Summary
Background and objectives
Higher body mass index (BMI) is paradoxically associated with lower mortality in persons with CKD, but whether cardiometabolic abnormalities modulate this association is unclear.
Design, setting, participants, & measurements
Participants with CKD from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study (n=4374) were analyzed. The harmonized criteria for metabolic syndrome were used to define metabolic health, and participants were categorized into one of six mutually exclusive categories defined by combined measures of metabolic health (metabolically healthy, <3 criteria for metabolic syndrome; metabolically unhealthy, ≥3 criteria) and weight status (normal weight, BMI 18.5–24.9 kg/m2; overweight, BMI 25–29.9 kg/m2; obese, BMI ≥30 kg/m2). Cox models were used to estimate the hazard ratio (HR) of death as a function of each category.
Results
A total of 683 deaths were observed over a mean 4.5 years of follow-up. In analyses adjusted for age, race, sex, and geographic region of residence, compared with metabolically healthy normal weight persons, the HRs of mortality in metabolically healthy overweight and obese persons were 0.68 (95% confidence interval [95% CI], 0.53 to 0.87) and 0.71 (95% CI, 0.51 to 0.98), respectively, whereas there were no statistically significant differences in survival among metabolically unhealthy overweight or obese individuals. After further adjustment for lifestyle, clinical and laboratory factors including markers of kidney function, the HR of mortality remained lower in metabolically healthy overweight individuals compared with metabolically healthy normal weight individuals (HR, 0.74; 95% CI, 0.57 to 0.96).
Conclusions
Metabolic abnormalities may attenuate the magnitude and strength of survival benefits associated with higher BMI in individuals with CKD.
Introduction
Although obesity is associated with adverse cardiovascular and kidney disease outcomes, there is increasing evidence that the magnitude and direction of these associations depend, in part, on the clustering of metabolic and cardiovascular risk factors (1). For example, certain individuals meet standard clinical criteria for obesity (i.e., body mass index [BMI] ≥30 kg/m2) yet appear to have healthy metabolic profiles, characterized by normal insulin sensitivity, BP, and lipid parameters. Alternatively, there are individuals who are classified as normal weight (BMI 18.5–25 kg/m2) yet manifest numerous cardiometabolic risk factors. Population-based studies suggest that “metabolically healthy” obese individuals may have similar or lower risks for cardiovascular disease and mortality as their nonobese counterparts, whereas “metabolically unhealthy” nonobese individuals may have similar cardiovascular risk as obese individuals with abnormal metabolic profiles (2).
Consideration of metabolic profile may be of particular importance in individuals with CKD. This is because studies have shown that there is a paradoxical relationship between higher BMI and outcomes in CKD populations. Individuals with kidney disease who have a high BMI, even when in the morbid range (>40 kg/m2), have better survival than their lower weight counterparts (3–8), although most of these data have been generated in ESRD populations. This relationship has largely been attributed to the high prevalence of protein-energy wasting and inflammation in CKD patients, factors that often result in weight loss and are strongly linked to metabolic dysfunction and increased mortality (4,9,10). In addition, BMI reflects not only fat mass but also muscle mass, and higher muscle mass may mediate some of the protective effects observed with higher BMI (6). As such, individuals with CKD who can maintain higher BMI, even at levels beyond those characterized as normal, while maintaining normal metabolic function, may have better nutritional status, and thus better long-term health outcomes. The primary goal of this study was to assess this possibility by examining the associations of metabolically healthy and unhealthy subtypes with all-cause mortality in normal weight, overweight, and obese individuals with CKD.
Materials and Methods
Study Participants
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a population-based investigation of stroke incidence in black and white US adults aged ≥45 years. Details of the study design were reviewed elsewhere (11). Briefly, participants were recruited from the 48 contiguous US states and the District of Columbia. The study was designed to provide approximately equal representation of men and women, and an oversampling of blacks and persons living in the “stroke belt/buckle” of the United States, both groups who have excess stroke mortality. Trained interviewers conducted computer-assisted telephone interviews to obtain information including participants’ sociodemographics, cardiovascular risk factors, tobacco usage, physical activity, and use of medications. After this call, an in-home study visit was conducted that included an electrocardiograph (ECG) recording, inventory of medications, and collection of blood and urine samples.
Overall, 30,239 black and white adults were enrolled between January 2003 and October 2007 (42% black, 55% women). For this study, we limited the analysis to participants with CKD (defined as an estimated GFR [eGFR] <60 ml/min per 1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation (12) or an albumin to creatinine ratio [ACR] ≥30 mg/g, both obtained from blood/urine collected on a single occasion) who were not receiving renal replacement therapy at baseline (n=6009). We excluded individuals who were lost to follow-up (n=92), had a BMI <18.5 kg/m2 (n=132), were not fasting or were missing data on fasting status at the time of blood draw (n=1015), or were missing data on any of the metabolic parameters of interest (n=396), leaving 4374 participants in the final analytic sample. The REGARDS study protocol was approved by the institutional review boards governing research in human subjects at the participating centers and all participants provided informed consent.
Primary Exposures
The exposures of interest were categories of metabolically healthy and unhealthy subtypes by weight status (normal weight, overweight, and obese). Metabolic subtypes were defined in accordance with the harmonized criteria for metabolic syndrome (13). Specifically, individuals who had evidence of ≥3 of the following criteria for the metabolic syndrome were categorized as having an unhealthy metabolic profile: elevated waist circumference (>102 cm for men; >88 cm for women), elevated fasting triglycerides (≥150 mg/dl), reduced fasting HDL (<40 mg/dl in men; <50 mg/dl in women), elevated BP (systolic ≥130 mmHg and/or diastolic ≥85 mmHg or current treatment with antihypertensive therapy), and elevated fasting glucose (≥100 mg/dl) or known history of diabetes (ascertained by self-report or current therapy with diabetes medications). Waist circumference (in centimeters) was measured during the in-home visit using a tape measure positioned midway between the lowest rib and the iliac crest with the participant standing. Systolic and diastolic BPs were defined as the average of two seated measures taken after a 5-minute rest. Each participant was categorized into one of six mutually exclusive categories: (1) normal weight (BMI 18.5–24.9 kg/m2) and healthy metabolic profile (<3 criteria for metabolic syndrome), (2) normal weight and unhealthy metabolic profile (≥3 criteria for metabolic syndrome), (3) overweight (BMI 25–29.9 kg/m2) and healthy metabolic profile, (4) overweight and unhealthy metabolic profile, (5) obese (BMI ≥30 kg/m2) and healthy metabolic profile, and (6) obese and unhealthy metabolic profile.
Ascertainment of Outcomes
The outcome of interest was death, ascertained from contact with proxies provided by the participant upon recruitment or during follow-up and by searching the Social Security Death Index or through the National Death Index. The REGARDS study staff confirmed dates of death through the Social Security Death Index, death certificates, or the National Death Index. Follow-up time for each participant was calculated from the date of the in-home visit to the date of death or last telephone follow-up for nondeceased participants, updated through March 31, 2011.
Covariates of Interest
Age, race, sex, annual family income, educational attainment, and tobacco and alcohol use history were determined by self-report. Physical activity was assessed through a single question: “How many times per week do you engage in intense physical activity, enough to work up a sweat,” with response options as follows: none, 1–3 times per week, or >4 times per week. History of coronary heart disease (CHD) was defined as having any of the following: evidence of myocardial infarction on the baseline ECG, self-report of a prior history of a cardiac procedure (coronary artery bypass surgery or percutaneous angioplasty), or self-reported history of myocardial infarction. History of stroke was ascertained by self-report.
Statistical Analyses
Standard descriptive statistics were used to examine baseline demographic, clinical, and laboratory characteristics of CKD participants according to weight and metabolic subtype categories. Next, mortality rates were calculated by each weight and metabolic subtype category. After confirming the proportionality of hazards assumption, Cox regression models were used to estimate the hazard ratio (HR) of mortality as a function of each weight and metabolic subtype category in sequential models, with metabolically healthy normal weight individuals being the referent group in all models. Model 1 was unadjusted. Model 2 was adjusted for age, sex, and geographic region of residence (stroke belt, stroke buckle, or other). Model 3 was adjusted for variables in model 2 plus lifestyle factors (self-reported physical activity, current tobacco usage), comorbidities (history of heart disease and stroke), educational achievement (< versus ≥high school diploma), annual family income (< versus ≥$20,000 per year), eGFR and natural log-transformed urinary ACR. We did not adjust for diabetes or hypertension in these models because they are used in defining the metabolically unhealthy phenotype. In prespecified analyses, we examined for effect modification by race, sex, and age by testing the statistical significance of interaction terms. We also conducted the following sensitivity analyses: (1) adding elevated high-sensitivity C-reactive protein (hsCRP) concentration (≥3 mg/L) as an extra criterion for the metabolic syndrome, (2) using World Health Organization (WHO) criteria to define metabolic syndrome (13), (3) further adjusting the models for hsCRP and LDL cholesterol concentrations (4), using the combined creatinine and cystatin C equation for estimating GFR to define CKD (14), and (5) further subdividing the obese category into morbid (≥40 kg/m2) and nonmorbid (30–39.9 kg/m2) classifications. A two-tailed P value <0.05 was considered statistically significant for all analyses. All analyses were conducted using SAS software version 9.2 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
The mean age of the study sample was 69±10 years, and 47% of participants were men and 45% were black. The mean eGFR was 68.5±25.0 ml/min per 1.73 m2, and the median urinary ACR was 44.4 mg/g (interquartile range, 13.9–113.5 mg/g). Table 1 depicts the baseline demographic and clinical characteristics and Table 2 depicts individual metabolic parameters of study participants according to the six categories of weight and metabolic subtype. In general, within each weight category, individuals classified as metabolically unhealthy were more likely to have lower income, were more likely to have a history of CHD and stroke, and were more likely to report lower physical activity than individuals classified as metabolically healthy. In addition, among overweight and obese individuals, participants categorized as metabolically unhealthy had lower mean eGFR and higher median urinary ACR than those categorized as metabolically healthy.
Table 1.
Participant characteristics by weight status and metabolic subtype
| Characteristics | Normal Weight (BMI 18.5–24.9 kg/m2) | Overweight (BMI 25–29.9 kg/m2) | Obese (BMI ≥30 kg/m2) | |||
|---|---|---|---|---|---|---|
| Metabolically Healthy (n=777) | Metabolically Unhealthy (n=178) | Metabolically Healthy (n=771) | Metabolically Unhealthy (n=752) | Metabolically Healthy (n=425) | Metabolically Unhealthy (n=1471) | |
| Age (yr) | 72±10.1 | 72±8.7 | 70±9.3 | 70±8.9 | 66±9.4 | 66±8.8 |
| Men | 47 | 48 | 57 | 51a | 46 | 41 |
| Black | 33 | 33 | 40 | 41 | 62 | 53a |
| Low household income | ||||||
| <$20,000 per year | 21 | 22 | 18 | 25a | 20 | 28a |
| ≥$20,000 per year | 64 | 61 | 68 | 63 | 69 | 59 |
| Education | ||||||
| <High school diploma | 13 | 12 | 12 | 19b | 18 | 20 |
| ≥High school diploma | 87 | 88 | 88 | 81 | 82 | 80 |
| History of coronary heart disease | 27 | 31 | 24 | 32b | 20 | 28b |
| History of stroke | 10 | 16a | 9 | 12 | 6 | 11a |
| Medication use | ||||||
| Statins | 33 | 37 | 40 | 45a | 35 | 47b |
| Antihypertensives | 55 | 75b | 58 | 81b | 62 | 84b |
| Current smoking | 21 | 20 | 12 | 15 | 10 | 14 |
| Alcohol consumption | ||||||
| None | 62 | 66 | 64 | 71a | 70 | 75 |
| Mild | 32 | 29 | 33 | 25 | 26 | 23 |
| Heavy | 6 | 5 | 3 | 3 | 3 | 2 |
| Physical activity (times per week) | ||||||
| None | 38 | 52b | 34 | 43b | 34 | 47b |
| 1–3 | 30 | 21 | 33 | 32 | 40 | 33 |
| >4 | 32 | 27 | 33 | 26 | 25 | 20 |
| Geographic region of residence | ||||||
| Stroke belt | 47 | 39a | 49 | 45 | 49 | 43 |
| Stroke buckle | 25 | 20 | 20 | 19 | 21 | 23 |
| Non-stroke belt/buckle | 28 | 41 | 31 | 35 | 30 | 34 |
| UACR (mg/g) | 40.9 (12.1–86.9) | 42.3 (12.9–180.6) | 36.2 (9.3–76.6) | 46.7 (15.8–126.1)b | 41.3 (16.7–98.0) | 55.6 (22.6–144.6)b |
| eGFR (ml/min per 1.73 m2) | 67.7±23.1 | 62.8±21.9a | 67.9±23.6 | 64.9±24.6a | 74.4±25.5 | 70.1±26.5a |
| >60 | 49 | 42a | 47 | 45a | 59 | 55a |
| 45–60 | 34 | 43 | 39 | 35 | 31 | 29 |
| 30–44.9 | 13 | 10 | 11 | 15 | 8 | 12 |
| <30 | 3 | 5 | 3 | 5 | 2 | 5 |
Data are presented as the mean ± SD, median (interquartile range), or frequency. US states in the stroke belt/buckle include North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas. UACR, urinary albumin/creatinine ratio; eGFR, estimated GFR.
P<0.05 for difference between metabolic subtypes within individual weight categories.
P<0.001 for difference between metabolic subtypes within individual weight categories.
Table 2.
Metabolic parameters by weight status and metabolic subtype
| Characteristics | Normal Weight (BMI 18.5–24.9 kg/m2) | Overweight (BMI 25–29.9 kg/m2) | Obese (BMI ≥30 kg/m2) | |||
|---|---|---|---|---|---|---|
| Metabolically Healthy (n=777) | Metabolically Unhealthy (n=178) | Metabolically Healthy (n=771) | Metabolically Unhealthy (n=752) | Metabolically Healthy (n=425) | Metabolically Unhealthy (n=1471) | |
| Waist circumference (cm) | 82.5±9.3 | 89.4±10.1a | 91.9±9.4 | 97.9±9.1a | 103.7±13.6 | 112.3±12.8a |
| Elevated waist circumferenceb | 4.3 | 27.5 | 21.0 | 65 | 72.2 | 96.1 |
| Fasting triglycerides (mg/dl) | 103.0±45.9 | 198.6±88.9a | 108.6±49.7 | 188.5±145.3a | 99.6±34.5 | 168.9±107.3a |
| Triglycerides ≥150 mg/dl | 10.6 | 70.8 | 11.3 | 58.8 | 4.0 | 46.9 |
| HDL (mg/dl) | 58.5±16.9 | 42.4±13.1a | 55.4±16.0 | 43.1±13.0a | 56.9±14.7 | 44.7±13.8a |
| Low HDLc | 16.6 | 74.2 | 17.9 | 66.2 | 11.1 | 61.3 |
| Fasting glucose (mg/dl) | 93.6±24.0 | 121.5±58.1a | 98.6±31.8 | 118.2±47.9 | 97.0±33.9 | 126.4±52.9a |
| Elevated fasting glucosed | 21.2 | 72.5 | 23.7 | 71.9 | 16.5 | 82.0 |
| SBP (mmHg) | 128.5±19.0 | 133.8±17.3a | 130.0±18.1 | 134.7±17.8a | 130.9±16.3 | 135.7±18.2a |
| DBP (mmHg) | 74.6±10.5 | 75.7±9.3 | 76.5±10.0 | 76.8±10.3 | 79.1±10.2 | 78.9±11.0 |
| Elevated BPe | 70.4 | 94.9 | 72.4 | 94.1 | 73.4 | 95.0 |
| hsCRP (mg/L) | 1.6 (0.7–4.5) | 2.7 (1.3–6.9)a | 2.1 (0.9–4.5) | 2.5 (1.2–5.7)f | 3.2 (1.5–6.5) | 4.2 (2.0–8.3)a |
| LDL (mg/dl) | 108.3±33.9 | 107.2±37.0 | 112.5±35.3 | 112.1±37.2 | 114.9±33.6 | 109.6±35.7f |
Data are presented as the mean ± SD, median (interquartile range), or frequency. SBP, systolic BP; DBP, diastolic BP; hsCRP, high-sensitivity C-reactive protein.
P<0.001 for difference between metabolic subtypes within individual weight categories.
Defined as waist circumference >102 for men or >88 cm for women.
Defined as <40 mg/dl for men or <50 mg/dl for women.
Defined as fasting glucose ≥100 mg/dl, history of diabetes, or current antidiabetic medication use.
Defined as SBP ≥130 mmHg, DBP ≥85 mmHg, and/or current antihypertensive medication use.
P<0.05 for difference between metabolic subtypes within individual weight categories.
Associations of Weight and Metabolic Subtype with Mortality
A total of 683 deaths were observed over a mean follow-up of 4.5 years. Table 3 presents total mortality events and mortality rates per 1000 person-years of follow-up (95% confidence intervals [CIs]) according to weight and metabolic subtype categories. In general, mortality rates were higher in normal weight individuals than in overweight or obese individuals, irrespective of metabolic health.
Table 3.
Mortality rates per 1000 person-years of follow-up by weight and metabolic subtype overall and stratified by race
| Participant Mortality Rates | Normal Weight (BMI 18.5–24.9 kg/m2) | Overweight (BMI 25–29.9 kg/m2) | Obese (BMI ≥30 kg/m2) | |||
|---|---|---|---|---|---|---|
| Metabolically Healthy | Metabolically Unhealthy | Metabolically Healthy | Metabolically Unhealthy | Metabolically Healthy | Metabolically Unhealthy | |
| Participants (n) | 777 | 178 | 771 | 752 | 425 | 1471 |
| Events | 153 | 49 | 106 | 126 | 47 | 202 |
| Mortality rate | 44.2 (37.7 to 51.7) | 61.6 (46.5 to 81.5) | 29.2 (24.2 to 35.4) | 35.5 (29.8 to 42.2) | 23.9 (18.0 to 31.9) | 30.4 (26.4 to 34.8) |
Data are presented as the frequency or mortality rate (95% confidence interval).
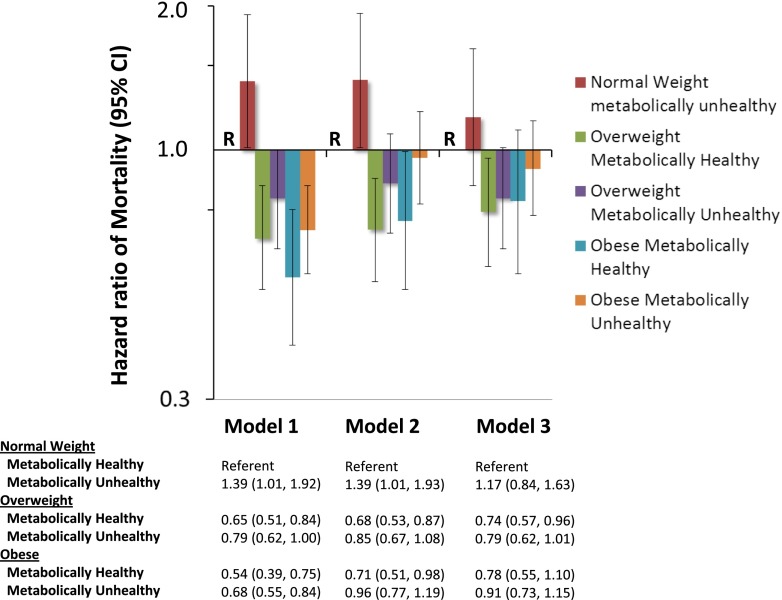
Figure 1 depicts unadjusted and multivariable-adjusted Cox proportional hazard models examining the association of weight and metabolic subtype categories with death. In the unadjusted analysis (model 1), compared with metabolically healthy normal weight individuals, the HR of mortality was significantly lower in overweight and obese individuals irrespective of metabolic subtype. After adjustment for age, race, sex, and geographic region of residence (model 2), the risk of mortality remained significantly lower among metabolically healthy overweight (HR, 0.68; 95% CI, 0.53 to 0.87) and obese individuals (HR, 0.71; 95% CI, 0.51 to 0.98) compared with metabolically healthy normal weight individuals, whereas the lower risk of mortality associated with higher weight in metabolically unhealthy individuals was attenuated and no longer statistically significant. When further adjusted for educational achievement, annual household income, physical activity, tobacco use, CHD, stroke, eGFR, and natural log-transformed urinary ACR (model 3), compared with metabolically healthy normal weight individuals, the risk of death remained significantly lower among metabolically healthy overweight individuals (HR, 0.74; 95% CI, 0.57 to 0.96) but not metabolically healthy obese individuals (HR, 0.78; 95% CI, 0.55 to 1.10).
Figure 1.
Unadjusted and multivariable-adjusted hazard ratios of mortality (95% confidence intervals) according to weight and metabolic subtypes. Model 1 is unadjusted. Model 2 is adjusted for age, sex, and geographic region of residence. Model 3 is adjusted for variables in model 2 plus lifestyle factors (self-reported physical activity, current smoking), comorbidities (history of coronary heart disease and stroke), educational achievement (< versus ≥ high school diploma), annual family income (< versus ≥$20,000 per year), natural log-transformed urinary albumin to creatinine ratio, and estimated GFR. Metabolically healthy individuals with normal weight are the referent group (R) in all models. Vertical bars represent 95% confidence intervals.
The interaction between metabolic subtype/weight categories and race on all-cause mortality was of borderline statistical significance (P=0.08). In analyses stratified by race, the magnitude and strength of the association between higher BMI and lower mortality among metabolically healthy individuals appeared to be greater among blacks than whites—in fully adjusted models among blacks, the risk of death was significantly lower among metabolically healthy overweight (HR, 0.57; 95% CI, 0.37 to 0.88) and obese individuals (HR, 0.62; 95% CI, 0.39 to 0.99) compared with metabolically healthy normal weight individuals, whereas there were no statistically significant differences in survival in any metabolic subtype/weight categories among whites (Supplemental Table 1).
Sensitivity Analyses
The results were not materially changed when we used alternative criteria for defining the metabolic syndrome (i.e., adding an elevated hsCRP concentration as an extra criterion for the metabolic syndrome or using WHO criteria), after further adjusting for serum LDL cholesterol and hsCRP concentrations in multivariable models, when using the combined creatinine and cystatin C equation for estimating GFR to define CKD (14), or when restricting the study sample to individuals with an eGFR <60 ml/min per 1.73 m2 (data not shown). Supplemental Table 2 depicts the results of the analyses after further subdividing the obese category into morbid and nonmorbid ranges. In the unadjusted model, there was no statistically significant association between morbid obesity and mortality. However, in the fully adjusted model, metabolically unhealthy participants who were morbidly obese had a significantly higher HR of mortality than metabolically healthy normal weight participants (HR, 1.49; 95% CI, 1.03 to 2.13). Adjustment for age was primarily responsible for the reversal of the direction of this association.
Discussion
In this population-based study of black and white adults with predialysis CKD, compared with metabolically healthy normal weight individuals, metabolically healthy overweight individuals had a significantly lower risk of all-cause mortality. In contrast, no differences in survival were observed in metabolically unhealthy overweight or obese individuals compared with their metabolically healthy normal weight counterparts in fully adjusted analyses. These findings suggest that metabolic abnormalities such as insulin resistance, lipid disorders, excess visceral adiposity, and hypertension may mitigate the survival benefits associated with higher BMI in persons with CKD.
Previous studies have shown that higher BMI is associated with improved survival in both ESRD and predialysis CKD populations (3–8). Our results add to these prior findings by showing that these relationships appear to depend in part on the presence or absence of cardiometabolic risk factors. Indeed, the magnitude and statistical strength of the survival benefit associated with higher BMI was greater among overweight individuals with a healthy metabolic profile than among individuals with an unhealthy metabolic profile. A previous study examined the effect of metabolic parameters on the association between obesity and survival in individuals with predialysis CKD (7). In this study involving participants of the Atherosclerosis Risk in Communities study, no association was observed between BMI and survival in a multivariable model adjusted for age, sex, race, cancer, smoking, and alcohol use. However, after further adjustment for indices of the metabolic syndrome, a statistically significant association of higher BMI with improved survival emerged in individuals with CKD, suggesting that the deleterious effects of metabolic syndrome factors may be outweighed by potential protective effects of obesity in CKD. This may partly explain why we did not observe a net increased risk of mortality in metabolically unhealthy individuals in this study.
Comparable to what has been shown in other cohorts investigating all-cause mortality (1,15), we observed that the metabolically healthy phenotype comprised 22% of the obese participants and 51% of the overweight participants. The extent to which one can be “obese” yet metabolically healthy has remained controversial for over a decade (16). Variability in the metabolic activity of different adipose storage sites may be key to understanding these relationships. Visceral adiposity is more metabolically active than other adipose tissue sites (17) and appears to contribute to many metabolic abnormalities across populations (18–26). Therefore, it is plausible that excess visceral adipose tissue may partly explain the differential association of obesity with survival in metabolically healthy versus metabolically unhealthy individuals. In support of this, we previously reported that increased waist circumference was a stronger predictor of mortality risk than BMI in REGARDS participants with CKD (6). In addition, differences in survival in metabolically healthy obese individuals compared with metabolically healthy normal weight individuals was recently shown to be attributed to cardiorespiratory fitness, such that a greater risk of morbidity and mortality among the obese was attenuated when fitness indices were included in the analyses (27). Assessing the role of fitness may be of value in future studies on this topic.
We also observed potential racial differences in the association of metabolic health/weight subtypes and death, although the interaction was of borderline statistical significance. The reason for these potential differences is unclear. A recent study utilizing data from the Southern Community Cohort Study found that adult-onset obesity among blacks was not associated with the same excess mortality risk seen among whites, suggesting the possibility of a differential effect of body composition apart from BMI, such as variation in fat distribution, differences in metabolic pathways, or a combination of both (28). Blacks in general have less visceral fat than whites at a similar BMI, which may at least partly explain the race-specificity of the relationships identified herein (29). Nevertheless, although our findings are intriguing, given the borderline statistical significance of these results, future studies with larger sample sizes will need to confirm whether associations of metabolic health and mortality are indeed modified by race.
Our study had limitations. BMI as a measure of obesity cannot distinguish between fat and lean tissue, preventing a potential mechanistic explanation of race-related differences. Similarly, waist circumference may reflect varying levels of abdominal visceral fat, particularly among older populations (30,31). Next, whether age-, race-, or sex-specific thresholds for the individual criteria of the metabolic syndrome are more appropriate for defining the metabolic syndrome than the criteria used herein remains unclear and should be the focus of future studies. In addition, we only had a single measurement of ACR and eGFR, which may have led to exposure misclassification for some study participants. Furthermore, the proportion of participants with eGFR <30 ml/min per 1.73 m2 was relatively low in this cohort, making the results most applicable to individuals with mild to moderate CKD. In addition, we were missing or had incomplete data on other potentially relevant confounders such as serum albumin, serum phosphorus, dietary intake, and comorbidities such as a verified history of cancer. Finally, we had relatively few events in certain weight/metabolic subtype categories, which likely limited our ability to detect statistically significant associations with mortality for some of these categories.
In summary, the results of this study suggest that the metabolic profile of CKD patients may be critical for properly assessing the effect of weight status on health outcomes. Additional studies with specific body composition measures and/or interventional studies examining the health benefits/risks of weight loss in CKD patients will likely add valuable insights into survival benefits by weight status, including how these relationships may differ by race.
Disclosures
D.G.W. is a member of the Amgen National Nephrology Advisory Board. O.M.G., P.M., W.M.M., and D.G.W. have received research support from the Amgen Corporation.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
This study was supported by a cooperative agreement (U01 NS041588) from the National Institutes of Health National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. In addition, L.J.H. was supported by Grant T32DK007545 and O.M.G. was supported by Grants K23DK081673 and R03DK095005 from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases.
Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation of the manuscript. The manuscript was sent to Amgen Corporation for review before submission for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00140113/-/DCSupplemental.
References
- 1.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR: The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 168: 1617–1624, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP: Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 20: 651–659, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal R: Body mass index-mortality paradox in hemodialysis: Can it be explained by blood pressure? Hypertension 58: 1014–1020, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R, Anker SD: The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc 85: 991–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 49: 581–591, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Kramer H, Shoham D, McClure LA, Durazo-Arvizu R, Howard G, Judd S, Muntner P, Safford M, Warnock DG, McClellan W: Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis 58: 177–185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwan BC, Murtaugh MA, Beddhu S: Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol 2: 992–998, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ: Body size and outcomes on peritoneal dialysis in the United States. Kidney Int 64: 1838–1844, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB: Survival advantages of obesity in dialysis patients. Am J Clin Nutr 81: 543–554, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Kovesdy CP, Kalantar-Zadeh K: Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol 29: 3–14, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G: The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology 25: 135–143, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr, International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity : Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blüher M: The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 21: 38–43, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Lee CD, Blair SN, Jackson AS: Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr 69: 373–380, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Jensen MD: Adipose tissue and fatty acid metabolism in humans. J R Soc Med 95[Suppl 42]: 3–7, 2002 [PMC free article] [PubMed] [Google Scholar]
- 18.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX: Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes 46: 456–462, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Berg AH, Scherer PE: Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96: 939–949, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Bonora E: Relationship between regional fat distribution and insulin resistance. Int J Obes Relat Metab Disord 24[Suppl 2]: S32–S35, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Colberg SR, Simoneau JA, Thaete FL, Kelley DE: Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest 95: 1846–1853, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Després JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, Thériault G, Pinault S, Bouchard C: Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes 38: 304–309, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Ding J, Visser M, Kritchevsky SB, Nevitt M, Newman A, Sutton-Tyrrell K, Harris TB: The association of regional fat depots with hypertension in older persons of white and African American ethnicity. Am J Hypertens 17: 971–976, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ross R, Fortier L, Hudson R: Separate associations between visceral and subcutaneous adipose tissue distribution, insulin and glucose levels in obese women. Diabetes Care 19: 1404–1411, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Ross R, Aru J, Freeman J, Hudson R, Janssen I: Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab 282: E657–E663, 2002 [DOI] [PubMed] [Google Scholar]
- 26.You T, Ryan AS, Nicklas BJ: The metabolic syndrome in obese postmenopausal women: Relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab 89: 5517–5522, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN: The intriguing metabolically healthy but obese phenotype: Cardiovascular prognosis and role of fitness. Eur Heart J 34: 389–397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen SS, Signorello LB, Cope EL, McLaughlin JK, Hargreaves MK, Zheng W, Blot WJ: Obesity and all-cause mortality among black adults and white adults. Am J Epidemiol 176: 431–442, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R: Visceral fat, waist circumference, and BMI: Impact of race/ethnicity. Obesity (Silver Spring) 16: 600–607, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Harris TB, Visser M, Everhart J, Cauley J, Tylavsky F, Fuerst T, Zamboni M, Taaffe D, Resnick HE, Scherzinger A, Nevitt M: Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women. The Health, Aging and Body Composition Study. Ann N Y Acad Sci 904: 462–473, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER: Racial differences in amounts of visceral adipose tissue in young adults: The CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr 69: 381–387, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.