Summary
Background and objectives
In carefully selected individuals, receiving expanded criteria donor (ECD) kidneys confer a survival advantage over remaining on dialysis. However, wait lists for ECD kidneys often include a significant proportion of young patients, who have no predictable survival benefit from ECD kidneys. This study hypothesized that educational and socioeconomic factors might influence a younger patient’s decision to accept an ECD kidney.
Design, setting, participants, & measurements
This study was a retrospective analysis of all first single-kidney transplants in the Scientific Registry of Transplant Recipients database from 2000 to 2009 in patients aged 18–40 years and waitlisted <3 years. The primary outcome measured was the odds of receiving an ECD kidney compared with an standard criteria donor kidney in different demographic subgroups. Race, income, and education were analyzed in main-effect and two-way interaction models, corrected for candidate panel reactive antibodies and sex.
Results
Of 13,615 ECD transplants, 591 kidneys (4.3%) went to recipients aged between 18 and 40 years who were waitlisted <3 years. African Americans (odds ratio, 1.71; 95% confidence interval, 1.26 to 2.33) or those with low education (odds ratio, 2.32; 95% confidence interval, 1.38 to 3.89) were more likely to receive an ECD kidney than Caucasians or those with a college degree, respectively. However, African Americans with higher education levels did not have significantly higher odds of receiving an ECD kidney than Caucasians with a college degree.
Conclusions
In patients aged <40 years and waitlisted <3 years, African Americans and those with lower educational status and low income are more likely to receive an ECD kidney than Caucasians or those with higher education. It is important that health care providers and patients understand such disparities to facilitate a more rational use of ECD kidneys.
Introduction
The survival advantages of renal transplantation over dialysis, coupled with the shortage of available organs (1–3), have driven attempts to increase the recovery and utilization of less-than-ideal kidneys. Kauffman et al. proposed the term expanded criteria donor kidneys to describe transplantable organs that did not meet the criteria for standard donor organs (4). In November 2001, the Organ Procurement and Transplantation Network (OPTN) approved the definition of an expanded criteria donor (ECD) kidney as any kidney from a donor aged >60 years or between the ages of 50 and 60 years with any two of the following three criteria: terminal creatinine >1.5 mg/dl, cerebrovascular accident (CVA) as a cause of death, or a history of hypertension (5). Although ECD kidneys have a 70% increased risk of graft loss compared with kidneys from a standard criteria donor (SCD) (6), in carefully selected individuals, transplantation with an ECD kidney will confer a survival advantage over remaining on dialysis (while waiting for the optimal kidney).
When the ECD policy was implemented in October 2002, older patients, patients with diabetes, and those with limited vascular access were considered appropriate candidates for an ECD kidney (7). However, for an individual patient, it is difficult to predict who will benefit from accepting a higher risk of graft loss for a shorter time on dialysis. On a transplant waiting list, older patients have a higher mortality risk than younger patients and are predicted to benefit from ECD kidneys. The Eurotransplant Seniors Program, which selectively allocates donor kidneys from persons aged >65 years to recipients who are also aged >65 years, demonstrated good graft and patient survival (8). Schold and Meier-Kriesche showed that patients aged <40 years who accepted an ECD kidney after 2 years on dialysis had worse outcomes than those who received a SCD kidney after 4 years on dialysis (9). Like older patients with limited life expectancy, patients with diabetes have dismal survival on dialysis (10–12) and are expected to benefit from accepting an ECD kidney in exchange for shorter waiting times on dialysis. However, Merion et al. did not find this benefit in patients with diabetes aged <40 years or in Hispanics (13). Another important factor in the decision process is the projected waiting time for a SCD kidney. Merion et al. found that patients in centers with a projected waitlist time >1350 days had a 27% decrease in mortality when accepting an ECD kidney. This mortality benefit increased to 31% in those aged >40 years (13). Merion et al. proposed that ECD kidneys should be allocated to non-Hispanics aged >40 years and to persons with diabetes or a projected transplant wait time >1350 days. Recently, a study by Gram et al. validated this algorithm and determined that patients predicted to benefit from ECD kidneys have a survival advantage with ECD kidney transplants, whereas there was a higher risk of death in low-risk individuals who received ECD kidneys (14).
Transplant centers have varied outcomes, likely due to wide variations in practice between and within centers (9,14). Whereas some centers do not perform ECD kidney transplants at all, other centers either offer them selectively to only high-risk patients or all eligible renal transplant patients. Studies have shown that despite the current scientific evidence, ECD kidney lists often include a large percentage of patients who are unlikely to benefit from an ECD kidney (14). Because the decision to accept an ECD kidney or wait for a SCD donor is a complicated one, we hypothesized that education, income, and other demographic variables might influence the decision to accept an ECD kidney even when a survival advantage is not present. To clearly identify a group that would not have a survival advantage with ECD kidneys, we restricted our analysis to those individuals aged <40 years that had been waitlisted <3 years.
Materials and Methods
We examined data from the national Scientific Registry of Transplant Recipients (SRTR) database from adults (aged ≥18 years) that received transplants between January 1, 2000 and November 1, 2009. We included adults who were aged ≤40 years, had been on the waiting list for ≤3 years, and had received an ECD kidney. We only included patients who received a single- or first-organ transplant. The Internal Revenue Service provided adjusted gross income (AGI) data from 2001. The AGI was based on the candidates’ zip code. Only candidates’ records that contained a valid zip code were included in this study.
Independent Variables
We examined the effect of recipient demographic variables including education, income, race, sex, and presence of panel reactive antibodies (PRAs) on the likelihood of being allocated an ECD kidney. Education was categorized into four levels: associate’s, bachelor’s, or postgraduate degree, technical degree, high school diploma, and grade school, none, or unknown. We grouped those whose educational status was unknown along with the grade school or less category, because a preliminary analysis indicated no differences in outcomes of unknown education as a group unto itself. Races were categorized as Caucasian, African American, Asian, Hispanic, or other minority. Transplant centers were assigned a numerical code in the database without reference to the name or location of the center. On the basis of these codes, a center effect variable was created relating to the frequency of ECD allocation. The AGI classification was determined by a univariate procedure of interquartile ranges to yield the following four groups (in $1000s): ≤31, 31–38, 38–50, and >50.
Outcome Measures
We analyzed the transplant recipients’ likelihood of receiving an ECD kidney. If the SRTR expanded criteria variable was labeled as “0” (non-ECD kidney recipient) or missing, the patient record was defined as a nonrecipient of an ECD kidney. We also analyzed the likelihood of encountering allograft failure following SRTR-recommended methodologies (15).
Statistical Analyses
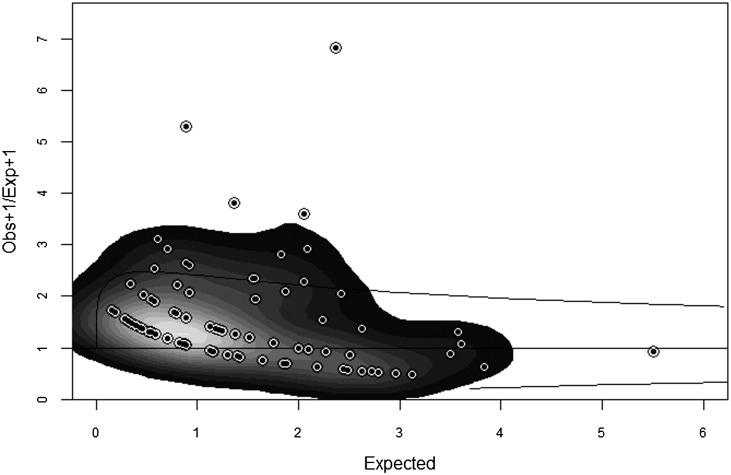
Multivariate logistic models were used to assess the likelihood of receiving an ECD kidney transplant and were adjusted for associated factors including race, education, AGI, sex, current PRA, and OPTN transplant center. Utilizing the same covariates as the main-effect model, two-way interaction models were developed for race by education and race by AGI. The two-way adjusted Cox proportional hazard models assessed the likelihood of allograft failure after transplant. Outcomes were measured by Cox multivariate proportional hazard models adjusting for the same covariates as the logistic model and with univariate Kaplan–Meier models. Adjusted Cox proportional hazard models were also used to generate plots to examine the time to allograft failure for the main-effects model. Proportional hazard assumptions were tested by visually assessing log-log survival curves. The exact method was used to estimate tied outcome occurrences. To examine a center effect, an expected number of ECD transplants were calculated for each of the 88 centers transplanting ECDs, based on the number of total transplants performed in the study population assuming a chi-squared distribution. A funnel plot with 95% confidence intervals (95% CIs) was generated by plotting the observed by expected ECD transplants at these centers (Figure 1). To further define the quality of the donor organ, we also examined the cause of death for the donors and included it as a covariate in the analysis.
Figure 1.
The funnel plot of observed/expected ECD kidney transplants in the study population. The plot shows the observed/expected ratio and 95% confidence interval for each of the study centers transplanting ECDs in the study population. The intensity of gray indicates the amount of deviation from an observed/expected ratio of 1. Most of the centers are at or close to 1. ECD, expanded criteria donor.
All analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC), and a type-one error probability of 0.05 indicated statistical significance.
Results
Of the kidneys allocated to the study population (n=14,163), 1.68% of the kidneys were from ECDs. The number of ECD kidneys peaked in 2005 and declined thereafter. The percentage of ECD kidneys of total kidneys transplanted in this age group dropped from 4.1% in 2001 to 1.6% in 2003. Thereafter, the percentage of ECD kidneys remained steady at 1.6%.
The demographic data of the study population are summarized in Table 1. The racial distribution was 50% Caucasian, 26% African American, 18% Hispanic, and the remaining were Asian and other minorities. Of those who received ECD kidneys, African Americans were the largest group (43%), followed by Caucasians (37%), Hispanics (16%), Asians (3%), and other minorities (2%).
Table 1.
Demographic data of the study population
| Characteristic | Standard Criteria Donor, n (%) | Expanded Criteria Donor, n (%) | P Value |
|---|---|---|---|
| Population (n) | 13,925 | 238 | |
| Race/ethnicity | <0.001 | ||
| Asian | 663 (5) | 8 (3) | |
| African American | 3550 (25) | 102 (43) | |
| Hispanic | 2497 (18) | 37 (16) | |
| Other minority | 245 (2) | 4 (2) | |
| Caucasian | 6970 (50) | 87 (37) | |
| Education level | <0.001 | ||
| Grade school, none, or unknown | 2168 (16) | 58 (24) | |
| High school | 5931 (43) | 109 (46) | |
| Technical | 3354 (24) | 49 (21) | |
| Associate’s, bachelor’s, or postgraduate degree | 2449 (18) | 22 (9) | |
| Income ($1000s) | 0.06 | ||
| ≤31 | 3533 (25) | 74 (31) | |
| 32–37 | 3451 (25) | 66 (28) | |
| 38–49 | 3368 (24) | 50 (21) | |
| ≥50 | 3572 (26) | 48 (20) | |
| Panel reactive antibody level | <0.001 | ||
| 0–9 | 9263 (67) | 193 (81) | |
| 10–79 | 1018 (7) | 23 (10) | |
| ≥80 | 271 (2) | 5 (2) | |
| Missing | 3373 (24) | 17 (7) | |
| Sex | 0.02 | ||
| Women | 5784 (42) | 81 (34) | |
| Men | 8141 (58) | 157 (66) | |
| Recipient diabetes status | <0.001 | ||
| No | 11715 (84) | 182 (76) | |
| Type I | 1026 (7) | 15 (6) | |
| Type II | 502 (4) | 16 (7) | |
| Other/unknown | 682 (5) | 25 (11) | |
| Primary diagnosis of recipients | <0.001 | ||
| Glomerular diseases | 5604 (40) | 61 (25) | |
| Hypertensive nephrosclerosis | 2916 (21) | 80 (34) | |
| Other kidney diseases | 1956 (14) | 33 (14) | |
| Diabetes | 1675 (11) | 37 (15) | |
| Polycystic kidney disease | 575 (4) | 13 (5) | |
| Tubular and interstitial disease | 575 (4) | 8 (3) | |
| Congenital, familial, and metabolic renal diseases | 575 (4) | 5 (2) | |
| Insurance type | <0.001 | ||
| Private | 6623 (48) | 54 (23) | |
| Public | 7267 (52) | 182 (76) | |
| Other | 35 (0) | 2 (1) |
The main-effect multivariate analysis (Table 2) controlling for sex, education, PRA, and AGI indicated that African Americans aged ≤40 years and waitlisted <3 years were 71% more likely to receive an ECD kidney (adjusted odds ratio [OR], 1.71; 95% CI, 1.26 to 2.33; P<0.001) compared with Caucasians (reference group). The African-American population was the only racial group significantly different from the reference population. Those recipients with the lowest education level (grade school, none, or unknown education) were twice as likely to receive an ECD kidney compared with those with the highest educational level (associate’s, bachelor’s, or postgraduate degree) (OR, 2.32; 95% CI, 1.38 to 3.89; P=0.002). When controlling for all variables, income alone did not have an effect on the likelihood of receiving an ECD kidney as opposed to an SCD kidney.
Table 2.
Demographic factors affecting odds of receiving an ECD kidney transplant
| Parameter | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Race | ||
| Caucasian | Reference | |
| Asian | 1.13 (0.53 to 2.37) | 0.76 |
| African American | 1.71 (1.26 to 2.33) | <0.001 |
| Hispanic | 1.14 (0.76 to 1.72) | 0.52 |
| Other minority | 1.23 (0.43 to 3.49) | 0.70 |
| Education | ||
| Associate’s, bachelor’s, or postgraduate degree | Reference | |
| Grade school, none, or unknown | 2.32 (1.38 to 3.89) | 0.002 |
| High school | 1.59 (0.99 to 2.55) | 0.06 |
| Technical | 1.43 (0.85 to 2.40) | 0.17 |
| Income group ($1000s) | ||
| ≤31 | 1.20 (0.81 to 1.78) | 0.35 |
| 32–37 | 1.15 (0.78 to 1.70) | 0.49 |
| 38–49 | 1.05 (0.69 to 1.58) | 0.84 |
| ≥50 | Reference | |
| Candidate sex | ||
| Women | Reference | |
| Men | 1.35 (1.01 to 1.80) | 0.05 |
| Panel reactive antibody level | ||
| 0–9 | Reference | |
| 10–79 | 1.00 (0.63 to 1.59) | >0.99 |
| ≥80 | 0.82 (0.33 to 2.09) | 0.68 |
| Missing | 0.32 (0.19 to 0.53) | <0.001 |
A two-way analysis of interaction between race and education (Table 3) suggested that the strong effect of African-American race persists across almost all strata of education. African Americans with an education below the highest level (associate’s, bachelor’s, or postgraduate degree) had a higher likelihood of receiving ECD kidneys than the reference group (Caucasian with an associate’s, bachelor’s, or postgraduate degree). The strong association of education level and odds of receiving an ECD kidney was evident in that races other than African Americans. For example, Asians (adjusted OR, 3.99; 95% CI, 1.24 to 12.90) and Hispanics (adjusted OR, 2.59; 95% CI, 1.23 to 5.47) with a grade school or lower education were more likely to receive an ECD kidney compared with Caucasians with a college degree. Other race-by-education interactions were not significant. Two-way interactions between race and AGI (Table 4) showed that African Americans across all strata of income, except those living in an area with an average income >$38,000, were more likely to receive an ECD kidney. In other races, income did not have a statistically significant effect.
Table 3.
Odds of receiving an ECD kidney transplant based on two-way interaction of race and education
| Education | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| African American | ||
| Associate’s, bachelor’s, or postgraduate degree | 0.98 (0.37 to 2.59) | 0.96 |
| High school | 2.29 (1.24 to 4.24) | 0.01 |
| Technical | 2.16 (1.09 to 4.26) | 0.03 |
| Grade school, none, or unknown | 2.95 (1.45 to 6.00) | 0.003 |
| Hispanic | ||
| Associate’s, bachelor’s, or postgraduate degree | 1.59 (0.35 to 7.17) | 0.55 |
| High school | 1.18 (0.56 to 2.49) | 0.66 |
| Technical | 1.25 (0.40 to 3.88) | 0.70 |
| Grade school, none, or unknown | 2.59 (1.23 to 5.47) | 0.01 |
| Caucasian | ||
| Associate’s, bachelor’s, or postgraduate degree | Reference | |
| High school | 1.23 (0.65 to 2.30) | 0.53 |
| Technical | 1.18 (0.59 to 2.36) | 0.64 |
| Grade school, none, or unknown | 1.45 (0.69 to 3.07) | 0.33 |
Table 4.
Odds of receiving an ECD kidney transplant based on two-way interaction of race and income
| Income ($1000s) | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| African American | ||
| ≤31 | 2.49 (1.45 to 4.28) | 0.001 |
| 32–37 | 1.88 (1.03 to 3.44) | 0.04 |
| 38–49 | 1.85 (0.95 to 3.58) | 0.07 |
| ≥50 | 1.74 (0.88 to 3.47) | 0.11 |
| Hispanic | ||
| ≤31 | 1.62 (0.81 to 3.25) | 0.17 |
| 32–37 | 1.69 (0.78 to 3.68) | 0.19 |
| 38–49 | 1.30 (0.54 to 3.13) | 0.56 |
| ≥50 | 1.32 (0.52 to 3.36) | 0.56 |
| Caucasian | ||
| ≤31 | 1.13 (0.57 to 2.23) | 0.72 |
| 32–37 | 1.31 (0.73 to 2.34) | 0.36 |
| 38–49 | 1.15 (0.63 to 2.11) | 0.65 |
| ≥50 | Reference |
The majority of ECD donors died due to CVA (82%). Trauma accounted for only 9% of deaths. Other causes of death included anoxia (6%). Three percent of ECD deaths were unspecified. There was wide variation in ECD kidney transplantation between centers. As a percentage of total transplants at each individual center (limited to the study population), ECD kidney transplants ranged from 0% to 17%. Eight centers performed ECD transplants on patients that fit the study population criteria in >9% of their transplant patients. Eighteen centers performed such transplants on 5%–9% of their transplant recipients. Eighteen other centers performed ECD transplants on patients that fit the study population criteria on 3%–5% of their transplant recipients. Our results showed that 210 centers performed such transplants on <3% of their transplant patients. The funnel plot with 95% CIs generated by plotting the observed to expected ECD transplants at the 88 centers showed that the majority of the centers had an observed/expected ratio close to 1. The eight centers that did >9% of ECD kidney transplants in the study population at the corresponding centers accounted for 51 of the 237 (22%) ECD transplants in the study population. However, chi-squared statistics did not show a difference in the frequency of African Americans or low socioeconomic individuals at these centers (data not shown).
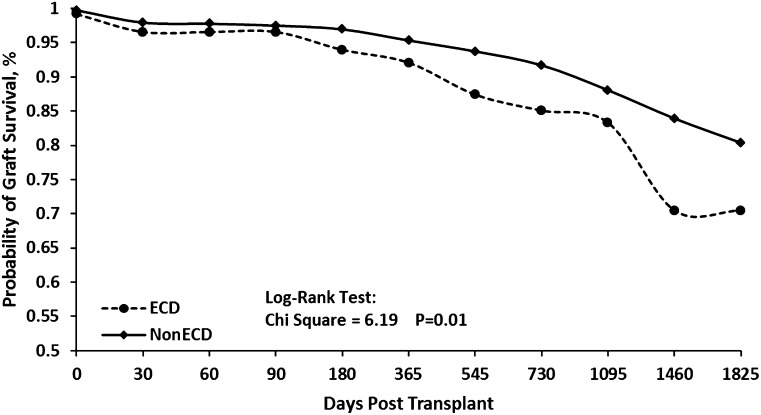
Multivariate analysis showed that in our study population, ECD kidneys were twice as likely to fail compared with SCD kidneys (OR, 2.03; 95% CI, 1.53 to 2.71; P<0.001) (Figure 2).
Figure 2.
Kaplan–Meier curves for graft survival in ECD versus SCD kidneys in the study population. ECD kidneys have a reduced long-term graft survival (P=0.01). ECD, expanded criteria donor; SCD, standard criteria donor.
Discussion
Previous studies have shown that significant racial disparities in renal transplantation exist, including the chances of being waitlisted, time on the waiting list, and outcomes (16–18). To our knowledge, our study is the first to look into possible disparities involving the allocation of ECD kidneys, particularly in young recipients with relatively short waiting times.
Even though the allocation of ECD organs to recipients aged <40 years is relatively rare, of the few ECD organs that were transplanted into young recipients, ECD kidneys are being disproportionately allocated to African Americans and those with less education. All racial groups have a mortality benefit with ECD kidneys compared with dialysis (13). However, Schnitzler and colleagues used expected quality-adjusted life years, a measure of survival and disease burden, to show that although an average patient could wait 3.2 years, African Americans could wait 4.4 years from the time they accepted an ECD kidney before any quality-adjusted life years gained over dialysis were negated (19). The strong effect of education was apparent in all races and subgroups. The United Network for Organ Sharing mandates that recipients be preconsented before receiving ECD kidneys. However, in a recent study, Gordon et al. showed that transplant consent forms are written in such a fashion that many transplant candidates have difficulty comprehending the different options available to them (20). Moreover, informed consent is seldom “educated consent,” and patients are not required to demonstrate an adequate understanding of their choices and proposed treatment plans. Current reimbursement and managed care practices have further limited the time most providers have for patient education. Although ECD kidneys are allocated from a supplementary list of all persons who have consented to one, surgeons and patients have the option to decline the allocated organ. In addition, transplant centers have a wide variation in listing practices for ECD kidneys (9). Center effects could account for some of the disparities we found in our study, because a few centers did account for a large percentage of ECD kidneys transplanted in our study population. However, the distribution of African Americans and those with low socioeconomic status at these centers was not statistically different. ECD kidneys have varied graft outcomes. In addition to age and history of hypertension, which are factored into the ECD algorithm, an important determinant of graft survival is the cause of the donor’s death. CVAs have been consistently associated with a higher risk of graft loss, even when adjusted for multiple covariates (21,22). In our study population, the vast majority of the kidneys were from donors who had died of a CVA.
Our study has a few limitations. To select a group that typically would not have a survival benefit from an ECD kidney, we focused our study on patients aged <40 years who were waitlisted <3 years and had received their first kidney transplant. However, these reduced criteria limit the number of individuals studied. Because we did not have access to individual patient records, we could not exclude the possibility that some of these patients might have had legitimate reasons to accept an ECD kidney, such as lack of vascular access for dialysis. However, even when we repeated our analysis and included patients without regard to waiting time and those without a valid zip code associated with AGI (data not shown), ECD kidney transplants were still associated with lower education and African-American race. We used income data from a zip code level of stratification. Although this method has been widely used in registry studies, extrapolating data from a geographically distributed variable to an individual level raises the possibility of an ecologic fallacy (23).
The allocation of ECD kidneys is a complex problem. Given the scarcity of donor kidneys, we should maximize their use. Studies have shown that despite best efforts, patients who could benefit from ECD kidneys are still not being listed (14), and a disproportionate number of recovered organs are still being wasted (24). On the other hand, we have to make certain that such kidneys are being allocated to individuals who are most likely to benefit and not disproportionately allocated to minorities with low socioeconomic and educational status. Prospective studies that explore the reasons for such disparities are essential to address the inequalities in health care and improve patient outcomes.
Disclosures
None.
Acknowledgments
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under T32 DK07518-26 and Gatorade funds to the Division of Nephrology at the University of Florida. The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the National Institutes of Health. The study was approved by the institutional review board at the University of Florida.
Melissa A. Lamb of Lamb Consulting assisted in the editing of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ojo AO, Heinrichs D, Emond JC, McGowan JJ, Guidinger MK, Delmonico FL, Metzger RA: Organ donation and utilization in the USA. Am J Transplant 4[Suppl 9]: 27–37, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Langone AJ, Helderman JH: Disparity between solid-organ supply and demand. N Engl J Med 349: 704–706, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Delmonico FL, Sheehy E, Marks WH, Baliga P, McGowan JJ, Magee JC: Organ donation and utilization in the United States, 2004. Am J Transplant 5: 862–873, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kauffman HM, Bennett LE, McBride MA, Ellison MD: The expanded donor. Transplant Rev 11: 165–190, 1997 [Google Scholar]
- 5.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM: Expanded criteria donors for kidney transplantation. Am J Transplant 3[Suppl 4]: 114–125, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ: Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 74: 1281–1286, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Gaston RS, Danovitch GM, Adams PL, Wynn JJ, Merion RM, Deierhoi MH, Metzger RA, Cecka JM, Harmon WE, Leichtman AB, Spital A, Blumberg E, Herzog CA, Wolfe RA, Tyan DB, Roberts J, Rohrer R, Port FK, Delmonico FL: The report of a national conference on the wait list for kidney transplantation. Am J Transplant 3: 775–785, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Frei U, Noeldeke J, Machold-Fabrizii V, Arbogast H, Margreiter R, Fricke L, Voiculescu A, Kliem V, Ebel H, Albert U, Lopau K, Schnuelle P, Nonnast-Daniel B, Pietruck F, Offermann R, Persijn G, Bernasconi C: Prospective age-matching in elderly kidney transplant recipients—a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant 8: 50–57, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Schold JD, Meier-Kriesche HU: Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 1: 532–538, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Mailloux LU, Bellucci AG, Mossey RT, Napolitano B, Moore T, Wilkes BM, Bluestone PA: Predictors of survival in patients undergoing dialysis. Am J Med 84: 855–862, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Khauli RB, Steinmuller DR, Novick AC, Buszta C, Goormastic M, Nakamoto S, Vidt DG, Magnusson M, Paganini E, Schreiber MJ: A critical look at survival of diabetics with end-stage renal disease. Transplantation versus dialysis therapy. Transplantation 41: 598–602, 1986 [PubMed] [Google Scholar]
- 12.Schroijen MA, Dekkers OM, Grootendorst DC, Noordzij M, Romijn JA, Krediet RT, Boeschoten EW, Dekker FW, NECOSAD Study Group : Survival in dialysis patients is not different between patients with diabetes as primary renal disease and patients with diabetes as a co-morbid condition. BMC Nephrol 12: 69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Grams ME, Womer KL, Ugarte RM, Desai NM, Montgomery RA, Segev DL: Listing for expanded criteria donor kidneys in older adults and those with predicted benefit. Am J Transplant 10: 802–809, 2010 [DOI] [PMC free article] [PubMed]
- 15.Wolfe RA, Webb RL, Dickinson DM, Ashby VB, Dykstra DM, Hulbert-Shearon TE, McCullough KP: Analytical approaches for transplant research. Am J Transplant 3[Suppl 4]: 103–113, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Ellison MD, Edwards LB, Edwards EB, Barker CF: Geographic differences in access to transplantation in the United States. Transplantation 76: 1389–1394, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM: Racial disparities in access to renal transplantation–clinically appropriate or due to underuse or overuse? N Engl J Med 343: 1537–1544, 1532 p preceding 1537, 2000 [DOI] [PMC free article] [PubMed]
- 19.Schnitzler MA, Whiting JF, Brennan DC, Lin G, Chapman W, Lowell J, Boxerman S, Hardinger KL, Kalo Z: The expanded criteria donor dilemma in cadaveric renal transplantation. Transplantation 75: 1940–1945, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Gordon E, Bergeron A, McNatt G, Friedewald J, Wolf M: Reading levels of kidney transplant consent forms: A national study. Presented at the 42nd Annual Meeting and Scientific Exposition of the American Society of Nephrology, San Diego, CA, October 27 to November 1, 2009 [Google Scholar]
- 21.Pessione F, Cohen S, Durand D, Hourmant M, Kessler M, Legendre C, Mourad G, Noël C, Peraldi MN, Pouteil-Noble C, Tuppin P, Hiesse C: Multivariate analysis of donor risk factors for graft survival in kidney transplantation. Transplantation 75: 361–367, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Singhal AK, Sheng X, Drakos SG, Stehlik J: Impact of donor cause of death on transplant outcomes: UNOS registry analysis. Transplant Proc 41: 3539–3544, 2009 [DOI] [PubMed] [Google Scholar]
- 23.McCutcheon AL: Cross-level inference-Achen, CH, Shively,WP. Contemp Sociol 25: 276–277, 1996 [Google Scholar]
- 24.Cho YW, Shah T, Cho ES, Stadtler M, Simmons V, Mone T, Mendez R, Hutchinson IV, Gill J, Bunnapradist S: Factors associated with discard of recovered kidneys. Transplant Proc 40: 1032–1034, 2008 [DOI] [PubMed] [Google Scholar]