Summary
Background and objectives
Higher left ventricular volume is associated with death in patients with ESRD. This work investigated the effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling.
Design, setting, participants, & measurements
The Frequent Hemodialysis Network daily trial randomized 245 patients to 12 months of six times per week versus three times per week in-center hemodialysis; the Frequent Hemodialysis Network nocturnal trial randomized 87 patients to 12 months of six times per week nocturnal hemodialysis versus three times per week predominantly home-based hemodialysis. Left and right ventricular end systolic and diastolic volumes, left ventricular mass, and ejection fraction at baseline and end of the study were ascertained by cardiac magnetic resonance imaging. The ratio of left ventricular mass/left ventricular end diastolic volume was used as a surrogate marker of left ventricular remodeling. In each trial, the effect of frequent dialysis on left or right ventricular end diastolic volume was tested between predefined subgroups.
Results
In the daily trial, frequent hemodialysis resulted in significant reductions in left ventricular end diastolic volume (−11.0% [95% confidence interval, −16.1% to −5.5%]), left ventricular end systolic volume (−14.8% [−22.7% to −6.2%]), right ventricular end diastolic volume (−11.6% [−19.0% to −3.6%]), and a trend for right ventricular end systolic volume (−11.3% [−21.4% to 0.1%]) compared with conventional therapy. The magnitude of reduction in left and right ventricular end diastolic volumes with frequent hemodialysis was accentuated among patients with residual urine output<100 ml/d (P value [interaction]=0.02). In the nocturnal trial, there were no significant changes in left or right ventricular volumes. The frequent dialysis interventions had no substantial effect on the ratio of left ventricular mass/left ventricular end diastolic volume in either trial.
Conclusions
Frequent in-center hemodialysis reduces left and right ventricular end systolic and diastolic ventricular volumes as well as left ventricular mass, but it does not affect left ventricular remodeling.
Introduction
Cardiovascular disease is the leading cause of death in patients with ESRD, with rates of cardiovascular death 10- to >100-fold higher than in the age-matched general population (1). An increase in left ventricular (LV) cavity size is independently associated with mortality and cardiovascular morbidity in ESRD (2,3). Few interventions have been shown to reduce a pathologically enlarged LV cavity in the ESRD patient population (4). Putative risk factors leading to LV dilation in ESRD include volume overload, anemia, high-flow arteriovenous fistulae, and poorly controlled uremia (5).
Ventricular remodeling is defined as “molecular, cellular and interstitial changes that are manifested clinically as changes in size, shape and function of the heart after cardiac injury” (6). At a cellular level, the process of cardiac myocyte hypertrophy combined with altered interstitial fibrosis, apoptosis, or necrosis is influenced by hemodynamic and neurohormonal changes secondary to the injurious stimuli. Impact of interventions on LV remodeling has become an important outcome measure. As a result, in addition to LV cavity size, the ratio of LV mass to LV end diastolic volume (EDV; a marker of LV remodeling) has become an important prognostic indicator in the non-ESRD population (7). In the setting of ESRD, changes in BP and interdialytic weight gain as well as surrogate markers of clearance (e.g., serum phosphorus) may be of particular interest given the emerging literature linking these factors to progression of LV mass, LV cavity size, or both (8–12). The effects of frequent hemodialysis on ventricular volumes and the ratio of LV mass to LVEDV is unknown.
The Frequent Hemodialysis Network (FHN) trials aimed to examine the effects of frequent (six times per week) hemodialysis (in the form of in-center daily hemodialysis or nocturnal home hemodialysis) versus conventional three times weekly hemodialysis on multiple intermediate outcome measures (13–15). The objectives and protocol summaries of both trials have been previously published (15). Owing to limitations in sample size, the FHN trials were not designed to assess the effects of frequent hemodialysis on death or major health events. Nine prespecified domains were identified by the investigators, including one domain related to cardiac structure and function. We previously identified determinants of change in LV mass with frequent hemodialysis (11). However, changes in cardiac ventricular volume and LV remodeling had not been explored.
The primary objective of the present report was to describe the effects of frequent hemodialysis on LV and right ventricular (RV) volumes, LV remodeling (as determined by LV mass/LVEDV), and global systolic function (as assessed by ejection fraction) and explore which (if any) baseline patient characteristics seem to modify such an effect. We also aimed to explore potential mechanistic pathways (including control of hypertension, magnitude of ultrafiltration, residual kidney function, and control of retained uremic solutes) that might explain the therapeutic response to frequent hemodialysis.
Materials and Methods
FHN Trials
The FHN daily and nocturnal trials were multicenter, randomized, prospective trials of in-center daily hemodialysis and home nocturnal hemodialysis, respectively, sponsored by the National Institutes of Health, National Institutes Diabetes, Digestive and Kidney Diseases and the Center for Medicare and Medical Services. The designs and inclusion and exclusion criteria of both daily and nocturnal trials were described previously (15,16). Of note, the residual kidney function exclusion criterion was higher in the nocturnal trial (average of urea and creatinine clearance>10 ml/min per 1.73 m2) than the daily trial (urea clearance>3 ml/min per 35 L). Patients were enrolled between March of 2006 and May of 2009, and the trials concluded in May of 2010. Both trials were approved by the local institutional review board at each participating site. An independent Data Safety Monitoring Board provided oversight of both trials (ClinicalTrials.gov numbers: NCT00264758 and NCT 00271999).
Dialysis Intervention
Patients in the conventional arm of both trials remained on their usual three times per week hemodialysis prescription subject to a prescribed equilibrated Kt/Vurea>1.1, a standardized Kt/Vurea>2.0, and a treatment time≥2.5 h/session. Patients randomized to the frequent arm of the daily trial were targeted to an equilibrated Kt/Vn=0.9, where Vn=3.271×V2/3, provided that the length of the session was between 1.5 and 2.75 hours. Patients randomized to the frequent arm of the nocturnal trial followed hemodialysis prescriptions subject to a standardized Kt/Vurea≥4.0 and a treatment time≥6 hours (72 of 87 patients in the nocturnal trial received therapy at home rather than in center).
Cardiac Magnetic Resonance Imaging
We measured LV mass (LVM) and biventricular volumes by cardiac magnetic resonance imaging (CMRI) in all randomized patients at baseline and 12 months where feasible. All CMRI images were analyzed centrally in a blinded manner. CMRI was performed on 1.5 T MRI systems (minimum gradient performance: peak strength≥12 mT/m, slew rate≥40 mTm/s) with dedicated surface coils. Sites were required to use standardized protocols using breath-held, retrospective electrocardiogram-gated steady state free precession imaging in contiguous short-axis views (8-mm slice thickness and 2-mm gap) that were carefully prescribed from localizer long-axis images. Imaging parameters were adjusted on each specific CMRI scanner to provide 20–25 cardiac phases, with an in-plane spatial resolution superior≤2 mm and a temporal resolution<50 ms. Using validated software (Argus; Siemens Medical Solutions, Erlangen, Germany), we measured myocardial volume on end diastolic frames by manual tracing of endocardial and epicardial contours. We excluded papillary muscles from the calculation of myocardial mass. Subsequently, this volume was multiplied by the specific density of the myocardium (1.05 g/cm3) to obtain LVM (17). Similarly, we traced biventricular endocardial contours in end diastole and end systole to derive EDV and end systolic volume (ESV). We used the formula in the work by DuBois and DuBois (18) to index LVM to body surface area. We calculated anthropometric volume using the equation in the work by Watson et al. (19)
CMRI Outcome Measures
Change in LV and RV Volumes and LVM/LVEDV
We assessed the effect of frequent hemodialysis on LV and RV volumes by examining the differences in LV and RV ESVs and EDVs and the percent change of the geometric mean of these parameters at baseline and 12 months. The treatment effect of frequent hemodialysis on LV remodeling was assessed by examining the differences in LVM and LVM/LVEDV at baseline and 12 months.
Subgroup Analyses
We a priori categorized nine subgroups to explore whether baseline demographic or clinical variables modified the effect of frequent hemodialysis on change in cardiac volumes and LVM/LVEDV. These baseline subgroups were age (≤50 or >50 years), sex, anthropometric total body water volume (≤40 or >40 L), vintage of ESRD (<4 or ≥4 years), urine volume (<100 or ≥100 ml [daily trial] and ≤500 or >500 ml [nocturnal trial]), race (black or white), baseline LVM (<132 or ≥132 g), baseline congestive heart failure (yes or no), and diabetes status (yes or no). Given that medications may also influence ventricular volumes and LV remodeling, baseline use of β-blockers (yes or no) and renin-angiotensin blockade (yes or no) was also considered.
Intermediate Outcomes Considered in Correlational Analyses
We a priori defined intermediate measures reflecting each of the three proposed mechanistic pathways leading to cardiac dilation: predialysis systolic BP, interdialytic weight change, and predialysis serum phosphorus concentration.
Data Analysis
Descriptive statistics for continuous variables were summarized using mean ± SD or medians and 10th and 90th percentiles as appropriate. Categorical variables were summarized using frequencies and proportions. Descriptive summaries of changes in treatment-related variables are provided for the constant cohort with nonmissing values at baseline and 12 months after randomization. Because of positive skewness, the variables LVEDV, LVESV, RDEDV, and RVESV were each log-transformed before statistical analyses. For these outcomes, estimates of mean changes and treatment effects were expressed as percent differences in geometric means. The effects of randomized treatment assignment on cardiac volumes and LV remodeling were estimated by applying a mixed effects model to baseline and 12-month values using an unstructured covariance matrix with covariate adjustment, including a time interaction for the baseline outcome measures, plus prespecified covariates age, diabetes, and clinical center for the daily trial and age, diabetes, and baseline GFR for the nocturnal trial (11). Because outcomes were assessed at a single follow-up time, this model produced results essentially identical to an analysis of covariance relating the change in the outcome to treatment assignment controlling for the baseline value of the outcome.
We addressed the possible role of the timing of the MRI measurements in relation to the dialysis treatment schedule by summarizing the distribution of the time interval between the MRI measurement and the end of the preceding dialysis treatment; we compared this distribution with the distribution of the average interdialytic interval between dialysis treatments in the week preceding the baseline and 12-month kinetic modeling sessions.
For each of nine prespecified factors, we used separate linear regression analyses to relate the change in LV and RV EDVs to treatment assignment, prespecified baseline covariates, and corresponding interaction terms. The primary assessment of treatment interactions with quantitative subgroup factors was based on a test for linear interaction, which treated the subgroup factor as a continuous variable; estimated treatment effects are also provided for the subgroups defined by the above indicated cutoffs for descriptive purposes. In the daily trial, we present P values for the interactions without adjustment for multiple comparisons. Because of its limited sample size, we considered subgroup analyses in the nocturnal trial in an exploratory fashion without significance testing.
We depicted the association of changes in LV and RV EDVs with changes in average sessional interdialytic weight change, predialysis systolic BP, and predialysis phosphorus for individual patients using scatterplots with separate nonparametric local regression curves (20) for each treatment group. We also provided Pearson correlations between the changes for each group. The end of study levels of average sessional interdialytic weight change, predialysis systolic BP, and serum phosphorus were averaged over measurements at months 10–12.
We performed all analyses using SAS version 9.2. We considered two-tailed P values<0.05 as statistically significant.
Results
The FHN trials randomized 245 patients to 12 months of frequent versus conventional in-center hemodialysis and 87 patients to 12 months of frequent home nocturnal versus conventional (predominantly at home) hemodialysis. Selected baseline demographics, clinical characteristics, cardiovascular risk profile, medication use, and biochemical status are summarized in Table 1. In the daily trial, 5 patients died, and 11 patients were transplanted in the frequent arm; corresponding numbers were 9 and 13, respectively, in the conventional arm. In the nocturnal trial, two patients died, and three patients were transplanted in the frequent arm; corresponding numbers were one and zero, respectively, in the conventional arm. Baseline median volumetric and global contractile indices for the RV and LV are depicted in Table 2.
Table 1.
Characteristics during baseline for Frequent Hemodialysis Network (FHN) patients
| Variables | Daily Trial | Nocturnal Trial | ||
|---|---|---|---|---|
| Three Times/Wk (n=120; Conventional) | Six Times/Wk (n=125; Daily) | Three Times/Wk (n=42; Conventional) | Six Times/Wk (n=45; Nocturnal) | |
| Age (yr) | 52.0±14.1 | 48.9±13.6 | 54.0±12.9 | 51.7±14.4 |
| Men | 73 (60.8%) | 78 (62.4%) | 28 (66.7%) | 29 (64.4%) |
| Race/ethnicity | ||||
| Black | 53 (44.2%) | 49 (39.2%) | 11 (26.2%) | 12 (26.7%) |
| White | 46 (38.3%) | 43 (34.4%) | 21 (50.0%) | 27 (60.0%) |
| Native American, Aboriginal Canadian, Alaskan Native, First Nation | 4 (3.3%) | 4 (3.2%) | 2 (4.8%) | 1 (2.2%) |
| Asian | 5 (4.2%) | 11 (8.8%) | 7 (16.7%) | 5 (11.1%) |
| Native Hawaiian or other Pacific Islander | 3 (2.5%) | 1 (0.8%) | 0 (0%) | 0 (0%) |
| Other/mixed/unknown | 9 (7.5%) | 17 (13.6%) | 1 (2.4%) | 0 (0%) |
| Hispanic/Latino ethnicity | 31 (26%) | 38 (30%) | 0 (0%) | 0 (0%) |
| ESRD vintage (yr)a | 3.40 (0.58, 12.94) | 3.85 (0.69, 17.31) | 0.53 (0.10, 6.00) | 1.32 (0.09, 12.55) |
| Weekly standard Kt/V | 2.53±0.39 | 2.50±0.31 | 2.34±0.34 | 2.35±0.28 |
| Residual urinary volume (L/d)a | 0 (0, 0.54) | 0 (0, 0.60) | 0.54 (0, 1.25) | 0.40 (0, 1.33) |
| Residual renal urea clearance (ml/min) | ||||
| 0 | 72 (60.0%) | 90 (72.0%) | 11 (26.2%) | 13 (28.9%) |
| >0–1 | 19 (15.8%) | 18 (14.4%) | 9 (21.4%) | 7 (15.6%) |
| >1–3 | 27 (22.5%) | 15 (12.0%) | 14 (33.3%) | 14 (31.1%) |
| >3 | 2 (1.7%) | 2 (1.6%) | 8 (19.0%) | 11 (24.4%) |
| Hypertension | 111 (92.5%) | 117 (93.6%) | 39 (92.9%) | 41 (91.1%) |
| Coronary artery disease | 16 (13.3%) | 11 (8.8%) | 4 (9.5%) | 5 (11.1%) |
| Congestive heart failure | 24 (20.0%) | 25 (20.0%) | 7 (16.7%) | 5 (11.1%) |
| Atrial fibrillation | 9 (7.5%) | 5 (4.0%) | 0 (0.0%) | 6 (13.3%) |
| Peripheral arterial disease | 10 (8.33%) | 15 (12.0%) | 7 (16.7%) | 8 (17.8%) |
| Stroke | 9 (7.5%) | 9 (7.2%) | 1 (2.4%) | 1 (2.2%) |
| Diabetes | 50 (41.7%) | 50 (40.0%) | 18 (42.9%) | 19 (42.2%) |
| COPD | 5 (4.2%) | 6 (4.8%) | 2 (4.8%) | 2 (4.4%) |
| Liver disease | 1 (0.8%) | 1 (0.8%) | 1 (2.4%) | 0 (0%) |
| Dialysis access | ||||
| Fistula | 72 (59.2%) | 83 (65.8%) | 18 (41.9%) | 24 (51.1%) |
| Graft | 23 (18.9%) | 22 (17.5%) | 4 (9.3%) | 3 (6.4%) |
| Catheter | 27 (22.1%) | 21 (16.7%) | 21 (48.8%) | 20 (42.6%) |
| Antihypertensives | 105 (87.5%) | 109 (87.2%) | 35 (83.3%) | 38 (84.4%) |
| ACEI | 38 (31.7%) | 42 (33.6%) | 12 (28.6%) | 7 (15.6%) |
| ARB | 25 (20.8%) | 30 (24.0%) | 3 (7.1%) | 9 (20.0%) |
| Dihydropyridine CCB | 53 (44.2%) | 62 (49.6%) | 15 (35.7%) | 18 (40.0%) |
| Nondihydropyridine CCB | 6 (5.0%) | 6 (4.8%) | 3 (7.1%) | 2 (4.4%) |
| β-Blockers | 77 (64.2%) | 70 (56.0%) | 21 (50.0%) | 30 (66.7%) |
| Peripheral α-blockers | 4 (3.3%) | 1 (0.8%) | 4 (9.2%) | 2 (4.4%) |
| Centrally acting agents | 24 (20.0%) | 22 (17.6%) | 3 (7.1%) | 5 (11.1%) |
| Nonspecific vasodilators | 13 (10.8%) | 22 (17.6%) | 0 (0%) | 2 (4.4%) |
| Diuretics | 16 (13.3%) | 17 (13.6%) | 6 (14.3%) | 11 (24.4%) |
Results are shown as mean ± SD or frequency (%) as appropriate. Patients may have more than one hemodialysis access at baseline. COPD, chronic obstructive pulmonary disease; ACEI, angiotension converting enzyme inhibitor ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
For ESRD and residual urine volume, the 10th and 90th percentiles are given.
Table 2.
Changes in left and right ventricular volumes and associated parameters
| Variables | Times/Wk | Baseline Median (Q1, Q3) | F12 Median (Q1, Q3) | Adjusted Mean Changes from Baselinea (95% CI) | Treatment Effectsa (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Daily trial | ||||||
| LVEDV (ml) | 3 | 167.6 (114.8, 255.4) | 174.4 (112.4, 247.2) | −0.26% (−4.72% to 4.40%) | −10.96% (−16.08% to −5.52%) | <0.001 |
| 6 | 163.2 (102.8, 248.0) | 146.6 (102.6, 215.6) | −11.19% (−14.83% to −7.4%) | |||
| LVESV (ml) | 3 | 69.2 (38.6, 126.3) | 71.3 (43, 117.8) | 0.46% (−6.69% to 8.15%) | −14.84% (−22.66% to −6.23%) | 0.001 |
| 6 | 69.6 (35.8, 123.7) | 57.1 (33.4, 105.8) | −14.45% (−20.03% to −8.47%) | |||
| LV stroke volume (ml) | 3 | 93.2 (62.1, 151.3) | 94.1 (66.6, 140.9) | 0.32 (−4.68 to 5.32) | −10.90 (−17.14 to −4.66) | 0.001 |
| 6 | 95.5 (62.9, 142.0) | 86.9 (56.9, 118.7) | −10.58 (−15.19 to −5.97) | |||
| LV ejection fraction (%) | 3 | 58.3 (41.5, 69.2) | 58.6 (44.8, 68.3) | 0.14 (−1.95 to 2.23) | 1.42 (−1.22 to 4.05) | 0.29 |
| 6 | 59.0 (45.6, 68.1) | 61 (46.6, 71) | 1.56 (−0.37 to 3.49) | |||
| LVM/LVEDV (g/ml) | 3 | 76.0 (59.7, 102.5) | 74.2 (58.7, 108.7) | −2.04 (−6.11 to 2.02) | 3.42 (−1.91 to 8.75) | 0.21 |
| 6 | 80.4 (56.9, 110.2) | 78.4 (58.2, 116.5) | 1.38 (−2.33 to 5.10) | |||
| RVEDV (ml) | 3 | 159.8 (104.2, 240.9) | 140.4 (90.6, 225.8) | −9.08 (−14.98 to −2.76) | −11.63% (−19.02% to −3.57%) | 0.006 |
| 6 | 146.6 (96.5, 254.1) | 125.8 (78.9, 196.1) | −19.65 (−24.44 to −14.56) | |||
| RVESV (ml) | 3 | 69.4 (46, 118.5) | 63.1 (35, 104.5) | −12.25% (−20.00% to −3.74%) | −11.33% (−21.42% to 0.05%) | 0.05 |
| 6 | 72.2 (35.9, 114.5) | 53.7 (27.9, 97.3) | −22.19% (−28.51% to −15.31%) | |||
| RV stroke volume (ml) | 3 | 87.2 (50.4, 129.2) | 76.4 (50.1, 127.4) | −5.39 (−11.05 to 0.27) | −10.49 (−17.48 to −3.49) | 0.003 |
| 6 | 85.0 (51.3, 125.6) | 70.8 (42.5, 101.3) | −15.87 (−21.1 to −10.65) | |||
| RV ejection fraction (%) | 3 | 53.1 (40, 65.5) | 54.0 (43.4, 68.4) | 1.30 (−1.04 to 3.63) | 0.00 (−2.95 to 2.96) | >0.99 |
| 6 | 54.3 (43.3, 65.1) | 56.0 (42.0, 68.4) | 1.30 (−0.85 to 3.44) | |||
| Nocturnal trial | ||||||
| LVEDV (ml) | 3 | 141.7 (98.9, 214.6) | 138.3 (106.9, 228.5) | 3.94% (−5.03% to 13.76%) | −5.30% (−16.42% to 7.29%) | 0.39 |
| 6 | 166.8 (109.8, 249.4) | 160 (98.6, 255.6) | −1.57% (−10.27% to 7.97%) | |||
| LVESV (ml) | 3 | 59.2 (35.3, 109.5) | 60.4 (35.9, 115.6) | 2.60% (−9.66% to 16.52%) | −7.34% (−22.39% to 10.64%) | 0.40 |
| 6 | 75.7 (39.1, 138.2) | 67.5 (31.7, 117) | −4.93% (−16.56% to 8.32%) | |||
| LV stroke volume (ml) | 3 | 83.3 (52, 119.1) | 84.5 (59.9, 121.9) | 4.08% (−3.68 to 11.84) | −2.97 (−13.75 to 7.8) | 0.58 |
| 6 | 92.3 (55.8, 118.4) | 82.6 (63.9, 140.9) | 1.11% (−6.84 to 9.06) | |||
| LV ejection fraction (%) | 3 | 58.5 (42.1, 69.2) | 58.7 (44.4, 70.5) | 0.78 (−2.21 to 3.76) | 0.28 (−3.85 to 4.42) | 0.89 |
| 6 | 54.1 (37.8, 70.8) | 58.1 (35.6, 71.9) | 1.06 (−2.00 to 4.12) | |||
| LVM/LVEDV (g/ml) | 3 | 81.5 (62.9, 137.7) | 80.4 (58.3, 120.1) | −3.48 (−10.55 to 3.59) | −1.49 (−10.98 to 7.99) | 0.76 |
| 6 | 77.7 (58.4, 123) | 76.5 (54.5, 120.1) | −4.97 (−12.2 to 2.26) | |||
| RVEDV (ml) | 3 | 131.3 (79.8, 200) | 116.8 (80.6, 199.7) | −9.05% (−18.04% to 0.92%) | 0.83% (−12.66% to 16.41%) | 0.91 |
| 6 | 147.6 (89.8, 202.6) | 132 (75, 231) | −8.3% (−17.57% to 2.02%) | |||
| RVESV (ml) | 3 | 62.9 (31.9,114.7) | 54.8 (32.2, 113.4) | −9.85% (−20.74% to 2.53%) | −3.84% (−19.59% to 15.00%) | 0.66 |
| 6 | 73.6 (44.1, 101) | 61.3 (27.9, 117.1) | −13.31% (−24.03% to −1.08%) | |||
| RV stroke volume (ml) | 3 | 67.6 (39.3, 103.8) | 67.5 (39.9, 107.5) | −5.54 (−14.16 to 3.08) | 3.27 (−8.31 to 14.86) | 0.58 |
| 6 | 73.6 (47.5, 118) | 64.1 (46.9, 110) | −2.27 (−11.09 to 6.56) | |||
| RV ejection fraction (%) | 3 | 52.1 (34.1, 67.7) | 51.8 (38.1, 66.4) | 0.56 (−3.11 to 4.23) | 1.34 (−3.58 to 6.27) | 0.59 |
| 6 | 50.4 (40.9, 59.2) | 53.4 (41.9, 68.8) | 1.90 (−1.86 to 5.65) |
Q, quartile; F12, month 12 of trial; 95% CI, 95% confidence interval; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LV, left ventricular; LVM, left ventricular mass; RVEDV, right ventricular end diastolic volume; RVESV, right ventricular end systolic volume; RV, right ventricular.
Adjusted mean changes and treatment effects for LVEDV, LVESV, RDEDV, and RVESV expressed as percent difference based on log-transformed analysis.
Effect on Cardiac Volumes and LV Remodeling
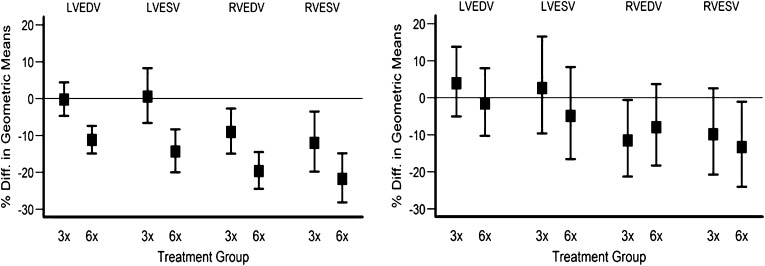
In the daily trial, frequent hemodialysis resulted in significant reductions in LVEDV (−11.0% [95% confidence interval, −16.1% to −5.5%]), LVESV (−14.8% [−22.7% to −6.2%]), and RVEDV (−11.6% [−19.0% to −3.6%]) and a trend for RVESV (−11.3% [−21.4% to 0.1%]) compared with the conventional group. There were no significant differences between groups in ejection fraction. In the nocturnal trial, there were no significant treatment differences in changes in LV and RV volumes or ejection fractions (Figure 1 and Table 2). Overall, there did not seem to be major differences in the timing of MRI scans relative to the preceding dialysis treatment at baseline or the end of the study in either trial (Supplemental Table 1).
Figure 1.
Relative changes in left ventricular end diastolic volume (LVEDV), right ventricular end diastolic volume (RVEDV), left ventricular end systolic volume (LVESV), and right ventricular end systolic volume (RVESV) in patients randomized to either conventional (three times per week) or intensive (six times per week) dialysis. Left panel shows results of the daily trial; right panel shows results of the nocturnal trial. Error bars show ± SD.
We have previously reported that, in the daily trial, frequent hemodialysis resulted in a significant relative reduction in LVM (−13.1 g [−5.0 to −21.3 g], P=0.002). LVM also seemed to fall in the nocturnal trial (−10.1 g [−23.7 to 1.8 g], P=0.09). However, the ratio of LVM to LVEDV did not change in either trial (Table 2).
Subgroup Analyses
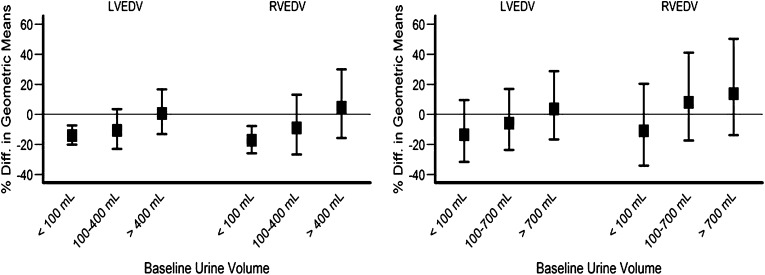
Among nine prespecified factors, only residual urine volume was shown to be an important effect modifier for LVEDV. In the daily trial, there was a consistent trend showing a more pronounced treatment effect among patients with lower baseline residual urine volume (P=0.02) (Figure 2 and Table 3).
Figure 2.
Effect of baseline residual urine volume on LVEDV, RVEDV, LVESV, and RVESV in the daily and nocturnal trials. Left panel shows results of the daily trial; right panel shows results of the nocturnal trial. Different scales were used for the vertical axis to accommodate wider confidence intervals for the nocturnal trial.
Table 3.
Subgroup trends in estimated treatment effects for left ventricular end diastolic volume (percent difference in geometric mean changes)
| Subgroup Factor | Daily Trial | Nocturnal Triala | |||
|---|---|---|---|---|---|
| Subgroup | Estimated Effect with 95% CIb | P Value Interaction | Subgroup | Estimated Effect with 95% CIb | |
| Age (yr) | ≤50 | −14.67 (−21.59 to −7.13) | 0.97 | ≤50 | −14.29 (−29.82 to 4.68) |
| >50 | −7.22 (−14.85 to 1.11) | >50 | −0.82 (−15.60 to 16.56) | ||
| Sex | Men | −9.51 (−15.97 to −2.56) | 0.41 | Men | −5.86 (−19.41 to 9.97) |
| Women | −14.12 (−22.15 to −5.25) | Women | −3.82 (−22.52 to 19.38) | ||
| Diabetic status | Nondiabetes | −11.44 (−18.05 to −4.3) | 0.82 | Nondiabetes | −3.83 (−18.36 to 13.29) |
| Diabetes | −10.18 (−18.18 to −1.39) | Diabetes | −7.82 (−23.51 to 11.10) | ||
| Race | White | −10.78 (−18.46 to −2.38) | 0.90 | White | −5.43 (−18.91 to 10.30) |
| Black | −11.5 (−19.12 to −3.15) | Black | −5.77 (−25.23 to 18.77) | ||
| Anthropometric volume (L) | ≤35 | −16.16 (−24.93 to −6.38) | 0.55 | ≤35 | 3.36 (−19.29 to 32.35) |
| >35 | −8.98 (−15.09 to −2.44) | >35 | −6.89 (−19.36 to 7.52) | ||
| Anthropometric volume (L) | ≤40 | −13.04 (−19.53 to −6.02) | 0.23 | ≤40 | 7.39 (−9.98 to 28.12) |
| >40 | −9.52 (−17.53 to −0.73) | >40 | −16.98 (−29.89 to −1.69) | ||
| Vintage (yr) | <4 | −8.2 (−15.17 to −0.66) | 0.35 | <2 | 1.23 (−13.23 to 18.10) |
| ≥4 | −14.27 (−22.03 to −5.73) | ≥2 | −17.02 (−32.98 to 2.75) | ||
| Urine volume (ml) | ≤100 | −14.2 (−20.15 to −7.81) | 0.02 | ≤500 | −11.52 (−25.48 to 5.04) |
| >100 | −3.25 (−13.01 to 7.6) | >500 | 5.08 (−12.45 to 26.13) | ||
| CHF | No CHF | −10.67 (−16.43 to −4.52) | 0.84 | No CHF | −11.34 (−21.79 to 0.51) |
| CHF | −12.14 (−23.64 to 1.1) | CHF | — | ||
| Baseline LVM (g) | <132 | −9.28 (−16.42 to −1.53) | 0.08 | <132 | −6.05 (−21.29 to 12.14) |
| ≥132 | −12.32 (−19.39 to −4.64) | ≥132 | −0.16 (−16.01 to 18.68) | ||
| β-Blockers use | No | −10.15 (−18.27 to −1.23) | 0.88 | No | −12.41 (−28.58 to 7.41) |
| Yes | −11.46 (−18.07 to −4.32) | Yes | −2.18 (−16.98 to 15.26) | ||
| ACEI or ARB use | No | −9.12 (−15.04 to −2.79) | 0.12 | No | −5.13 (−17.37 to 8.93) |
| Yes | −17.17 (−27.05 to −5.95) | Yes | −8.65 (−40.64 to 40.59) | ||
95% CI, 95% confidence interval; CHF, congestive heart failure; LVM, left ventricular mass; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Because of its limited sample size, we considered subgroup analyses in the nocturnal trial in an exploratory fashion without inference testing.
Treatment effects expressed as percent difference based on log-transformed analysis.
Correlational Analyses
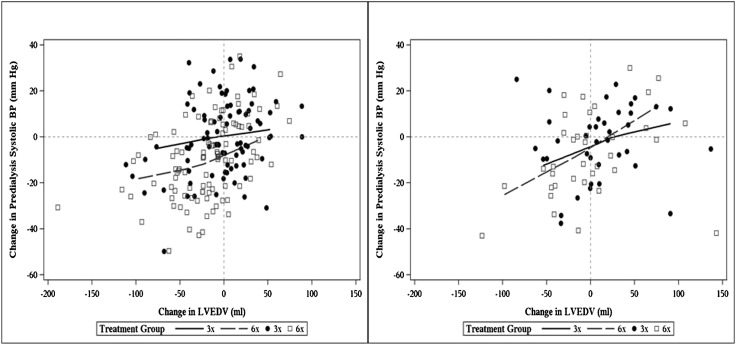
Within each treatment group in the daily and nocturnal trials, those patients with larger reductions in LVEDV tended to have larger reductions in predialysis systolic BP (Figure 3 and Table 4). There was no association between changes in LVEDV and changes in interdialytic weight gain or changes in serum phosphorus.
Figure 3.
Scatter plots relate change in LVEDV to change in predialysis systolic BP from baseline to 12 months with nonparametric loess regression lines. Left panel shows results of the daily trial; right panel shows results of the nocturnal trial.
Table 4.
Pooled correlational analyses among changes in left and right ventricular end diastolic volumes and changes in potential mediators adjusting for treatment group
| Interdialytic Weight Gain | Predialysis Systolic BP | Predialysis Phosphate | |
|---|---|---|---|
| Daily trial | |||
| Log LVEDV | R=0.08, P=0.28 | R=0.39, P<0.001 | R=0.05, P=0.49 |
| Log RVEDV | R=0.14, P=0.06 | R=0.23, P=0.002 | R=−0.11, P=0.13 |
| Nocturnal trial | |||
| Log LVEDV | R=−0.31, P=0.008 | R=0.28, P=0.02 | R=−0.22, P=0.07 |
| Log RVEDV | R=−0.34, P=0.004 | R=0.22, P=0.06 | R=−0.22, P=0.07 |
Partial Pearson correlations relating changes in left and right ventricular end diastolic volumes to changes in potential mediators from baseline to 12 months. Because the partial correlations are adjusted for treatment group, they reflect the average association within the two treatment groups, but they do not reflect differences in mean changes between the two groups. The 12-month values of interdialytic weight gain, predialysis systolic BP, and predialysis phosphate used to compute changes from baseline were taken as values from the 12-month visits without averaging values from neighboring months. LVEDV, left ventricular end diastolic volume; RVEDV, right ventricular end diastolic volume.
Similar relations were noted for changes in RVEDV and reduction in predialysis systolic BP in the daily and nocturnal trials. In addition, changes in RVEDV tended to track with changes in interdialytic weight gain in both trials (Table 4).
Evolution of LVM and LVEDV
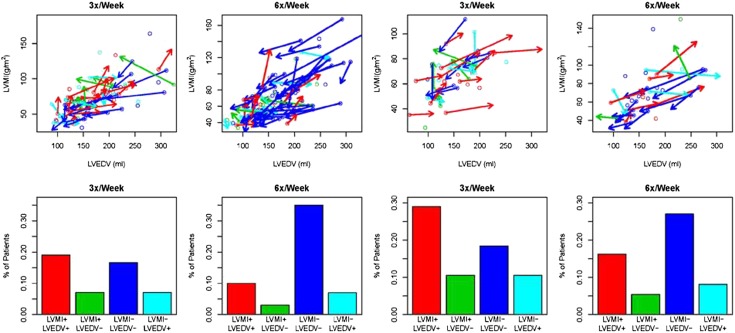
Within each trial, we examined the changes in LVM in concert with LVEDV. Overall, of the patients who received frequent hemodialysis, the highest proportion of these participants had reductions in both LVM and LVEDV in both daily and nocturnal trials (Figure 4).
Figure 4.
Changes from baseline to 12 months in LVEDV and LVMI. In upper panels, open circles designate the baseline LVEDV (x coordinate) and left ventricular mass index (LVMI) (y coordinate) values for each patient. For those patients with at least a 10% change in either LVEDV or LVMI, arrows are drawn from the baseline LVEDV and LVMI values to month 12 LVEDV and LVMI values. Open circles and arrows are colored red if both LVMI and LVEDV increased; they are colored blue if both decreased, green if LVMI increased but LVEDV decreased, and aquamarine if LVMI decreased but LVEDV increased. Lower panels contain corresponding bar charts indicating the percentage of patients falling into the four categories with at least a 10% change in one or both of LVMI and LVEDV within each treatment group.
Discussion
LV dilation is an independent determinant of cardiac mortality in patients with ESRD (21). The present study extends observations in these earlier studies and provides precise estimates of the magnitude of reduction in LV and RV volumes by frequent hemodialysis using CMRI. In addition, we documented the evolution of LVM and volume with frequent hemodialysis. Our data also provide insights into the determinants of changes in LV and RV volumes with frequent dialysis.
Considerable interest has been directed at the clinical implication of LV remodeling. As a result, the ratio of LVM to LVEDV has been used to describe the various patterns of LV remodeling (22). The clinical use of this ratio has been largely derived from population studies and studies involving patients treated for hypertension. For example, Cheng et al. (7) examined age-related differences in LV structure and function in 5004 participants without symptomatic cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Increased mass to volume ratio conferred a significant risk for total cardiovascular events, which was strongest among younger patients (<65 years; hazard ratio, 3.69 [1.34 to 10.10]). Similarly, in 4128 patients enrolled in the iPRESERVE Trial, higher LVM/LVEDV ratio was independently associated with the primary composite end point (death or protocol-specific cardiovascular hospitalization) and heart failure events. It is important to note that several of the previous studies have based their observations on two-dimensional echocardiography. Our present results represent a unique dataset, in which cardiac MRI is used to define changes in volume and LVM as a result of frequent hemodialysis. In contrast to most cardiovascular interventions, frequent hemodialysis simultaneously ameliorates hypertension and volume overload in patients with ESRD. Given that both LVM and LVEDV were reduced in patients treated with six times per week hemodialysis, it may not be surprising to note that the ratio was unaffected.
Observational studies in ESRD suggest that solute and water retention contribute to hypertension, LV hypertrophy, and congestive heart failure (23). The presence of a dilated ventricle (as measured by LVEDV) has been associated with much higher risk than the risk predicted by elevation in LVM alone (2,3). These prior studies were derived from echocardiography, which may overestimate LVM and/or cardiac volumes (24). CMRI used in the daily dialysis trials has obvious advantages for assessing changes in mass and small changes in volume in the ESRD population. LVM assessment by CMRI is independent of chamber dimensions or geometry (25,26). In a previous analysis of the daily dialysis trials, we showed that frequent daily hemodialysis potently reduced LVM, with a strong association between changes in BP and changes in LVM. In this analysis, we were interested in further understanding the magnitude of reduction of chamber volumes as measured by changes in both LV and RV volumes after dialysis and contrasting these changes in the daily and nocturnal trials. Our results show that the magnitudes of reductions in LV and RV volumes with frequent daily dialysis were sizeable and comparable with other direct valvular intervention in the non-ESRD population (27). Patients with larger reductions in LVEDV also tended to have larger reductions in predialysis systolic BP and LVM, further underscoring the fact that the pathophysiologic mechanisms that lead to LV hypertrophy may be inextricably linked to those mechanisms that mediate adverse LV dilation.
Recently, Agarwal et al. (28) showed in a randomized controlled trial that reduction in target postdialysis weight in patients on conventional daily dialysis occurs in conjunction with a substantial fall in systolic and diastolic BPs. More frequent hemodialysis, whether given daily in center or in long-session nocturnal mode, reduced the mean interdialytic weight change and BP. The acute dynamic effects of hemodialysis on LV end diastolic and end systolic dimensions have been measured by Drighil et al. (29). Seventeen patients were examined by tissue Doppler echocardiography before and after hemodialysis. After hemodialysis, there was a fall in LV end diastolic and end systolic dimensions by 20% and 12%, respectively. Our present results show that frequent daily hemodialysis reduced LV and RV end diastolic dimensions by 11% and 12%, respectively. In the daily trial, both LV and RV volumes were reduced in the six times per week group and occurred in conjunction with reductions in BP. It is logical to assume that reductions in biventricular volumes are related to the fall in extracellular fluid excess as a result of frequent hemodialysis. Kjellström et al. (30) evaluated 16 patients on conventional hemodialysis with continuous implantable hemodynamic monitors. Kjellström et al. (30) showed a dynamic direct association between increases in RV pressures (an average increment of 14%) and the extent of interdialytic weight gain. One may infer that the cyclic expansion and contraction of extracellular volume typically seen in conventional hemodialysis is directly transmitted to both LV and RV.
Residual urine volume has been shown to be an important determinant of cardiovascular morbidity and mortality in patients with ESRD. The effect of residual urine volume on the pathogenesis of left ventricular hypertrophy has been most extensively studied in patients on peritoneal dialysis (31). On average, frequent hemodialysis enabled patients with ESRD to preserve cardiac (left- and right-sided) ventricular geometry. We found a more pronounced reduction in LV and RV volumes with frequent relative to conventional hemodialysis among patients with lower levels of residual urine volume. Given that only a minority of patients in the nocturnal trial (27.6%) has minimal residual urine volume, the inclusion of incident patients with ESRD may, in part, explain the lack of effect of nocturnal hemodialysis on cardiac volumes. Patients with little or no residual urine volume may be more susceptible to the adverse cardiovascular effects of volume overload and/or retention of so-called middle molecules—solutes that are less well dialyzed than urea and other lower molecular weight solutes; indeed, these molecules are generally cleared far more efficiently by native kidneys than dialysis in contrast to urea and other smaller, uncharged water-soluble molecules. These analyses shed light on the profound health benefits afforded to patients who retain even slight degrees of residual kidney function relative to those patients who are completely anuric.
Strengths of the current study include the trial design—a multicenter randomized clinical trial with a broad range of patients in terms of age, sex, race/ethnicity, vintage, and primary causes of kidney disease as well as blinded centralized assessment of cardiac volumes. Moreover, adherence was excellent in the daily trial (13) and very good in the nocturnal trial (14). Thus, we endorse both the internal and external validity of our findings. However, there are several important weaknesses inherent in our approach. First, we did not collect data on dietary sodium intake, fluid intake, or ambulatory (continuous) BP, which might have helped to refine our analyses and provide additional detail useful to clinicians. Second, we conducted the MRI measurement at only one time point within the dialysis cycle; repeated scans would have allowed us to refine our estimates. Third, we are limited to ventricular volumes. Assessment of atrial volumes would have been helpful, especially in view of the accentuation of effect on ventricular volumes in those individuals with minimal urine volume. The present study did not aim to relate the intervention-induced changes in cardiac chamber volumes and geometry with clinical events. Nevertheless, in another trial enrolling patients on hemodialysis with dilated cardiomyopathy, a similar magnitude reduction in LVEDV translated into an improvement in overall survival (4).
In summary, the FHN trials show that frequent in-center hemodialysis results in reductions in LV and RV volumes. This effect was more pronounced in patients with little or no residual urine volume. LV remodeling was unaffected by frequent hemodialysis. The majority of patients randomized to six times per week hemodialysis exhibited either reduction or preservation of LVM, EDV, and ESV.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH), the National Institutes of Diabetes and Digestive and Kidney Diseases, the Center for Medicare and Medical Services, and the NIH Research Foundation. Contributors to the NIH Foundation in support of the Frequent Hemodialysis Network (FHN) trials included Amgen, Baxter, and Dialysis Clinics. Additional support was provided by DaVita, Dialysis Clinics, Fresenius Medical Care, Renal Advantage, Renal Research Institute, and Satellite Healthcare.
A list of members of the FHN trial group for each study has been published (13,14).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03280313/-/DCSupplemental.
See related editorial, “The Effects of Frequent Hemodialysis on Left Ventricular Mass, Volumes, and Geometry,” on pages 2025–2027.
References
- 1.Foley RN, Collins AJ: End-stage renal disease in the United States: An update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, Seminara G, Stancanelli B, Malatino LS: Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol 15: 1029–1037, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS: Left ventricular mass monitoring in the follow-up of dialysis patients: Prognostic value of left ventricular hypertrophy progression. Kidney Int 65: 1492–1498, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Cice G, Ferrara L, D’Andrea A, D’Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabrò R: Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol 41: 1438–1444, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Curtis BM, Parfrey PS: Congestive heart failure in chronic kidney disease: Disease-specific mechanisms of systolic and diastolic heart failure and management. Cardiol Clin 23: 275–284, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cohn JN, Ferrari R, Sharpe N: Cardiac remodeling—concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35: 569–582, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA: Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: The Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 2: 191–198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unsal A, Kose Budak S, Koc Y, Basturk T, Sakaci T, Ahbap E, Sinangil A: Relationship of fibroblast growth factor 23 with left ventricle mass index and coronary calcificaton in chronic renal disease. Kidney Blood Press Res 36: 55–64, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE: Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47: 186–192, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, Daugirdas JT, Kotanko P, Larive B, Levin NW, Mehta RL, Rocco M, Sanz J, Schiller BM, Yang PC, Rajagopalan S, Frequent Hemodialysis Network (FHN) Trial Group : Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging 5: 251–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan CT, Arab S, Carasso S, Moravsky G, Li GH, Liu PP, Rakowski H: Impact of frequent nocturnal hemodialysis on myocardial mechanics and cardiomyocyte gene expression. Circ Cardiovasc Imaging 5: 474–480, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS, FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocco MV, Lockridge RS, Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, Larive B, Chan CT, Chertow GM, Copland M, Hoy CD, Lindsay RM, Levin NW, Ornt DB, Pierratos A, Pipkin MF, Rajagopalan S, Stokes JB, Unruh ML, Star RA, Kliger AS, Kliger A, Eggers P, Briggs J, Hostetter T, Narva A, Star R, Augustine B, Mohr P, Beck G, Fu Z, Gassman J, Greene T, Daugirdas J, Hunsicker L, Larive B, Li M, Mackrell J, Wiggins K, Sherer S, Weiss B, Rajagopalan S, Sanz J, Dellagrottaglie S, Kariisa M, Tran T, West J, Unruh M, Keene R, Schlarb J, Chan C, McGrath-Chong M, Frome R, Higgins H, Ke S, Mandaci O, Owens C, Snell C, Eknoyan G, Appel L, Cheung A, Derse A, Kramer C, Geller N, Grimm R, Henderson L, Prichard S, Roecker E, Rocco M, Miller B, Riley J, Schuessler R, Lockridge R, Pipkin M, Peterson C, Hoy C, Fensterer A, Steigerwald D, Stokes J, Somers D, Hilkin A, Lilli K, Wallace W, Franzwa B, Waterman E, Chan C, McGrath-Chong M, Copland M, Levin A, Sioson L, Cabezon E, Kwan S, Roger D, Lindsay R, Suri R, Champagne J, Bullas R, Garg A, Mazzorato A, Spanner E, Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T, Pierratos A, Chan W, Regozo K, Kwok S, Frequent Hemodialysis Network (FHN) Trial Group : The effects of frequent nocturnal home hemodialysis: The Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 80: 1080–1091, 2011. 21775973 [Google Scholar]
- 15.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, Beck GJ, Gassman JJ, Eggers PW, Star RA, Ornt DB, Kliger AS, Frequent Hemodialysis Network Trial Group : Frequent Hemodialysis Network (FHN) randomized trials: Study design. Kidney Int 71: 349–359, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Rocco MV, Larive B, Eggers PW, Beck GJ, Chertow GM, Levin NW, Kliger AS, FHN Trial Group : Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. Am J Kidney Dis 57: 90–100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fieno DS, Jaffe WC, Simonetti OP, Judd RM, Finn JP: TrueFISP: Assessment of accuracy for measurement of left ventricular mass in an animal model. J Magn Reson Imaging 15: 526–531, 2002 [DOI] [PubMed] [Google Scholar]
- 18.DuBois D, DuBois EF: A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17: 863–871, 1916 [Google Scholar]
- 19.Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980 [DOI] [PubMed] [Google Scholar]
- 20.Cleveland ER, Johnson RK, Cunningham PJ: Correlated responses of carcass and reproductive traits to selection for rate of lean growth in swine. J Anim Sci 66: 1371–1377, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE: The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5: 2024–2031, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Gaasch WH, Zile MR: Left ventricular structural remodeling in health and disease: With special emphasis on volume, mass, and geometry. J Am Coll Cardiol 58: 1733–1740, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Chaignon M, Chen WT, Tarazi RC, Bravo EL, Nakamoto S: Effect of hemodialysis on blood volume distribution and cardiac output. Hypertension 3: 327–332, 1981 [DOI] [PubMed] [Google Scholar]
- 24.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE: Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277–1285, 1996 [PubMed] [Google Scholar]
- 25.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA: Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. AJR Am J Roentgenol 186[Suppl 2]: S357–S365, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Hunold P, Vogt FM, Heemann UW, Zimmermann U, Barkhausen J: Myocardial mass and volume measurement of hypertrophic left ventricles by MRI—study in dialysis patients examined before and after dialysis. J Cardiovasc Magn Reson 5: 553–561, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L, EVEREST II Investigators : Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 364: 1395–1406, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Agarwal R, Alborzi P, Satyan S, Light RP: Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension 53: 500–507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drighil A, Madias JE, Mathewson JW, El Mosalami H, El Badaoui N, Ramdani B, Bennis A: Haemodialysis: Effects of acute decrease in preload on tissue Doppler imaging indices of systolic and diastolic function of the left and right ventricles. Eur J Echocardiogr 9: 530–535, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Kjellström B, Braunschweig F, Löfberg E, Fux T, Grandjean PA, Linde C: Changes in right ventricular pressures between hemodialysis sessions recorded by an implantable hemodynamic monitor. Am J Cardiol 103: 119–123, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK, Sanderson JE: Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol 15: 2186–2194, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.