Summary
Failure of hemodialysis access is caused mostly by venous intimal hyperplasia, a fibro-muscular thickening of the vessel wall. The pathogenesis of venous neointimal hyperplasia in primary arteriovenous fistulae consists of processes that have been identified as upstream and downstream events. Upstream events are the initial events producing injury of the endothelial layer (surgical trauma, hemodynamic shear stress, vessel wall injury due to needle punctures, etc.). Downstream events are the responses of the vascular wall at the endothelial injury that consist of a cascade of processes including leukocyte adhesion, migration of smooth muscle cells from the media to the intimal layer, and proliferation. In arteriovenous fistulae, the stenoses occur in specific sites, consistently related to the local hemodynamics determined by the vessel geometry and blood flow pattern. Recent findings that the localization of these sites matches areas of disturbed flow may add new insights into the pathogenesis of neointimal hyperplasia in the venous side of vascular access after the creation of the anastomosis. The detailed study of fluid flow motion acting on the vascular wall in anastomosed vessels and in the arm vasculature at the patient-specific level may help to elucidate the role of hemodynamics in vascular remodeling and neointimal hyperplasia formation. These computational approaches may also help in surgical planning for the amelioration of clinical outcome. This review aims to discuss the role of the disturbed flow condition in acting as upstream event in the pathogenesis of venous intimal hyperplasia and in producing subsequent local vascular remodeling in autogenous arteriovenous fistulae used for hemodialysis access. The potential use of blood flow analysis in the management of vascular access is also discussed.
Introduction
Almost half a century after the pioneering hemodialysis using venipuncture (1) on a surgically created radial-cephalic arteriovenous fistula (AVF), maintaining adequate vascular access is a difficult challenge to face. Although there is general consensus in the literature and vascular access guidelines on the superiority of AVFs over arteriovenous grafts (AVGs) and central venous catheters in terms of patient complications and survival, the reality reveals a high nonmaturation rate after creation of the primary AVF. Allon and Robbin (2) showed that in studies performed between 1977 and 2002, the mean early failure rate of AVFs was 25% (range, 2%–53%), whereas the mean 1-year primary survival was 70% (range, 42%–90%).
The most common cause of vascular access failure is vascular stenosis (3,4) as a result of severe venous neointimal hyperplasia. It has been proposed that the pathogenesis of venous neointimal hyperplasia in vascular access is related to a cascade of events that can be divided into “upstream” and “downstream” events, as reported by Roy-Chaudhury et al. (5). Upstream events are the processes responsible for endothelial cell (EC) and smooth muscle cell (SMC) activation and injury that then set the downstream events into motion, which are complex interactions of adhesion molecules, inflammation mediators, and chemokines that result in venous neointimal hyperplasia.
Because stenoses in AVFs develop in specific locations (4,6), an important question is whether neointimal hyperplasia is related to local hemodynamic conditions (i.e., disturbed flow with low and oscillating wall shear stress [WSS]). This hypothesis was quite neglected in the past because a massive increase in blood flow is induced by the surgically created anastomosis—up to 20 times greater than preoperatively (7,8), with a consequent increase in WSS.
In this context, new computational tools, such as one-dimensional (1D) pulse wave propagation models and more detailed three-dimensional (3D), image-based, computational fluid dynamics (CFD) of blood flow, may help in elucidating the mechanisms of AVF failure. The aim of this review is to connect basic research and new concepts about the role of disturbed flow, a condition of low and reciprocating WSS that develops in specific sites upon surgery and acts as upstream event in the pathogenesis of venous neointimal hyperplasia in vascular access for hemodialysis.
Pathogenesis of Vascular Access Stenosis
Mechanisms of Intimal Hyperplasia
Neointimal hyperplasia is a fibro-muscular thickening of the vascular wall due to SMCs that migrate from the media to the intimal layer and then proliferate into the subintimal layer. The phases of upstream events leading to neointimal hyperplasia can be broadly classified into platelet activation and adhesion, leukocyte recruitment and transmigration, and SMC migration and proliferation (9). The endothelium has a central role in this cascade of events. Activated or injured ECs release inflammatory mediators that trigger platelet aggregation and recruitment of leukocytes to this area. In activated ECs, increased expression of growth factors at the gene and protein levels (such as HDAC3 and PDGF-2) promote SMC migration from the media to the intima as well as proliferation (10). Proliferation of SMCs in the intima is associated with deposition of extracellular matrix, a process analogous to scar formation (9,11). The overall result is the rapid formation of a neointimal layer over the site of injury. The possibility of adventitial migration of fibroblasts to the media and ongoing phenotypic switching within the media and intima were shown in patients with advanced CKD and ESRD with severe neointimal hyperplasia (12) as well as in patients with a new AVF (13,14). In fact, recent studies have shown that the adventitia may have an important role in neointimal hyperplasia lesion formation, as a hub for recruitment of inflammatory cells that may directly stimulate medial SMC migration and proliferation and as a source of neointimal precursor cells that contribute to the cellular mass of developing neointimal hyperplasia lesions (15). In such “outside–in” mechanisms of cell migration, a subpopulation of fibroblasts from adventitia undergoes a phenotypic switch into migratory myofibroblasts. These converted cells travel to the site of the injured vasculature to mitigate vessel damage.
Factors like aging, obesity, underlying diabetes, and cardiovascular disease may lead to arteriosclerotic changes of blood vessels in uremic patients. In patients undergoing AVF creation, preexisting radial artery neointimal hyperplasia (16) and increased intima-media thickness in the radial artery correlated with early failure of radiocephalic AVFs (17). In these patients, there is preexisting neointimal hyperplasia on the cephalic vein (12,18) and major changes in the vascular SMC component (19) are present even before AVF construction, making arterial vessel less compliant. Thus, previous conditions of arm blood vessels strongly influence the outcome of vascular access, in early as well as in late AVF failure.
Neointimal Hyperplasia in Vascular Access
Although several studies have reported venous neointimal hyperplasia before dialysis access surgery as mentioned above, Allon et al. (20) did not find any preexisting neointimal hyperplasia in 50 hemodialysis patients. However, the authors found severe venous neointimal hyperplasia in six patients with nonmaturing AVFs that clearly was not present in the samples obtained at surgery, a strong demonstration that venous neointimal hyperplasia does develop de novo after AVF creation. In patients with failing AVFs, the exuberant healing response leads to neointimal hyperplasia that reduces vessel lumen and causes stenosis and subsequent thrombosis (21). The presence of a stenosis results in an increase in resistance and in the pressure gradient over the stenosis as well as a subsequent decline in access blood flow. Low flow causes intimal hyperplasia, thus establishing a vicious cycle: low flow generates obstructive neointima layers, which in turn further reduce the flow. The histology of neointimal hyperplasia and its relation with WSS have been characterized in patients with AVF creation for hemodialysis and early failure (13). Cellular phenotype studies showed that the majority of cells within the neointimal hyperplasia region were myofibroblasts and a smaller number were contractile SMCs. The luminal shapes at the sites of stenoses were eccentric in the majority of cases, consistent with a role of hemodynamic stresses in the development of neointimal hyperplasia.
Role of Hemodynamics in the Pathogenesis of Neointimal Hyperplasia
Physiologic adaptation of the vascular tree occurs when there are sustained changes in the hemodynamic stimuli. Thus, an increase in the blood flow rate increases WSS on ECs that produce mediators of vascular cell proliferation and matrix deposition, resulting in enlargement of the vessel lumen and a consequent reduction of WSS to the physiologic range. An increase in intraluminal pressure regulates wall thickness through its effect on wall tension and SMC response to mechanical stimulation (22). Locally, if the vessel is injured, the arterial remodeling can be either maladaptive or adaptive. Maladaptive remodeling is inward (negative) growth that leads to a reduction in the lumen diameter, whereas adaptive remodeling is outward (positive) growth that maintains the lumen diameter (23,24). Vascular remodeling in the AVF aims to accommodate blood vessels at the new hemodynamic condition in such a way to restore the basal levels of tensile and shear stresses (7,8).
Disturbed Flow versus Laminar Flow
The previously mentioned response of the vasculature to hemodynamic changes may act in the major part of the vascular tree where blood flow is laminar. However, this mechanism cannot act in specific regions of the vasculature, like branching and curvatures of large arteries where disturbed flow may develop. The most frequent sites for these flow patterns are the carotid and coronary bifurcations and the branching of renal and femoral arteries from abdominal aorta. The pattern of disturbed flow is irregular; it features secondary and recirculation eddies that may change in direction with time and space, and hence it exerts low and oscillating WSS on the ECs (25). Localization of atherosclerosis and thrombosis within these specific sites, in humans and experimental animals, suggests a significant role of the local disturbed flow in the pathogenesis of the vascular wall (26).
Experimental investigations have demonstrated that in laminar flow, the WSS induces quiescence of ECs and of the adjacent SMCs by controlling production of several vasoactive compounds (10). Thus, sustained laminar WSS within a physiologic range and a predominant direction activates signaling pathways that induce expression of several atheroprotective and antithrombogenic genes, encoding products that have antioxidant, anti-inflammatory, anticoagulant, and antiapoptotic functions. Krüppel-like factor-2 (KLF2) is one of the most important transcription factors regulated by WSS acting on ECs (27). The exposure of ECs to physiologic shear stress increases gene expression for KLF2 and endothelial nitric oxide synthase even to a larger extent than statins, inducing a protective effect on ECs (26). The mechanism by which KLF2 mRNA expression is increased by shear forces is related to activation of calmodulin-dependent kinases and subsequent phosphorylation of histone deacetylase 5 (28). This activates myocyte enhancer factor-2 with consequent increased gene expression of several vasoactive molecules. Pulsatile flow increases KLF2 expression compared with steady flow. On the basis of in vivo observations, it has been reported that the inner surface of branches of the thoracic aorta that are exposed to low WSS show lower KLF2 expression compared with the outer surface where higher flow and WSS occur (29). In general, these protective effects of laminar WSS are mediated via several mechanisms, including the inhibition of cytokine-induced EC signaling, suppression of EC proliferation, and modulation of the SMC phenotype toward a contractile quiescent state.
In contrast to laminar flow, disturbed flow acting on ECs in vitro, with low and reciprocating WSS, induces selective expression of atherogenic and thrombogenic genes (pro-oxidant, proinflammatory, procoagulant, and proapoptotic) (10). It was then proposed that WSS waveforms may be identified as atheroprone or atheroprotective. Dai et al. (30) compared in vitro the effects of two different atheroprone and atheroprotective shear stress patterns, demonstrating an important effect of low and oscillating WSS on the EC cytoskeleton, interleukin production, and nuclear translocation of transcription factor NF-κB that is related to enhanced expression of adhesion molecules (30).
Disturbed Flow as an Upstream Event in Neointimal Hyperplasia
Disturbed flow itself stimulates neointimal hyperplasia by promoting an inflammatory and thrombotic phenotype on arterial ECs and also stimulates SMC migration and proliferation (31). Because the stenoses occur mainly on the venous side in AVFs for hemodialysis access, more attention should be focused on venous ECs. The response of venous ECs to disturbed flow resulting from reflux through incompetent valves and stasis in patients with chronic venous diseases (32) resembles the response of arterial ECs in atherogenesis, in terms of adhesion and transmigration of leukocytes, elevated expressions of adhesion molecules, and production of reactive oxygen species. There are, however, differences between ECs from veins and arteries. For example, venous ECs have greater capacity to mount an inflammatory response compared with arterial ECs, and this may enhance the development of venous thrombosis and vein graft atherosclerosis (10). Furthermore, the elevated pressure in the venous system can activate proinflammatory signaling pathways (32).
It has been shown that low or near-zero shear stress itself also promotes neointimal growth in vivo. The mechanism of cell proliferation in these areas of the vessel wall is directly related to increased binding of monocyte chemotactic protein-1 to the cysteine-cysteine receptor (33). Thus, low WSS activates a cascade of molecular events that lead to leukocyte adhesion and migration into the intimal layer of the vasculature. Hemodynamically induced neointimal hyperplasia has been related not only to low WSS but also to stagnation points of the circulation (11). That low WSS is the major determinant of intimal thickness was demonstrated in vivo in dogs by Morinaga and colleagues in 1985 (34), who clearly showed that the change in WSS, but not the rate of blood flow, is the essential hemodynamic factor related to neointimal hyperplasia in autogenous vein grafts. Recently, a direct relation between low WSS profiles and patterns of neointimal hyperplasia was demonstrated in vivo in a pig model of AVF (35).
Hemodynamic Conditions in Vascular Access
The above-mentioned phenomena are of crucial importance in AVF function. Creating a well functioning AVF means placing a nonphysiologic, high blood volume flow conduit into a dysfunctional vascular bed that must initiate a remodeling process to adapt to these new hemodynamic conditions. To make things even more complex, two different types of vessels are involved in this adaptation process, the artery and the vein, which are linked by sewing the anastomosis (36).
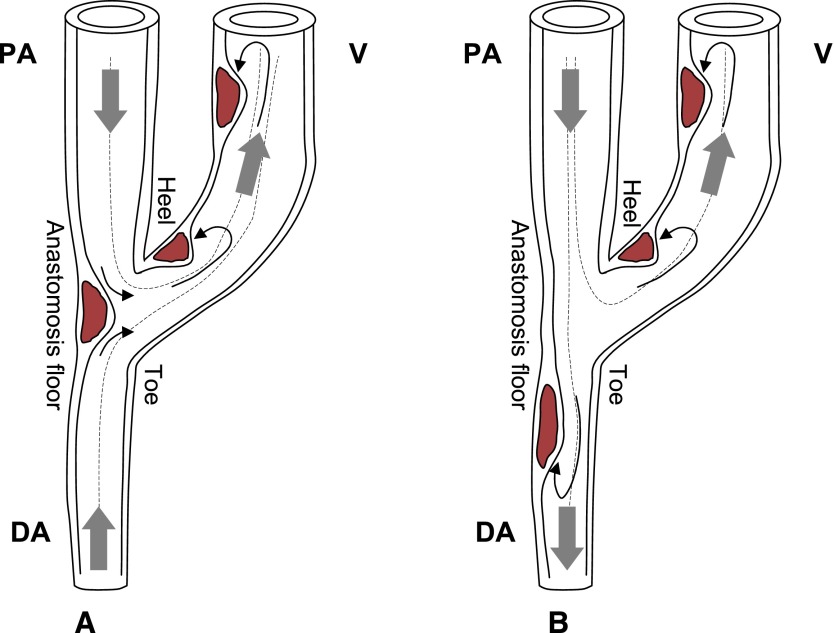
The first factor influencing hemodynamics in these vessels is the surgically created anastomosis, which bypasses the peripheral resistances and consequently greatly increases blood flow. The AVF-induced high flows and complex hemodynamic conditions, very different from the physiologic state, initiate the process of vascular adaptation and local remodeling. Second, the nonuniform geometry near the anastomosis forces blood to rapidly change direction. In end-to-side anastomoses, as presented schematically in Figure 1, blood flow is directed from the proximal artery to the vein, and the flow in the distal artery may be retrograde (Figure 1A) if blood is directed from the hand to the anastomosis or antegrade (Figure 1B) if blood flows toward the hand (37,38). If the anastomosis is end-to-end, blood flows from the artery to the vein in a bending, U-shaped structure. In such geometries, acceleration or deceleration of blood due to bending curves and variations of the cross-sectional area, implying recirculation vortexes and secondary flows, entails energy dissipation and nonuniform WSS across the circumference of the vessel. Disturbed flow with low and reciprocating WSS may develop on the internal wall of the juxta-anastomotic vein and on the arterial side in the vicinity of the anastomosis floor and triggers formation of neointimal hyperplasia at these sites (see Figure 1). Distal from the anastomosis, blood flow becomes more laminar and the hemodynamic shear stress is more uniform and within the physiologic range. Although intimal hyperplasia occurs mainly on the venous limb, a frequency of arterial stenoses up to 16.6% was reported by Badero et al. (4), and Asif et al. reported an even higher frequency up to 35% (39). Even if thickening of the vessel wall occurs on the arterial limb of the AVF, these stenoses seem nonprogressive (6) and hence may lead to impairment of blood flow seldom with respect to the venous stenoses; however, they often may contribute to the development of steal syndrome (38). In this context, the evidence of deleterious effects of low and oscillating shear stress in vessel wall injury poses some questions. Despite high blood flow in the AVF, what type of flow develops on the vessel wall? Are there areas of low and oscillating shear stress?
Figure 1.
Schematic illustration of typical end-to-side anastomoses, blood flow pathways, and location of intimal hyperplasia in AVFs for hemodialysis. (A) An AVF with retrograde blood flow in the DA. (B) An AVF with antegrade flow in the DA. Thick gray arrows indicate blood flow direction, dashed lines represent flow streamlines, and continuous line black arrows represent recirculation vortexes and stagnation points at the sites where disturbed flow develops. Disturbed flow with low and reciprocating WSS triggers formation of neointimal hyperplasia on the AVF walls. AVF, arteriovenous fistula; PA, proximal artery; DA, distal artery; V, vein.
Disturbed Flow Patterns in the AVF
Among the upstream events that may contribute to neointimal hyperplasia formation, the hemodynamic shear stress at the AVF anastomosis was investigated in several CFD studies in the last decade (40–43). Numerical analysis of flow velocity has been validated with established experimental data (44), even for high values of the Reynolds number, an index of flow instability or turbulence. As in good functioning AVFs the Reynolds number is high, CFD has been validated against in vitro measurements by particle image velocimetry (42) in AVF models. All of these studies found high WSS, especially very high near the anastomosis, but did not find disturbed flow patterns on the AVF walls. To answer this question, we used pulsatile CFD simulations applied to idealized models of AVF anastomoses to compute velocity field and related WSS. Despite the high flow rate, we found areas on the juxta-anastomotic vein in which WSS is low and oscillating, both in end-to-side and end-to-end anastomosis (45). On the basis of these findings, in a parametric idealized model of end-to-side AVF, we further studied whether the anastomosis angle might influence the pattern of disturbed flow (46). Quantification of these areas showed that the smaller the angle, the smaller is the area of low and oscillating WSS, as quantified by the relative residence time (47). These results suggest that an acute anastomosis angle should be preferred to minimize the risk of neointimal hyperplasia on the juxta-anastomotic vein.
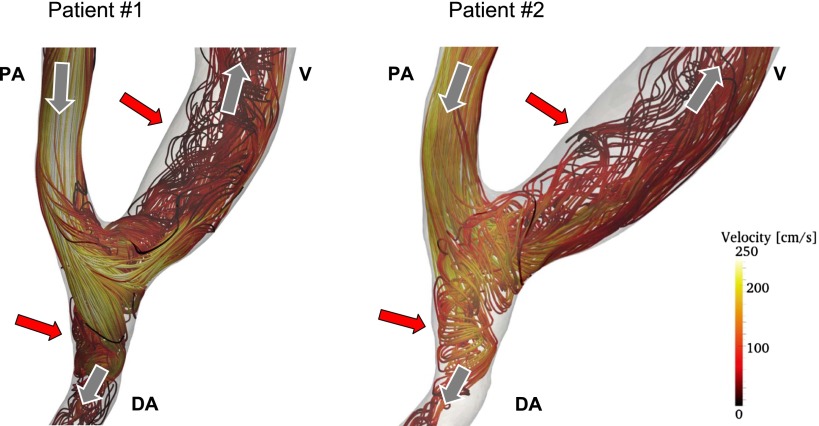
Recently, we performed image-based CFD studies in patient-specific models of AVF, simulating pulsatile, realistic blood flow conditions (A. Remuzzi and B. Ene-Iordache, unpublished observations). We generated the 3D meshes using contrast-enhanced magnetic resonance imaging of normal functioning AVFs at 4 weeks after surgery. Representative velocity streamlines for the peak systolic blood flow for the AVFs of two patients are reported in Figure 2. In these CFD simulations, we assumed an unsteady laminar flow regime because the Reynolds numbers based on the maximum velocity in the proximal artery and in the vein for the two patients with AVFs were 1200 and 900 and 1400 and 1100, respectively. The velocity streamlines reveal laminar flow in the proximal artery and secondary flows and local vortexes in the venous and distal artery segment. This pattern was similar for both patients. Due to the pulsatility of flow during the cardiac cycle, recirculation and reattachment flow with low velocity develops near the wall, inducing disturbed flow with low and reciprocating WSS on the inner surface of the juxta-anastomotic segment and on the distal artery (red arrows in Figure 2). These preliminary data indicate that the results obtained with idealized models of AVFs (45,46) are representative of the real condition in patients with AVF.
Figure 2.
Blood velocity streamlines inside two patient-specific radial-cephalic AVFs, representative of the peak systolic blood volume flow instance. Streamlines are colored by velocity magnitude scale; dark colors (brown) indicate low velocities and bright colors (yellow) indicate high velocities. Gray arrows indicate the direction of blood flow in PA, DA, and V. The red arrows indicate areas of vessel wall where disturbed flow with low and oscillating shear stress develops. AVF, arteriovenous fistula; PA, proximal artery; DA, distal artery; V, vein.
Vascular Adaptation in the AVF
Besides intimal hyperplasia, it is well known that WSS is the physiologic stimulus for vascular adaptation upon changes in blood flow. We have shown that the radial artery diameter increases in response to a chronic increase in blood flow in patients with end-to-end AVF (8). In that study, we calculated the WSS time waveform using Womersley’s theory (48). Although mean WSS increased five times after surgery, the peak value of WSS remained quite constant. Similar findings were reported by Dammers et al. in the brachial artery of patients undergoing vascular access surgery (7,49). These findings indicate that in hemodialysis patients, the peak shear stress rather than the average, as previously considered, is the key factor in driving vessel dilatation upon chronic increase in blood volume flow. Although it originated in arteries of uremic patients, this hypothesis is in line with previous results on the temporal gradient versus the steady shear obtained in ECs in vitro (50,51).
On the basis of these observations, we recently developed a network model of blood circulation, including a vascular adaptation algorithm, to simulate blood flow after vascular access surgery over time (52). Briefly, the model is based on a 0D/1D pulse wave propagation model of lumped parameter segments with serial arrangement of the resistor and inductor, representing detailed arm circulation together with the radiocephalic end-to-end anastomosis, whereas the aorta and the vessels in the lower body, contralateral arm, and head are partially modeled and ended with three-element Windkessel segments (53). In this model, the dilation of the vessel lumen is assumed to be proportional to the difference between actual peak WSS and a baseline reference value. We also assumed that vessel remodeling varies along the AVF anastomosis due to the presence of the suture line, being absent at the anastomosis and increasing toward the arterial and venous limbs by a quadratic law. The average errors between model-predicted and measured values of radial artery diameter and blood flow at 100 days postsurgery were 10.3% and 11.6%, respectively (52), confirming that peak WSS is indeed the physical stimulus for vascular adaptation.
Clinical Applications and Perspectives
Minimization of Disturbed Flow Regions Created by the Surgical Intervention
One example of clinical application is the design and improvement of vascular surgery. Optimization of the factors considered in this review and their introduction as recommendations that now lack in the vascular access guidelines (54,55) can significantly improve the outcome. By using computational modeling, we have shown how a sharp anastomotic angle (approximately 30°) minimizes the disturbed flow patterns in AVF (46). Although our finding regarding the angle must be confirmed in future experimental studies, recent studies are in line with this indication. Bharat et al. (56) performed a clinical study in patients undergoing radiocephalic AVF comparing three different anastomotic techniques. They proposed a novel technique of vascular anastomosis (the piggyback straight-line onlay technique) that was characterized by a very small anastomosis angle and that had very few juxta-anastomotic stenosis events respect to the traditional end-to-side and side-to-side techniques. Such results are obtained by minimizing two upstream events of neointimal hyperplasia. By using the piggyback straight-line onlay technique, the disturbed flow is reduced due to the acute angle and the venous wall injury produced by the traditional torsion of the juxta-anastomotic vein (56,57) is minimized.
The next step in these investigations should be the use of image-based CFD analyses to document WSS patterns in patient-specific models of AVF during vascular access follow-up (58,59), aimed at identifying areas at risk for development of neointimal hyperplasia. Moreover, with the advent of parallel processors power and new CFD solvers including fluid-structure interaction, vessel wall elasticity could be modeled in pulsatile simulations toward the understanding of the influence of wall compliance on the pattern of disturbed flow in AVF. Another useful application of image-based CFD studies would be the study of disturbed flow inside the AVG. Despite differences between natural and graft material, it was shown that in the venous neointima of AVGs the cellular phenotypes are similar to those in AVF (14) and neointimal hyperplasia occurs in specific anatomic locations (11). We might speculate that disturbed flow may be an upstream event in the pathogenesis of neointimal hyperplasia also in AVG, but this hypothesis has to be confirmed.
AVF Surgery Planning
CFD simulations on 3D models are used to study locally detailed hemodynamics (e.g., velocity field, WSS, pressure, and flow). Disadvantages of such an approach are the difficulty in creating the 3D models and in defining proper patient-specific boundary conditions, as well as the long calculation time. For this purpose, 1D pulse wave propagation models are more suitable (60). In these models, the vascular system is divided into segments representing local blood and vessel wall properties that are serially connected based on the anatomic configuration.
We recently validated the previously mentioned computational model (52) for prediction of AVF surgery outcome based on preoperative patient-specific parameters (61). Briefly, we implemented a clinical application based on the 0D/1D theoretical model of arm circulation (53), which was validated experimentally using an in vitro model of silicone tubes to mimic the aorta and arm vasculature before and after AVF surgery (62). We improved the model with a nonlinear resistance algorithm of the anastomosis based on parametric CFD analysis with different anastomosis angles and vessel sizes. The model includes the previously described vascular adaptation algorithm (52). On the basis of demographic and clinical data, it is possible to predict the expected blood flow along the vascular network that will develop with time. We recently reported the results of the validation of this application (63), comparing the calculated vascular access flow with that measured in a group of 63 patients during a follow-up period of 6 weeks. We thus propose using this approach in the clinical routine to perform computer-based planning of AVF surgery using a fast and distributed computer infrastructure (http://avfsim.herokuapp.com). The clinical operator is allowed to identify the best location and type of anastomosis at the patient-specific level in order to obtain an optimal flow rate for hemodialysis. The use of this approach for AVF surgery planning may reduce nonmaturation events, as well as late failure rates, and may avoid creation of upper arm vascular access with very high blood flows that are at risk for heart failure.
Summary and Conclusions
Despite the massive increase in blood flow, some zones of the AVF, especially juxta-anastomotic sites, are characterized by disturbed flow with low and reciprocating WSS, a condition that predisposes the venous wall to development of neointimal hyperplasia. In many uremic patients having already impaired endothelial function due to the final stage of CKD and risk factors such as aging, cardiovascular disease, diabetes, and obesity, the disturbed flow will act as an additional upstream event for the pathogenesis of neointimal hyperplasia, further enhancing its development. In such patients, the surgical creation of the AVF may trigger the downstream events for development of neointimal hyperplasia and luminal stenosis, leading to immediate failure or to nonmaturation of the fistula.
The use of new computational modeling tools can help us to understand the role of disturbed flow in the pathophysiology of vascular access disease and to design surgical techniques toward minimization of the influence of hemodynamics on neointimal hyperplasia formation in dialysis access. At the same time, these tools may predict vascular adaptation, at the patient-specific level, and may be used to identify the best surgical procedure to obtain optimal blood flow in the vascular access.
Disclosures
None.
Acknowledgments
The authors acknowledge funding of this research through the ARCH project funded by the Seventh Framework Program (FP7-ICT-2007-2) of the European Commission under grant agreement number 224390.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Brescia MJ, Cimino JE, Appel K, Hurwich BJ: Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med 275: 1089–1092, 1966 [DOI] [PubMed] [Google Scholar]
- 2.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, Roy-Chaudhury P: Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol 6: 575–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badero OJ, Salifu MO, Wasse H, Work J: Frequency of swing-segment stenosis in referred dialysis patients with angiographically documented lesions. Am J Kidney Dis 51: 93–98, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Sivanesan S, How TV, Bakran A: Sites of stenosis in AV fistulae for haemodialysis access. Nephrol Dial Transplant 14: 118–120, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Dammers R, Tordoir JH, Welten RJ, Kitslaar PJ, Hoeks AP: The effect of chronic flow changes on brachial artery diameter and shear stress in arteriovenous fistulas for hemodialysis. Int J Artif Organs 25: 124–128, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Ene-Iordache B, Mosconi L, Antiga L, Bruno S, Anghileri A, Remuzzi G, Remuzzi A: Radial artery remodeling in response to shear stress increase within arteriovenous fistula for hemodialysis access. Endothelium 10: 95–102, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Mitra AK, Gangahar DM, Agrawal DK: Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol 84: 115–124, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chiu JJ, Chien S: Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haruguchi H, Teraoka S: Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J Artif Organs 6: 227–235, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P: Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant 26: 2264–2270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, Samaha A, Munda R: Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis 50: 782–790, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, Heffelfinger S, Arend L: Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant 24: 2786–2791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM, Jr: The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs 195: 73–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YO, Song HC, Yoon SA, Yang CW, Kim NI, Choi YJ, Lee EJ, Kim WY, Chang YS, Bang BK: Preexisting intimal hyperplasia of radial artery is associated with early failure of radiocephalic arteriovenous fistula in hemodialysis patients. Am J Kidney Dis 41: 422–428, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Kim YO, Choi YJ, Kim JI, Kim YS, Kim BS, Park CW, Song HC, Yoon SA, Chang YS, Bang BK: The impact of intima-media thickness of radial artery on early failure of radiocephalic arteriovenous fistula in hemodialysis patients. J Korean Med Sci 21: 284–289, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wali MA, Eid RA, Dewan M, Al-Homrany MA: Intimal changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. J Smooth Muscle Res 39: 95–105, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Wali MA, Eid RA, Al-Homrany MA: Smooth muscle changes in the cephalic vein of renal failure patients before use as an arteriovenous fistula (AVF). J Smooth Muscle Res 38: 75–85, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 58: 437–443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P: Biology of arteriovenous fistula failure. J Nephrol 20: 150–163, 2007 [PubMed] [Google Scholar]
- 22.Pries AR, Secomb TW: Control of blood vessel structure: insights from theoretical models. Am J Physiol Heart Circ Physiol 288: H1010–H1015, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD: Vascular remodeling. Hypertension 28: 505–506, 1996 [PubMed] [Google Scholar]
- 24.Gibbons CP: Primary vascular access. Eur J Vasc Endovasc Surg 31: 523–529, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Davies PF: Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimbrone MA, Jr, García-Cardeña G: Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol 22: 9–15, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu Y, Young A, Yuan S, Nguyen P, Wu CC, Chien S: Shear stress regulation of Krüppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun 341: 1244–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG: Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 115: 2971–2979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ: Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood 100: 1689–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, García-Cardeña G, Gimbrone MA, Jr: Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A 101: 14871–14876, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan L, Karino T: Effect of a disturbed flow on proliferation of the cells of a hybrid vascular graft. Biorheology 47: 31–38, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B: Chronic venous disease. N Engl J Med 355: 488–498, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Jiang Z, Yu P, Tao M, Ifantides C, Ozaki CK, Berceli SA: Interplay of CCR2 signaling and local shear force determines vein graft neointimal hyperplasia in vivo. FEBS Lett 583: 3536–3540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morinaga K, Okadome K, Kuroki M, Miyazaki T, Muto Y, Inokuchi K: Effect of wall shear stress on intimal thickening of arterially transplanted autogenous veins in dogs. J Vasc Surg 2: 430–433, 1985 [PubMed] [Google Scholar]
- 35.Krishnamoorthy M, Roy-Chaudhury P, Wang Y, Sinha Roy A, Zhang J, Khoury S, Munda R, Banerjee R: Measurement of hemodynamic and anatomic parameters in a swine arteriovenous fistula model. J Vasc Access 9: 28–34, 2008 [PubMed] [Google Scholar]
- 36.Konner K, Lomonte C, Basile C: Placing a primary arteriovenous fistula that works—more or less known aspects, new ideas. Nephrol Dial Transplant 28: 781–784, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Sivanesan S, How TV, Bakran A: Characterizing flow distributions in AV fistulae for haemodialysis access. Nephrol Dial Transplant 13: 3108–3110, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Scheltinga MR, Bruijninckx CM: Haemodialysis access-induced distal ischaemia (HAIDI) is caused by loco-regional hypotension but not by steal. Eur J Vasc Endovasc Surg 43: 218–223, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Asif A, Gadalean FN, Merrill D, Cherla G, Cipleu CD, Epstein DL, Roth D: Inflow stenosis in arteriovenous fistulas and grafts: a multicenter, prospective study. Kidney Int 67: 1986–1992, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Krueger U, Zanow J, Scholz H: Computational fluid dynamics and vascular access. Artif Organs 26: 571–575, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Kharboutly Z, Fenech M, Treutenaere JM, Claude I, Legallais C: Investigations into the relationship between hemodynamics and vascular alterations in an established arteriovenous fistula. Med Eng Phys 29: 999–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Kharboutly Z, Deplano V, Bertrand E, Legallais C: Numerical and experimental study of blood flow through a patient-specific arteriovenous fistula used for hemodialysis. Med Eng Phys 32: 111–118, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Carroll GT, McGloughlin TM, Burke PE, Egan M, Wallis F, Walsh MT: Wall shear stresses remain elevated in mature arteriovenous fistulas: a case study. J Biomech Eng 133: 021003, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Botti L, Piccinelli M, Ene-Iordache B, Remuzzi A, Antiga L: An adaptive mesh refinement solver for large-scale simulation of biological flows. Int J Numer Methods Biomed Eng 26: 86–100, 2010 [Google Scholar]
- 45.Ene-Iordache B, Remuzzi A: Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: low and oscillating shear stress locates the sites of stenosis. Nephrol Dial Transplant 27: 358–368, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Ene-Iordache B, Cattaneo L, Dubini G, Remuzzi A: Effect of anastomosis angle on the localization of disturbed flow in ‘side-to-end’ fistulae for haemodialysis access. Nephrol Dial Transplant 28: 997–1005, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH: Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol 286: H1916–H1922, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Womersley JR: Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J Physiol 127: 553–563, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dammers R, Tordoir JH, Kooman JP, Welten RJ, Hameleers JM, Kitslaar PJ, Hoeks AP: The effect of flow changes on the arterial system proximal to an arteriovenous fistula for hemodialysis. Ultrasound Med Biol 31: 1327–1333, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Bao X, Lu C, Frangos JA: Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: role of NO, NF kappa B, and egr-1. Arterioscler Thromb Vasc Biol 19: 996–1003, 1999 [DOI] [PubMed] [Google Scholar]
- 51.White CR, Haidekker M, Bao X, Frangos JA: Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation 103: 2508–2513, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Manini S, Passera K, Huberts W, Botti L, Antiga L, Remuzzi A: Computational model for simulation of vascular adaptation following vascular access surgery in haemodialysis patients [published online ahead of print January 3, 2013]. Comput Methods Biomech Biomed Engin doi:10.1080/10255842.2012.745857 [DOI] [PubMed] [Google Scholar]
- 53.Huberts W, Bode AS, Kroon W, Planken RN, Tordoir JH, van de Vosse FN, Bosboom EM: A pulse wave propagation model to support decision-making in vascular access planning in the clinic. Med Eng Phys 34: 233–248, 2012 [DOI] [PubMed] [Google Scholar]
- 54.National Kidney Foundation: KDOQI Clinical practice guidelines for vascular access. Update 2006. Am J Kidney Dis 48 [Suppl 1]: S177–S247, 2006 [Google Scholar]
- 55.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Bharat A, Jaenicke M, Shenoy S: A novel technique of vascular anastomosis to prevent juxta-anastomotic stenosis following arteriovenous fistula creation. J Vasc Surg 55: 274–280, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Konner K: The initial creation of native arteriovenous fistulas: surgical aspects and their impact on the practice of nephrology. Semin Dial 16: 291–298, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Sigovan M, Rayz V, Gasper W, Alley HF, Owens CD, Saloner D: Vascular remodeling in autogenous arterio-venous fistulas by MRI and CFD. Ann Biomed Eng 41: 657–668, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He Y, Terry CM, Nguyen C, Berceli SA, Shiu YT, Cheung AK: Serial analysis of lumen geometry and hemodynamics in human arteriovenous fistula for hemodialysis using magnetic resonance imaging and computational fluid dynamics. J Biomech 46: 165–169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reymond P, Perren F, Lazeyras F, Stergiopulos N: Patient-specific mean pressure drop in the systemic arterial tree, a comparison between 1-D and 3-D models. J Biomech 45: 2499–2505, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Bode A, Caroli A, Huberts W, Planken N, Antiga L, Bosboom M, Remuzzi A, Tordoir J; ARCH project consortium: Clinical study protocol for the ARCH project - computational modeling for improvement of outcome after vascular access creation. J Vasc Access 12: 369–376, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Huberts W, Van Canneyt K, Segers P, Eloot S, Tordoir JH, Verdonck P, van de Vosse FN, Bosboom EM: Experimental validation of a pulse wave propagation model for predicting hemodynamics after vascular access surgery. J Biomech 45: 1684–1691, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Caroli A, Manini S, Antiga L, Passera K, Ene-Iordache B, Rota S, Remuzzi G, Bode A, Leermakers J, van de Vosse FN, Vanholder R, Malovrh M, Tordoir J, Remuzzi A: Validation of a patient-specific hemodynamic computational model for surgical planning of vascular access in hemodialysis patients [published online ahead of print May 29, 2013]. Kidney Int doi:10.1038/ki.2013.188 [DOI] [PubMed] [Google Scholar]