Patients
The mean age at diagnosis of the enrolled seronegative (n = 12) rheumatoid arthritic patients was 49.0 ± 15.5 (range 18–66 years). The subset included one man and 11 women. The mean disease duration at inclusion was 4.3 ± 4.2 months. Of 12 examined patients, 11 had early RA, with disease duration from 1 to 5 months; the disease duration of one patient was 17 months. All of the patients had Steinbrocker's radiographic stage II, both at baseline and after 24 months of follow-up. All of the patients were treated with MTX, while 5 out of 12 received a combination of MTX and methylprednisolone. Only 3 out of 12 patients were ACPA-positive both at baseline and after 24 months. Most patients (8 out of 12) presented high disease activity (DAS28 > 5.1) at study entry (Table 2). The DAS28 index decreased significantly after 24 months of follow-up (P = 0.001). At the end of the study, the majority of patients had moderate disease activity (3.2 < DAS28 < 5.1), while four patients (33%) fulfilled the remission criteria (DAS28 < 2.6). These findings were associated with significant decreases in morning stiffness and in the number of swollen and tender joints. Only one patient out of 12 exhibited erosions both at the beginning and at the end of the study, while the joint space narrowing score increased over the course of the follow-up period (P = 0.003).
Table 2.
Clinical, immunological, and radiological parameters and therapeutic response in seronegative rheumatoid arthritic patients.
| Baseline n = 12 |
24 months n = 12 |
P (Wilcoxon matched pairs t-test) | |
|---|---|---|---|
| IgM RF, mU/mL | 9.5 [9.5; 9.5] | 9.5 [9.5; 9.5] | 1.00 |
| ACPA, U/mL | 0.35 [0.15; 50.3] | 1.0 [0.5; 44] | 0.19 |
| C-reactive protein, mg/L | 12.51 [6.4; 30] | 4.68 [1.5; 12.1] | 0.001 |
| DAS28 | 5.37 [4.5; 5.8] | 3.29 [1.8; 3.5] | 0.001 |
| DAS28 < 2.6 | 0 | 4 (33%) | — |
| 2.6 < DAS28 < 3.2 | 1 (8%) | 1 (8%) | — |
| 3.2 < DAS28 < 5.1 | 3 (25%) | 7 (58%) | 0.57 |
| DAS28 > 5.1 | 8 (67%) | 0 | 0.01 |
| Morning stiffness, min | 150 [75; 210] | 10 [0; 30] | 0.002 |
| Swollen joints | 8 [6; 10.5] | 1 [0; 2] | 0.001 |
| Tender joints | 8.5 [6.5; 11.5] | 2 [0; 3] | 0.001 |
| Number of patients with erosions, % | 8.3 (1/12) | 8.3 (1/12) | — |
| Joint space narrowing score | 8 [5.5; 11] | 13 [8.5–17] | 0.003 |
The mean age at diagnosis of the seropositive (n = 21) rheumatoid arthritic patients was 46.2 ± 13.7 (range 18–68 years). This subset included 4 men and 17 women. The mean disease duration at inclusion was 9.2 ± 6.5 months; a total of 18 patients had early RA, with a disease duration from one to 12 months, while four patients had established RA with a disease duration of 18–23 months. All of the patients had Steinbrocker's radiographic stage II at baseline. After 24 months of follow-up, 18 patients maintained stage II, while three patients developed radiographic stage III. All of the patients were treated with MTX, while 6 out of 21 were treated with a combination of MTX and methylprednisolone. Only 3 out of 21 patients were ACPA-negative both at baseline and after 24 months of follow-up. Most patients (15 out of 21) presented high disease activity (DAS28 > 5.1) at baseline (Table 3). The DAS28 index decreased significantly after 2 years (P = 0.0002). At the end of the study, the majority of patients had moderate disease activity (3.2 < DAS28 < 5.1) while five patients (24%) fulfilled the remission criteria (DAS28 < 2.6). These findings were associated with significant decreases in morning stiffness and in the numbers of swollen and tender joints. Five patients out of 21 exhibited erosions at the beginning of the study; by the end of the study, 10 patients out of 21 had erosions, and the erosion score significantly increased (P = 0.003) after two years of follow-up. The joint space narrowing score also increased significantly over the course of the follow-up period (P = 0.001).
Table 3.
Clinical, immunological, and radiological parameters and therapeutic response in seropositive rheumatoid arthritic patients.
| Baseline n = 21 | 24 months n = 21 | P (Wilcoxon matched pairs t-test) | |
|---|---|---|---|
| IgM RF, mU/mL | 83.1 [58.5; 304.5] | 76.7 [26.9; 250.1] | 0.24 |
| ACPA, U/mL | 100 [20.3; 100] | 100 [68.7; 100] | 0.03 |
| C-reactive protein, mg/L | 13.8 [3.7; 20.8] | 6.2 [4.4; 11.4] | 0.03 |
| DAS28 | 5.56 [4.7; 6.4] | 3.5 [2.4; 4.1] | 0.0002 |
| DAS28 < 2.6 | 0 | 5 (24%) | — |
| 2.6 < DAS28 < 3.2 | 1 (5%) | 2 (9%) | 0.30 |
| 3.2 < DAS28 < 5.1 | 5 (24%) | 11 (52%) | 0.34 |
| DAS28 > 5.1 | 15 (72%) | 3 (14%) | 0.002 |
| Morning stiffness, min | 60 [30; 180] | 16.5 [0; 27] | 0.002 |
| Swollen joints | 8 [5.5; 13] | 2 [0; 6] | 0.001 |
| Tender joints | 9 [3; 18.5] | 2.5 [0; 8] | 0.006 |
| Number of patients with erosions, % | 23.8% (5/21) | 47.6% (10/21) | 0.05 |
| Erosion score | 0 [0; 0.5] | 1.5 [0; 5] | 0.003 |
| Joint space narrowing score | 13 [7; 24] | 23 [15.5; 31.5] | 0.001 |
Direct comparison of the clinical, immunological, and radiological parameters between the groups showed that, at baseline, seropositive patients exhibited significantly higher RF (P < 0.001) and ACPA (P = 0.01) values compared with seronegative RA subjects. After 24 months the seropositive RA patients also showed significantly elevated RF (P < 0.001) and ACPA (P = 0.006) versus seronegative subjects. In addition, at the end of the study, seropositive patients exhibited higher joint space narrowing (P = 0.006) values compared with seronegative RA patients, and there was a higher number of seropositive patients than seronegative patients with bone erosions (P = 0.02). However, no significant differences in the manifestation of other examined parameters were noted between the analyzed groups of RA patients both at baseline and at the end of the follow-up period (Tables 2 and 3).
4. Discussion
Many aspects of a disease phenotype are produced by pathophysiological processes driven by genes and their products [62]. Therefore, comparison of gene expression signatures between RA patients and healthy subjects may reveal important insights into mechanistic differences and unravel the fundamental nature of the disease. Moreover, this approach might also be useful in the evaluation of the response to RA treatment, which is supposed to restore normal cellular metabolism and should arguably aim to restore gene expression to levels comparable to healthy controls.
As it has been noted that more homogenous patient groups produce more consistent results [63], we analyzed the value of RF status on the outcome of MTX therapy in a sample of RA patients during a 24-month follow-up in relation to changes in the expression of genes involved in basic cellular processes and joint function, as measured in the peripheral blood. We found that, in the majority of the examined patients, the disease activity in the subsets of seropositive and seronegative RA patients significantly decreased from high levels at baseline to moderate levels at the end of the follow-up. This decrease was accompanied by a significant decrease in the morning stiffness and the number of swollen and tender joints in both subsets and a significant downregulation of TNFα gene expression in the blood compared with baseline levels. Moreover, TNFα gene expression became equal to that of control subjects. These results support previous observations that MTX treatment decreases TNFα production in T cells from RA patients [64, 65].
RF status did not affect the remission frequency (P = 0.29) in the examined RA patients; in both subsets, the remission criteria were fulfilled by 24% of the examined seropositive patients and by 33% of the seronegative RA patients. The inability of RF positivity to predict the outcome based on clinical parameters has also been observed previously [12–15, 66, 67].
The significantly increased erosion and joint space narrowing scores observed at the end of the follow-up period in the seropositive RA patients support previous observations that many RA patients exhibit radiographic progression, even though clinically they are in a state of low disease activity [68, 69]. Worsening of radiological parameters in these patients at the end of the study was associated with significant upregulation of MMP-9 and cathepsin K gene expression in the peripheral blood compared with baseline values. In contrast, the less severe joint destruction noted in the seronegative RA patients was accompanied by fewer alterations in MMP-9 and cathepsin K gene expression in the blood at the end of the follow-up. Therefore, upregulation of MMP-9 and cathepsin K gene expression might serve as blood-based biomarker of increased joint destruction activity in RA patients treated with MTX.
The difference in the observed response to MTX treatment might be partially caused by ACPA positivity in the majority of the examined seropositive RA patients compared with seronegative subjects. Some studies have reported previously that ACPA positivity was related to resistance to DMARDs and was inversely associated with remission at 24 months [70, 71].
The decrease in the disease activity at the end of the follow-up was accompanied by downregulation of mTOR gene expression in seronegative RA patients to the level observed in healthy controls. This outcome is important in MTX therapy, as mTOR upregulation has been shown to be associated with interleukin (IL)-1 [72], TNFα [40] production, synovial fibroblast proliferation [73], and osteoclast formation [74].
However, expression of the other examined genes (namely, p21, caspase-3, and ULK1 which are also required for maintenance of basic cellular processes) did not show significant changes in RA patients over the course of treatment, remaining significantly upregulated compared with healthy controls at the end of the study. This result might indicate that MTX treatment does not ameliorate basic cellular functions associated with apoptosis, autophagy, and cell cycle control, which are disturbed in RA.
5. Conclusions
We have shown that a significant reduction in the disease activity of the examined RA patients treated with methotrexate during a 24-month follow-up was associated with the significant downregulation of TNFα gene expression in the blood compared with baseline, which became equal to that in healthy subjects. Nevertheless, rheumatoid factor-positive RA patients exhibited significantly increased joint destruction accompanied by significant upregulation of MMP-9 and cathepsin K gene expression in the peripheral blood compared with baseline levels. These analyses may be of value in better characterizing disease activity and joint degeneration in rheumatoid arthritic patients.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgments
This study was supported by the Russian Foundation for Basic Research (Project no. 12-04-00038a to Elena V. Tchetina). The sponsor had no role in the study design or execution, data analysis, writing of the paper, or the decision to submit the paper for publication.
References
- 1.Matteson EL, Weyand CM, Fulbright JW, Christianson TJH, McClelland RL, Goronzy JJ. How aggressive should initial therapy for rheumatoid arthritis be? Factors associated with response to ‘non-aggressive’ DMARD treatment and perspective from a 2-yr open label trial. Rheumatology. 2004;43(5):619–625. doi: 10.1093/rheumatology/keh135. [DOI] [PubMed] [Google Scholar]
- 2.Scott DL. Evidence for early disease-modifying drugs in rheumatiod arthritis. Arthritis Research and Therapy. 2004;6(1):15–18. doi: 10.1186/ar1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinblatt ME. Efficacy of methotrexate in rheumatoid arthritis. British Journal of Rheumatology. 1995;34(supplement 2):43–48. [PubMed] [Google Scholar]
- 4.Katchamart W, Trudeau J, Phumethum V, Bombardier C. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Annals of the Rheumatic Diseases. 2009;68(7):1105–1112. doi: 10.1136/ard.2008.099861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis & Rheumatism. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 6.Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. The Lancet. 2008;372(9636):375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 7.de Vries-Bouwstra JK, Goekoop-Ruiterman YPM, Verpoort KN, et al. Progression of joint damage in early rheumatoid arthritis: association with HLA-DRB1, rheumatoid factor, and anti-citrullinated protein antibodies in relation to different treatment strategies. Arthritis & Rheumatism. 2008;58(5):1293–1298. doi: 10.1002/art.23439. [DOI] [PubMed] [Google Scholar]
- 8.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis & Rheumatism. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 9.Möttönen T, Paimela L, Leirisalo-Repo M, Kautiainen H, Ilonen J, Hannonen P. Only high disease activity and positive rheumatoid factor indicate poor prognosis in patients with early rheumatoid arthritis treated with “sawtooth” strategy. Annals of the Rheumatic Diseases. 1998;57(9):533–539. doi: 10.1136/ard.57.9.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vittecoq O, Pouplin S, Krzanowska K, et al. Rheumatoid factor is the strongest predictor of radiological progression of rheumatoid arthritis in a three-year prospective study in community-recruited patients. Rheumatology. 2003;42(8):939–946. doi: 10.1093/rheumatology/keg257. [DOI] [PubMed] [Google Scholar]
- 11.Bukhari M, Lunt M, Harrison BJ, Scott DGI, Symmons DPM, Silman AJ. Rheumatoid factor is the major predictor of increasing severity of radiographic erosions in rheumatoid arthritis: results from the norfolk arthritis register study, a large inception cohort. Arthritis & Rheumatism. 2002;46(4):906–912. doi: 10.1002/art.10167. [DOI] [PubMed] [Google Scholar]
- 12.Hider SL, Silman AJ, Thomson W, Lunt M, Bunn D, Symmons DPM. Can clinical factors at presentation be used to predict outcome of treatment with methotrexate in patients with early inflammatory polyarthritis? Annals of the Rheumatic Diseases. 2009;68(1):57–62. doi: 10.1136/ard.2008.088237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saevarsdottir S, Wallin H, Seddighzadeh M, et al. Predictors of response to methotrexate in early DMARD naïve rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Annals of the Rheumatic Diseases. 2011;70(3):469–475. doi: 10.1136/ard.2010.139212. [DOI] [PubMed] [Google Scholar]
- 14.Hodkinson B, Musenge E, Ally M, Meyer PWA, Anderson R, Tikly M. Response to traditional disease-modifying anti-rheumatic drugs in indigent South Africans with early rheumatoid arthritis. Clinical Rheumatology. 2012;31(4):613–619. doi: 10.1007/s10067-011-1900-5. [DOI] [PubMed] [Google Scholar]
- 15.Forslind K, Hafström I, Ahlmén M, Svensson B. Sex: a major predictor of remission in early rheumatoid arthritis? Annals of the Rheumatic Diseases. 2007;66(1):46–52. doi: 10.1136/ard.2006.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Möttönen T, Hannonen P, Korpela M, et al. Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis & Rheumatism. 2002;46(4):894–898. doi: 10.1002/art.10135. [DOI] [PubMed] [Google Scholar]
- 17.Combe B, Cantagrel A, Goupille P, et al. Predictive factors of 5-year health assessment questionnaire disability in early rheumatoid arthritis. Journal of Rheumatology. 2003;30(11):2344–2349. [PubMed] [Google Scholar]
- 18.Aletaha D, Smolen JS. The rheumatoid arthritis patient in the clinic: comparing more than 1300 consecutive DMARD courses. Rheumatology. 2002;41(12):1367–1374. doi: 10.1093/rheumatology/41.12.1367. [DOI] [PubMed] [Google Scholar]
- 19.Hoekstra M, van Ede AE, Haagsma CJ, et al. Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2003;62(5):423–426. doi: 10.1136/ard.62.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maradit-Kremers H, Nicola PJ, Crowson CS, O’Fallon WM, Gabriel SE. Patient, disease, and therapy-related factors that influence discontinuation of disease-modifying antirheumatic drugs: a population-based incidence cohort of patients with rheumatoid arthritis. Journal of Rheumatology. 2006;33(2):248–255. [PubMed] [Google Scholar]
- 21.da Mota LM, dos Santos Neto LL, de Carvalho JF, et al. The presence of anti-citrullinated protein antibodies (ACPA) and rheumatoid factor on patients with rheumatoid arthritis (RA) does not interfere with the chance of clinical remission in a follow-up of 3 years. Rheumatology International. 2012;32(12):3807–3812. doi: 10.1007/s00296-011-2260-9. [DOI] [PubMed] [Google Scholar]
- 22.Wessels JAM, van der Kooij SM, le Cessie S, et al. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis & Rheumatism. 2007;56(6):1765–1775. doi: 10.1002/art.22640. [DOI] [PubMed] [Google Scholar]
- 23.Gossec L, Dougados M, Goupille P, et al. Prognostic factors for remission in early rheumatoid arthritis: a multiparameter prospective study. Annals of the Rheumatic Diseases. 2004;63(6):675–680. doi: 10.1136/ard.2003.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felson DT. Epidemiology of the rheumatic diseases. In: Koopman WJ, editor. Arthritis and Allied Conditions. Textbook of Rheumatology. 14th edition. Philadelphia, Pa, USA: Lippincott, Williams & Wilkins; 2001. pp. 3–38. [Google Scholar]
- 25.van der Pouw Kraan TCTM, van Gaalen FA, Huizinga TWJ, Pieterman E, Breedveld FC, Verweij CL. Discovery of distinctive gene expression profiles in rheumatoid synovium using cDNA microarray technology: evidence for the existence of multiple pathways of tissue destruction and repair. Genes and Immunity. 2003;4(3):187–196. doi: 10.1038/sj.gene.6363975. [DOI] [PubMed] [Google Scholar]
- 26.Shou J, Bull CM, Li L, et al. Identification of blood biomarkers of rheumatoid arthritis by transcript profiling of peripheral blood mononuclear cells from the rat collagen-induced arthritis model. Arthritis Research and Therapy. 2006;8(1, article R28) doi: 10.1186/ar1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moncrieffe H, Hinks A, Ursu S, et al. Generation of novel pharmacogenomic candidates in response to methotrexate in juvenile idiopathic arthritis: correlation between gene expression and genotype. Pharmacogenetics and Genomics. 2010;20(11):665–676. doi: 10.1097/FPC.0b013e32833f2cd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreas K, Häupl T, Lübke C, et al. Antirheumatic drug response signatures in human chondrocytes: potential molecular targets to stimulate cartilage regeneration. Arthritis Research & Therapy. 2009;11(1, article R15) doi: 10.1186/ar2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu J, Märker-Hermann E, Baeten D, et al. A 588-gene microarray analysis of the peripheral blood mononuclear cells of spondyloarthropathy patients. Rheumatology. 2002;41(7):759–766. doi: 10.1093/rheumatology/41.7.759. [DOI] [PubMed] [Google Scholar]
- 30.Olsen NJ, Sokka T, Seehorn CL, et al. A gene expression signature for recent onset rheumatoid arthritis in peripheral blood mononuclear cells. Annals of the Rheumatic Diseases. 2004;63(11):1387–1392. doi: 10.1136/ard.2003.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Pouw Kraan TCTM, Wijbrandts CA, van Baarsen LGM, et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Annals of the Rheumatic Diseases. 2007;66(8):1008–1014. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas CS, Creighton CJ, Pi X, et al. Identification of genes modulated in rheumatoid arthritis using complementary DNA microarray analysis of lymphoblastoid B cell lines from disease-discordant monozygotic twins. Arthritis & Rheumatism. 2006;54(7):2047–2060. doi: 10.1002/art.21953. [DOI] [PubMed] [Google Scholar]
- 33.Tu Z, Wang L, Xu M, Zhou X, Chen T, Sun F. Further understanding human disease genes by comparing with housekeeping genes and other genes. BMC Genomics. 2006;7, article 31 doi: 10.1186/1471-2164-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grcevic D, Jajic Z, Kovacic N, et al. Peripheral blood expression profiles of bone morphogenetic proteins, tumor necrosis factor-superfamily molecules, and transcription factor Runx2 could be used as markers of the form of arthritis, disease activity, and therapeutic responsiveness. Journal of Rheumatology. 2010;37(2):246–256. doi: 10.3899/jrheum.090167. [DOI] [PubMed] [Google Scholar]
- 35.Catrina AI, Ulfgren AK, Lindblad S, Grondal L, Klareskog L. Low levels of apoptosis and high FLIP expression in early rheumatoid arthritis synovium. Annals of the Rheumatic Diseases. 2002;61(10):934–936. doi: 10.1136/ard.61.10.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raza K, Scheel-Toellner D, Lee C-Y, et al. Synovial fluid leukocyte apoptosis is inhibited in patients with very early rheumatoid arthritis. Arthritis Research and Therapy. 2006;8(4, article R120) doi: 10.1186/ar2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eguchi K. Apoptosis in autoimmune diseases. Internal Medicine. 2001;40(4):275–284. doi: 10.2169/internalmedicine.40.275. [DOI] [PubMed] [Google Scholar]
- 38.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes and Development. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura N, Ohmoto Y, Yasui H, et al. The direct effect of FK506 and rapamycin on interleukin 1(β) and immunoglobulin production in vitro. Transplantation. 1994;57(12):1815–1818. [PubMed] [Google Scholar]
- 40.Foey AD, Feldmann M, Brennan FM. CD40 ligation induces macrophage IL-10 and TNF-α production: differential use of the PI3K and p42/44 MAPK-pathways. Cytokine. 2001;16(4):131–142. doi: 10.1006/cyto.2001.0954. [DOI] [PubMed] [Google Scholar]
- 41.Carlson RP, Hartman DA, Tomchek LA, et al. Rapamycin, a potential disease-modifying antiarthritic drug. Journal of Pharmacology and Experimental Therapeutics. 1993;266(2):1125–1138. [PubMed] [Google Scholar]
- 42.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nature Reviews Molecular Cell Biology. 2005;6(6):439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 43.Lleo A, Invernizzi P, Selmi C, et al. Autophagy: highlighting a novel player in the autoimmunity scenario. Journal of Autoimmunity. 2007;29(2-3):61–68. doi: 10.1016/j.jaut.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin Y-J, Han S-H, Kim D-S, et al. Autophagy induction and CHOP under-expression promotes survival of fibroblasts from rheumatoid arthritis patients under endoplasmic reticulum stress. Arthritis Research and Therapy. 2010;12(1, article R19) doi: 10.1186/ar2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grassi F, Cristino S, Toneguzzi S, Piacentini A, Facchini A, Lisignoli G. CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts: CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. Journal of Cellular Physiology. 2004;199(2):244–251. doi: 10.1002/jcp.10445. [DOI] [PubMed] [Google Scholar]
- 46.Kaneko M, Tomita T, Nakase T, et al. Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rhematoid arthritis. Rheumatology. 2001;40(3):247–255. doi: 10.1093/rheumatology/40.3.247. [DOI] [PubMed] [Google Scholar]
- 47.Brömme D, Lecaille F. Cathepsin K inhibitors for osteoporosis and potential off-target effects. Expert Opinion on Investigational Drugs. 2009;18(5):585–600. doi: 10.1517/13543780902832661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim KS, Choi HM, Lee Y-A, et al. Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatology International. 2011;31(4):543–547. doi: 10.1007/s00296-010-1592-1. [DOI] [PubMed] [Google Scholar]
- 49.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Frontiers in Bioscience. 2006;11(1):529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 50.Skoumal M, Haberhauer G, Kolarz G, et al. The imbalance between osteoprotegerin and cathepsin K in the serum of patients with longstanding rheumatoid arthritis. Rheumatology International. 2008;28(7):637–641. doi: 10.1007/s00296-007-0506-3. [DOI] [PubMed] [Google Scholar]
- 51.Richardson VJ. Divergent and synergistic regulation of matrix metalloprotease production by cytokines in combination with C-C chemokines. International Journal of Immunopathology and Pharmacology. 2010;23(3):715–726. doi: 10.1177/039463201002300305. [DOI] [PubMed] [Google Scholar]
- 52.Huang J-L, Wu S-Y, Xie X-J, Wang M-X, Zhu S, Gu J-R. Inhibiting effects of Leflunomide metabolite on overexpression of CD147, MMP-2 and MMP-9 in PMA differentiated THP-1 cells. European Journal of Pharmacology. 2011;670(1):304–310. doi: 10.1016/j.ejphar.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 53.Vandooren B, Kruithof E, Yu DTY, et al. Involvement of matrix metalloproteinases and their inhibitors in peripheral synovitis and down-regulation by tumor necrosis factor α blockade in spondylarthropathy. Arthritis & Rheumatism. 2004;50(9):2942–2953. doi: 10.1002/art.20477. [DOI] [PubMed] [Google Scholar]
- 54.Matsushita I, Uzuki M, Matsuno H, Sugiyama E, Kimura T. Rheumatoid nodulosis during methotrexate therapy in a patient with rheumatoid arthritis. Modern Rheumatology. 2006;16(6):401–403. doi: 10.1007/s10165-006-0522-2. [DOI] [PubMed] [Google Scholar]
- 55.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 56.Prevoo MLL, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LBA, van Riel PLCM. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis & Rheumatism. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 57.Prevoo MLL, van Gestel AM, van ’t Hof MA, van Rijswijk MH, van de Putte LBA, van Riel PLCM. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. British Journal of Rheumatology. 1996;35(11):1101–1105. doi: 10.1093/rheumatology/35.11.1101. [DOI] [PubMed] [Google Scholar]
- 58.Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. Journal of the American Medical Association. 1949;140(8):659–662. doi: 10.1001/jama.1949.02900430001001. [DOI] [PubMed] [Google Scholar]
- 59.van der Heijde DM, van Riel PL, Nuver-Zwart IH, Gribnau FW, van de Putte LB. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. The Lancet. 1989;1(8646):1036–1038. doi: 10.1016/s0140-6736(89)92442-2. [DOI] [PubMed] [Google Scholar]
- 60.Tchetina EV, Poole AR, Zaitseva EM, et al. Differences in Mammalian target of rapamycin gene expression in the peripheral blood and articular cartilages of osteoarthritic patients and disease activity. Arthritis. 2013;2013:14 pages. doi: 10.1155/2013/461486.461486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livak KJ. User Bulletin no. 2: ABI PRISM 7700 Sequence Detection System. Foster City, Calif, USA: PE Applied Biosystems; 1997. Comparative Ct method. [Google Scholar]
- 62.Verweij CL, Vosslamber S. New insight in the mechanism of action of rituximab: the interferon signature towards personalized medicine. Discovery Medicine. 2011;12(64):229–236. [PubMed] [Google Scholar]
- 63.Brkic Z, Olthof ED, Drexhage HA, Versnel MA. Monocyte gene expression signatures in rheumatic diseases: biomarkers for disease activity and tools for diagnosis and classification. The Open Rheumatology Journal. 2010;3:13–17. [Google Scholar]
- 64.Gerards AH, de Lathouder S, de Groot ER, Dijkmans BAC, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology. 2003;42(10):1189–1196. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- 65.Miranda-Carús M-E, Balsa A, Benito-Miguel M, Pérez De Ayala C, Martín-Mola E. Rheumatoid arthritis synovial fluid fibroblasts express TRAIL-R2 (DR5) that is functionally active. Arthritis & Rheumatism. 2004;50(9):2786–2793. doi: 10.1002/art.20501. [DOI] [PubMed] [Google Scholar]
- 66.Vázquez I, Graell E, Gratacós J, et al. Prognostic markers of clinical remission in early rheumatoid arthritis after two years of DMARDs in a clinical setting. Clinical and Experimental Rheumatology. 2007;25(2):231–238. [PubMed] [Google Scholar]
- 67.Ma MHY, Ibrahim F, Walker D, et al. Remission in early rheumatoid arthritis: predicting treatment response. Journal of Rheumatology. 2012;39(3):470–475. doi: 10.3899/jrheum.110169. [DOI] [PubMed] [Google Scholar]
- 68.Smolen JS, van der Heijde DMFM, St Clair EW, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis & Rheumatism. 2006;54(3):702–710. doi: 10.1002/art.21678. [DOI] [PubMed] [Google Scholar]
- 69.Emery P, Genovese MC, Kavanaugh AF, Cohen SB, Perez JL, Sasso EH. Adalimumab plus methotrexate results in less frequent and less severe radiographic progression than methotrexate alone at all levels of clinical response in early rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006;65(supplement 2):p. 88. [Google Scholar]
- 70.Forslind K, Ahlmén M, Eberhardt K, Hafström I, Svensson B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP) Annals of the Rheumatic Diseases. 2004;63(9):1090–1095. doi: 10.1136/ard.2003.014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mori S, Hirose J, Yonemura K. Contribution of HLA-DRB1*04 alleles and anti-cyclic citrullinated antibodies to development of resistance to disease-modifying antirheumatic drugs in early rheumatoid arthritis. Clinical Rheumatology. 2010;29(12):1357–1366. doi: 10.1007/s10067-010-1454-y. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimura N, Ohmoto Y, Yasui H, et al. The direct effect of FK506 and rapamycin on interleukin 1(β) and immunoglobulin production in vitro. Transplantation. 1994;57(12):1815–1818. [PubMed] [Google Scholar]
- 73.Migita K, Eguchi K, Aoyagi T, et al. The effects of the immunosuppressant rapamycin on the growth of rheumatoid arthritis (RA) synovial fibroblast. Clinical and Experimental Immunology. 1996;104(1):86–91. doi: 10.1046/j.1365-2249.1996.d01-651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. Journal of Biological Chemistry. 2005;280(5):3583–3589. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]


