Summary
Background and objectives
Parathyroid hormone, calcium, and phosphate have been independently associated with cardiovascular event risk. Because these parameters may be on the same causal pathway and have been proposed as quality measures, an integrated approach to estimating event risks is needed.
Design, setting, participants, & measurements
Prevalent dialysis patients were followed from August 31, 2005 to December 31, 2006. A two-stage modeling approach was used. First, the 16-month probabilities of death and composite end point of death or cardiovascular hospitalization were estimated and adjusted for potential confounders. Second, patients were categorized into 1 of 36 possible phenotypes using average parathyroid hormone, calcium, and phosphate values over a 4-month baseline period. Associations among phenotypes and outcomes were estimated and adjusted for the underlying event risk estimated from the first model stage.
Results
Of 26,221 patients, 98.5% of patients were in 22 groups with at least 100 patients and 20% of patients were in the reference group defined using guideline-based reference ranges for parathyroid hormone, calcium, and phosphate. Within the 22 most common phenotypes, 20% of patients were in groups with significantly (P<0.05) higher risk of death and 54% of patients were in groups with significantly higher risk of the composite end point relative to the in-target reference group. Increased risks ranged from 15% to 47% for death and from 8% to 55% for the composite. More than 40% of all patients were in the three largest groups with elevated composite end point risk (high parathyroid hormone, target calcium, and high phosphate; target high parathyroid hormone, target calcium, and high phosphate; and target high parathyroid hormone, target calcium, and target phosphate).
Conclusion
After adjusting for baseline risk, phenotypes defined by categories of parathyroid hormone, calcium, and phosphate identify patients at higher risk of death and cardiovascular hospitalization. Identifying common high-risk phenotypes may inform clinical interventions and policies related to quality of care.
Introduction
Hemodialysis patients experience exaggerated risks of mortality and cardiovascular morbidity that are not fully explained by the highly prevalent, more traditional cardiovascular risk factors (1). Numerous observational analyses suggest that disorders of bone and mineral metabolism, collectively known as CKD–mineral and bone disorder (CKD-MBD), are involved in the pathogenesis of this higher risk (2–5). These analyses typically estimate the risks associated with parathyroid hormone (PTH), calcium, and phosphate and sometimes use these results to establish the relative importance of each as a therapeutic target. Meta-analyses of these reports have further attempted to precisely identify risk thresholds for these individual parameters (6). National and international guidelines have attempted to set target values for individual parameters in an effort to provide clinicians with ranges within which optimal outcomes can be expected (7,8).
Using results from these statistical models to make inferences relating CKD-MBD laboratory parameters to morbid events may have limitations. In nearly every report, statistical models estimate the effects of these parameters independently through the inclusion of the others as covariates. This process can lead to overadjustment if all, or even part, of the effect of one parameter on the risk of a clinical event is mediated through its effect on increasing or decreasing levels of another parameter included in the model (e.g., if the risk of high PTH is mediated in part through an effect on causing high phosphate) (9). Overadjustment is of particular concern in CKD-MBD because the biologic response to changes in one of these parameters invariably affects the others (e.g., when PTH goes up, phosphate generally also goes up) (10). Furthermore, nearly every treatment option to modify one of the parameters also influences the others simultaneously (e.g., when activated vitamin D is given to lower PTH, serum calcium and phosphate tend to increase as well) (11). Thus, although it is tempting (and common) to attempt to estimate risks associated with each parameter individually, this resulting statistical overadjustment can obscure both biologic inter-relationships and therapeutic treatment effects. Also, because all of these parameters may be on the same causal pathway, this approach can result in bias in the observed effect estimates (12).
One way to address this issue is to classify patients within naturally occurring CKD-MBD groups (phenotypes), and then analyze event risks according to these mutually exclusive groups. This method has the advantages of avoiding assumptions of independence among CKD-MBD parameters and incorporating any interactions and dependencies that might naturally exist or occur with treatment. This study was conducted to determine the prevalence of CKD-MBD phenotypes in hemodialysis patients and estimate their associations with mortality as well as a composite end point of cardiovascular hospitalization or death. The purpose of conducting these analyses was to advance the discussion of quality metrics for CKD-MBD.
Materials and Methods
This observational study used a combined dataset from DaVita, Inc. and the US Renal Data System (USRDS) (13). The dataset consisted of a point prevalent dialysis population in August of 2004, several months after the introduction of cinacalcet into clinical practice. Patients were included in this study if they were alive on August 31, 2005 (i.e., survived at least 12 months), had received six or more dialysis sessions in August of 2005, and had not left the facility as of August 31, 2005. Patients had to have at least one PTH, calcium, and phosphate result in the 4-month period before baseline (May through August of 2005). The 4-month interval was chosen to ensure that most patients had at least two PTH results. Patients were followed from September 1, 2005 until the earliest of the following events: the end of available data (December 31, 2006), death, transplant, or discontinuation of dialysis. Patients who had a parathyroidectomy within 12 months before baseline were excluded.
Analytic Design
A two-stage modeling approach was used to efficiently adjust for confounding caused by non–CKD-MBD risk factors. In the first stage, data from the baseline period was used to estimate the predicted probability of each outcome for each patient (14). This probability was included in the second stage as a summary of each patients’ underlying risk given the non–CKD-MBD risk factors along with the CKD-MBD phenotypes as categorical exposures. This two-stage approach was used to increase statistical efficiency in estimating risks for CKD-MBD phenotypes by allowing inclusion of one summary variable in place of 34 individual covariates comprising the initial model.
Variables
Risk Factors.
Patients with ESRD have unique comorbidity profiles reflecting their exaggerated risk of both death and cardiovascular hospitalization. Liu et al. (15) recently published an improved comorbidity index using the USRDS data and validated its use in hemodialysis populations for predicting both mortality and hospitalizations. We added additional clinical variables available from DaVita, Inc. that are commonly used in other studies to estimate the non–CKD-MBD risk of events. These variables included albumin, hemoglobin, body mass index, Kt/V, hypertension, smoking, and vintage.
For sensitivity analyses, the consistent vitamin D user group was defined as any patient who received vitamin D in each of the 4 months before baseline (16).
Continuous Variables.
Monthly average values for all continuous variables (BP, laboratory assessments, and Kt/V) were used. The average of the (up to) 4 monthly values immediately preceding baseline (i.e., from May to August of 2005) was used as the baseline value for each variable. Body mass index was included as a continuous covariate.
Development of Composite CKD-MBD Phenotypes.
Average values for the CKD-MBD parameters (PTH, calcium, and phosphate) over the 4 months before baseline were calculated identically to the other continuous risk factors. Patients were then categorized into 1 of 36 possible phenotypes based on PTH, calcium, and phosphate categories. PTH categories were defined to be compatible with both the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, which recommend an upper limit of approximately 600 pg/ml (8), and the older Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, which recommend an upper limit of 300 pg/ml (7). The following PTH categories were used: low=0–149 pg/ml, low target=150–300 pg/ml, high target=301–600 pg/ml, and high=600+ pg/ml. Calcium was categorized into low, normal, and high using the DaVita Laboratory normal reference range (8.4–10.2 mg/dl). Phosphate was categorized into low, target, and high using 3.5–5.5 mg/dl as the target range, which consistent with KDOQI, because no specific target range was recommended by KDIGO.
For time to event analyses according to CKD-MBD exposure, we required at least 100 patients per exposure group, which meant that 407 patients (1.5% of all patients) in 14 groups were not reported in the final analyses. Results are presented for the remaining 22 CKD-MBD phenotypes representing 98.5% of the population.
Outcome Measures.
Cardiovascular hospitalizations were identified using the algorithm from the USRDS annual report that requires the presence of one of the following primary diagnosis codes for the hospitalization: 394–398.99, 401–405, 410–420, 421.9, 422.90, 422.99, 423–438, and 440–459 (17). Death was identified from the Medicare data. The composite end point was defined as the earlier event: death or cardiovascular hospitalization.
Analyses
Analysis file construction, descriptive analyses, and regression analyses were performed in SAS (version 9.3). Cox proportional hazards regression was used for assessing event risk. Predicted probabilities were estimated for each patient using the BASELINE statement in SAS PHREG.
Sensitivity analyses were conducted among all phenotypes with a sample size≥500 within strata of underlying risk, which were defined by the lowest 25%, the middle 50%, and the upper 25% of the distribution of all risk scores. Finally, analyses were conducted within cohorts of patients who used vitamin D consistently and cohorts of patients who did not use vitamin D consistently.
Results
Of 45,312 patients, 27,049 patients were alive, uncensored, and receiving regularly scheduled dialysis as of August 31, 2005. Of these patients, 26,253 patients had at least one value of PTH, calcium, and phosphate in the 4-month period before baseline. There were 32 patients who had a parathyroidectomy in the 12 months before baseline, and they were excluded, leaving 26,221 patients in the final cohort. Their demographic and clinical characteristics at baseline are summarized in Table 1.
Table 1.
Baseline demographic information: overall and by outcome
| Levels | Total (n=26,221) | Died (n=6070) | Cardiovascular Hospitalization or Death (n=11,622) |
|---|---|---|---|
| Age group, yr (%) | |||
| 0–29 | 2.1 | 0.5 | 1.4 |
| 30–39 | 6.1 | 1.9 | 3.8 |
| 40–49 | 12.8 | 6.8 | 10.0 |
| 50–59 | 21.4 | 15.3 | 18.6 |
| 60–64 | 12.3 | 10.8 | 12.2 |
| 65–69 | 12.3 | 13.3 | 13.3 |
| 70–79 | 21.0 | 28.6 | 24.4 |
| ≥80 | 12.0 | 22.9 | 16.4 |
| Sex (%) | |||
| Women | 53.9 | 46.8 | 47.1 |
| Men | 46.2 | 53.2 | 52.9 |
| Race (%) | |||
| White | 52.3 | 59.8 | 53.9 |
| Asian | 4.7 | 4.2 | 4.2 |
| African American | 38.7 | 32.2 | 38.3 |
| Native American | 2.9 | 2.7 | 2.6 |
| Other/unknown | 1.4 | 1.2 | 1.0 |
| ESRD primary cause (%) | |||
| Diabetes | 44.9 | 50.5 | 48.1 |
| Hypertension | 29.0 | 28.8 | 29.4 |
| GN/CK | 13.5 | 9.5 | 11.3 |
| Other | 12.6 | 11.3 | 11.2 |
| Comorbid conditions (%) | |||
| Atherosclerotic heart disease | 36.9 | 52.2 | 49.7 |
| Congestive heart failure | 42.6 | 60.1 | 56.2 |
| Cerebrovascular accident or transient ischemic attack | 15.6 | 24.8 | 21.7 |
| Peripheral vascular disease | 30.3 | 43.7 | 39.8 |
| Other cardiac | 23.5 | 34.5 | 33.4 |
| Chronic obstructive pulmonary disease | 17.0 | 28.0 | 24.8 |
| Gastrointestinal bleeding | 7.4 | 11.7 | 10.5 |
| Liver disease | 3.0 | 4.2 | 3.8 |
| Dysrhythmia | 21.5 | 34.6 | 30.9 |
| Cancer | 7.2 | 10.7 | 9.0 |
| Diabetes | 57.4 | 65.6 | 63.1 |
| Hypertension | 96.2 | 97.2 | 97.9 |
| Vintage, mo (%) | |||
| 12–24 | 23.7 | 23.6 | 22.6 |
| 25–48 | 32.8 | 31.9 | 33.2 |
| ≥49 | 43.5 | 44.5 | 44.2 |
| Smoking (current %) | |||
| Yes | 4.5 | 4.8 | 5.0 |
| Albumin, g/dl | |||
| Mean (SD) | 3.87 (0.35) | 3.69 (0.37) | 3.79 (0.37) |
| Hemoglobin, g/dl | |||
| Mean (SD) | 12.36 (0.91) | 12.20 (0.98) | 12.26 (0.95) |
| Body mass index, kg/m2 | |||
| Mean (SD) | 28.13 (6.57) | 26.71 (6.34) | 27.52 (6.39) |
| Kt/V | |||
| Mean (SD) | 1.66 (0.25) | 1.64 (0.25) | 1.64 (0.25) |
| Parathyroid hormone category, pg/ml (%) | |||
| Low (0–149) | 9.8 | 12.0 | 10.3 |
| Target low (150–300) | 35.7 | 37.1 | 34.9 |
| Target high (301–600) | 36.5 | 34.6 | 36.6 |
| High (≥601) | 18.0 | 16.4 | 18.2 |
| Calcium category, mg/dl (%) | |||
| Low (<8.4) | 3.1 | 2.4 | 2.8 |
| Normal (8.4–10.2) | 83.1 | 81.0 | 82.0 |
| High (>10.2) | 13.4 | 16.6 | 15.2 |
| Phosphate category, mg/dl (%) | |||
| Low (<3.5) | 2.7 | 4.1 | 3.2 |
| Target (3.5–5.5) | 50.5 | 54.7 | 50.5 |
| High (>5.5) | 46.8 | 41.3 | 46.3 |
| Parathyroid hormone, pg/ml | |||
| Mean (SD) | 434 (403) | 418 (413) | 436 (412) |
| Calcium, mg/dl | |||
| Mean (SD) | 9.60 (0.58) | 9.67 (0.59) | 9.64 (0.58) |
| Phosphate, mg/dl | |||
| Mean (SD) | 5.54 (1.26) | 5.40 (1.28) | 5.53 (1.28) |
GN/CK, glomerulonephritis or cystic kidney disease.
Hazard ratios underlying the first-stage models defining the predicted probabilities of the two study outcomes (death and a composite of the first outcome of death or cardiovascular hospitalization) are provided in Supplemental Tables 1 and 2. We observed excellent agreement between our results and the results shown in the work by Liu et al. (15) for the relative hazards of death. Strata of the predicted 16-month risk of death were strongly associated with observed mortality rates, and strata of predicted risk of the composite outcome were also strongly associated with observed mortality rates (Table 2). Distributions of these predicted probabilities are in Supplemental Figures 1 and 2.
Table 2.
Predicted probabilities and observed rate of death or the composite end point within levels of predicted probability
| Level of Predicted Event Probability (%) | Death | Composite (Death or Cardiovascular Hospitalization) | ||||
|---|---|---|---|---|---|---|
| Sample Size | Predicted Event Rate (%) | Observed Event Rate (%) | N | Predicted Event Rate (%) | Observed Event Rate (%) | |
| 0–9.9 | 5980 | 6.8 | 5.2 | 1108 | 17.3 | 15.0 |
| 10–19.9 | 8217 | 14.6 | 13.3 | |||
| 20–29.9 | 4903 | 24.5 | 25.5 | 5800 | 25.5 | 23.2 |
| 30–39.9 | 2862 | 34.6 | 36.5 | 6068 | 34.8 | 34.3 |
| 40–49.9 | 1732 | 44.5 | 46.3 | 4297 | 44.8 | 47.3 |
| 50–59.9 | 1078 | 54.6 | 53.8 | 3173 | 54.8 | 57.5 |
| 60–69.9 | 2477 | 64.8 | 64.6 | |||
| 70–79.9 | 1449 | 73.8 | 68.2 | 1681 | 74.7 | 73.9 |
| 80–100 | 1617 | 87.1 | 82.5 | |||
Results reflect the 16-month predicted probability of the event for each patient from each Cox proportional hazards model using the baseline command in SAS Proc PHREG.
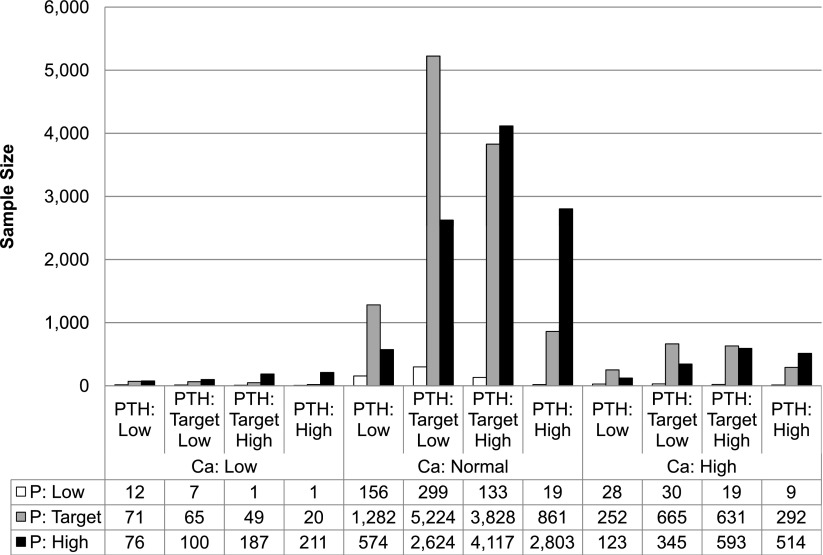
Of 36 possible CKD-MBD phenotypes, 22 phenotypes contained at least 100 patients and represented 98.5% of the sample. The largest MBD phenotype was the reference group of target low PTH, normal calcium, and target phosphate (n=5224, 20% of cohort). The second largest MBD phenotype was the group of target high PTH, normal calcium, and high phosphate (n=4117, 15.7% of cohort), whereas the third largest MBD phenotype was the phenotype of target high PTH with normal calcium and target phosphate (n=3828, 14.6% of the cohort). The top seven phenotypes all had patients in the normal calcium range, and they accounted for 79% of the population. Approximately 47% of all patients had an elevated phosphate level, 13% of patients had an elevated calcium level, 36% of patients had a PTH between 300 and 600 pg/dl, and 18% of patients had a PTH over 600 pg/dl. The sizes of all the possible CKD-MBD phenotypes are given in Figure 1.
Figure 1.
Population distribution according to CKD–mineral and bone disorder phenotypes. Ca, calcium; P, phosphate; PTH, parathyroid hormone.
After controlling for the background risk of death or the composite end point, the results showed that many categories of CKD-MBD exposure were associated with higher risk of events compared with the reference group of target low PTH, normal calcium, and target phosphate, particularly in the model of the composite end point. The results of the models are presented in Figure 2. The six statistically significant (P<0.05) increases in the relative risk of death ranged from 15% to 47%. For risks of the composite end point, the 10 statistically significant risk increases ranged from 8% to 55%.
Figure 2.
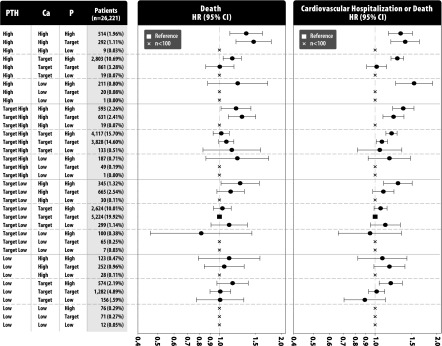
Hazard ratios (HRs) of death and the composite end point by CKD–mineral and bone disorder phenotype. The reference group is the target low PTH (PTH 150–300 pg/ml), target serum calcium (8.2–10.2 mg/dl), and target serum phosphate (3.5–5.5 mg/dl). Whiskers depict the 95% confidence intervals (95% CIs). Ca, calcium; P, phosphate; PTH, parathyroid hormone.
Several phenotypes showed notable increases in the risk of both death and the composite end point. Five of eight phenotypes with high calcium were associated with a higher risk of both end points, with only those phenotypes having low or target low PTH not being associated with higher risk of each outcome. Similarly, 5 of 11 phenotypes with target high or high PTH were associated with higher mortality, whereas 7 of 11 phenotypes were associated with a higher risk of the composite end point. Other than the reference group, the three largest phenotypes all had significantly elevated hazards of the composite end point, and together, they accounted for more than 40% of all patients (and 50% of all out-of-target patients). These phenotypes were high PTH, normal calcium, and high phosphate; target high PTH, normal calcium, and high phosphate; and target high PTH, normal calcium, and target phosphate.
In a sensitivity analysis, notable differences in the associations between MBD phenotypes and the relative risk of death were observed (Table 3) within different risk groups defined by the background risk of each event. CKD-MBD phenotypes were not clearly associated with higher risk of death among those patients at the lowest and highest levels of predicted risk based on the baseline comorbidity model. For the 50% of patients at intermediate risk of death, phenotypes associated with death were similar to those phenotypes seen in the total sample as described above. Among those patients at intermediate risk of death, two phenotypes very common in the overall cohort (greater than 25% of subjects) were associated with a substantially higher risk of death (31%–58%). These phenotypes were the target high PTH, normal calcium, and target phosphate and high PTH, normal calcium, and high phosphate. For the composite end point that included cardiovascular hospitalization, CKD-MBD phenotypes showed consistent associations with higher risk, regardless of the quartile of baseline risk.
Table 3.
Association between CKD–mineral and bone disorder phenotypes and outcomes within baseline risk groups defined by the distribution of the 16-month predicted outcome
| End Point and Sample Size | Exposure Group | Hazard Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|---|
| Parathyroid Hormone | Calcium | Phosphate | Quartile 1 (n=5929) | Quartiles 2–3 (n=11,858) | Quartile 4 (n=5929) | Overall (n=23,716) | |
| Death | |||||||
| 514 | High | High | High | 1.72 (1.31 to 2.25) | 1.35 (1.12 to 1.62) | ||
| 2803 | High | Normal | High | 1.58 (1.36 to 1.84) | 1.15 (1.04 to 1.28) | ||
| 861 | High | Normal | Target | ||||
| 593 | Target high | High | High | 1.43 (1.08 to 1.88) | 1.24 (1.00 to 1.55) | 1.20 (1.02 to 1.42) | |
| 631 | Target high | High | Target | 1.39 (1.15 to 1.69) | 1.29 (1.10 to 1.50) | ||
| 4117 | Target high | Normal | High | ||||
| 3828 | Target high | Normal | Target | 1.31 (1.14 to 1.50) | |||
| 665 | Target low | High | Target | ||||
| 2624 | Target low | Normal | High | ||||
| 5224 | Target low | Normal | Target | Reference category for all models | |||
| 574 | Low | Normal | High | 1.44 (1.09 to 1.91) | |||
| 1282 | Low | Normal | Target | ||||
| Cardiovascular hospitalization or death | |||||||
| 514 | High | High | High | 1.35 (1.11 to 1.64) | 1.40 (1.12 to 1.74) | 1.33 (1.16 to 1.52) | |
| 2803 | High | Normal | High | 1.33 (1.10 to 1.61) | 1.27 (1.15 to 1.41) | 1.33 (1.18 to 1.49) | 1.28 (1.20 to 1.38) |
| 861 | High | Normal | Target | ||||
| 593 | Target high | High | High | 1.87 (1.37 to 2.54) | 1.34 (1.11 to 1.61) | 1.32 (1.10 to 1.59) | 1.37 (1.22 to 1.55) |
| 631 | Target high | High | Target | 1.55 (1.07 to 2.25) | 1.26 (1.06 to 1.50) | 1.23 (1.09 to 1.38) | |
| 4117 | Target high | Normal | High | 1.26 (1.16 to 1.38) | 1.19 (1.07 to 1.32) | 1.20 (1.13 to 1.28) | |
| 3828 | Target high | Normal | Target | 1.11 (1.01 to 1.23) | 1.08 (1.01 to 1.15) | ||
| 665 | Target low | High | Target | ||||
| 2624 | Target low | Normal | High | ||||
| 5224 | Target low | Normal | Target | Reference category for all models | |||
| 574 | Low | Normal | High | 1.22 (1.00 to 1.47) | 1.19 (1.05 to 1.36) | ||
| 1282 | Low | Normal | Target | ||||
Only statistically significant (P<0.05) results are shown to facilitate readability.
Stratifying patients into groups defined by nonconsistent and consistent use of active vitamin D did not substantively alter the relationships between phenotype and clinical outcome.
Discussion
Our results confirm a substantial association of CKD-MBD with the underlying risk of death and cardiovascular hospitalization among patients receiving hemodialysis. Unlike most previous work, we have specifically not attempted to assess the independent effect of any single parameter on these important outcomes, because it is our belief that such analyses are likely to be influenced by overadjustment bias and may not reflect the known pathophysiology of CKD-MBD. The inter-relationships among the biologic variables of PTH, calcium, and phosphate are such that changes in any one of these variables, whether de novo or with treatment, invariably affect the others, and as such, the variables are almost certain to share common elements on the causal pathway to adverse outcomes. Using statistical adjustment for each variable to identify target values for any single parameter is, thus, likely to result in inaccurate estimates of risk and reflect potentially biologically implausible combinations of biochemical parameters. By using naturally occurring groups of patients (i.e., CKD-MBD phenotypes) and estimating incremental risk from a well defined baseline risk of events, we may more accurately estimate the effect of CKD-MBD on outcomes.
To define CKD-MBD phenotypes, it was necessary to establish a target range for each variable. We chose to use thresholds that reflected both KDOQI and KDIGO guideline recommendations; however, the levels chosen are not meant to suggest that these levels are optimal levels. Indeed, future analyses might consider data-driven thresholds to define higher risk, and it would be reasonable for such analyses to consider physiologic normal ranges. It is likely, for some variables, that there is variation in risk within the category. However, to design our analyses to take full advantage of the naturally occurring phenotypes, these tradeoffs were required.
It was not our goal to define a precise relative risk associated with each phenotype; rather, we attempted to estimate the additional contribution of CKD-MBD beyond the background risk for well defined clinical end points. It is notable that only 20% of patients were our reference population (PTH=150–300 pg/dl, normal serum calcium, and phosphate=3.5–5.5 mg/dl), whereas 80% of patients were uncontrolled in some way. Even with a broader PTH target that extends to 600 pg/dl, only 35% of patients were in a guideline-based target range.
There are many phenotypes of abnormal PTH, calcium, and phosphate, each with its own risk of death and cardiovascular hospitalization. To improve the quality of dialysis care, it is important to consider not only the risk associated with each phenotype, but also the number of patients exposed to the risk. Based on these analyses, using guideline-based target values, approximately 20% of patients are well controlled. We observed a large concentration of patients in three groups with elevated risk (high PTH, target calcium, and high phosphate; target high PTH, target calcium, and high phosphate; and target high PTH, target calcium, and target phosphate). It is notable that two of these groups involve both elevated PTH and phosphate. Improving outcomes for just these groups, which account for more than one half of the 80% of patients out of our target range, could have a substantial population-level impact.
We find it of interest that those phenotypes with high calcium and PTH>300 pg/ml consistently had a higher risk of death or the composite end point of death or cardiovascular hospitalization. This finding may suggest that these phenotypes are of particular concern and that analyses using naturally occurring groups may be more useful in defining quality care in this clinical arena. We strongly believe that analyses (or meta-analyses of analyses) that attempt to identify target values for individual biochemical parameters of CKD-MBD should not be used for establishing quality metrics given the biologic interdependencies among these parameters that occur naturally as well as with treatment.
In the United States, the inclusion of oral phosphate binders and calcimimetics into the bundled payment system will represent a profound shift in the economics of therapy for CKD-MBD (18). There is an urgent need to develop quality metrics that reflect overall care in CKD-MBD to protect the quality of health care delivered to Medicare beneficiaries. One potentially important contribution of this work may be that the paradigm of CKD-MBD phenotypes can be used to identify quality care and discriminate among facilities and among dialysis providers over time. Regardless of the specific thresholds chosen to define the CKD-MBD phenotypes, the characterization of high-risk patterns of PTH, calcium, and phosphate can help to identify populations at higher risk of adverse outcomes and their overall contribution to mortality in dialysis patients.
It has previously been shown using a much simpler classification system that CKD-MBD may account for as much as 20% of the overall mortality risk in hemodialysis patients (5). A better approach may be to estimate the contribution of each category of CKD-MBD phenotype to the overall risk of death and/or cardiovascular hospitalization by integrating the size of the population with the magnitude of the risk. Our finding that the CKD-MBD phenotype has prognostic importance for all patients, including those patients at the lowest and highest risks of the composite end point (but not the mortality end point), further clarifies the most vulnerable populations. In addition, the consistent relationships among CKD-MBD phenotypes and the composite end point support the hypothesized causal pathway of disturbed mineral metabolism. This finding strongly suggests that economic analyses incorporating cardiovascular events should be considered when evaluating the overall impact of the inclusion of CKD-MBD medications into the bundled payment system.
Our analysis has several important limitations. We have studied a prevalent cohort of only hemodialysis patients who were, by design, required to survive a 12-month baseline period. However, similar analyses conducted by Liu et al. (15) have showed the effectiveness of the comorbidity index in predicting death and hospitalization using the same administrative dataset in both incident and prevalent patients (16). In addition, we have arbitrarily defined thresholds to take advantage of the naturally occurring groupings of PTH, calcium, and phosphate. It is likely that the risk within each category is not homogenous; however, this tradeoff is necessary to take advantage of biologically occurring groups. As a result, some of our phenotypes have very small sample sizes, and the significance may be underestimated as a result. Studies with larger sample sizes may help to clarify the risks associated with the smaller groups. There is likely to be some selection bias in these results (that is, patients in the reference group may also reflect better overall care or fewer high-risk behaviors in ways that are not captured by our covariates). Finally, there may be effect modification from the use of vitamin D, cinacalcet, or phosphate binders (i.e., the risk in a category may depend on the therapy received). Future work may wish to explore such effect modification within some of the larger phenotypes.
Future research should focus on several areas. First, although the current targets are based on current clinical practice guidelines, the evaluation of other target ranges may be helpful, particularly for calcium, because most patients were within the normal range. Second, the phenotypes might be reduced to a smaller set of homogeneous groups that are more amenable to quality guidelines by collapsing groups according to either similar risk or similar phenotype. The use of a net reclassification index may be useful in assessing such reclassification schemes. Third, the prevalence of the resulting groups should be compared across facilities to evaluate variations in practice patterns and outcomes.
In summary, we have presented a novel method for establishing the important contribution of CKD-MBD to mortality and cardiovascular hospitalization. Using naturally occurring groups of CKD-MBD parameters, we have shown that many specific phenotypes, particularly those phenotypes with high calcium and PTH>300 pg/dl, are consistently associated with a higher risk of these events. We suggest that characterization of patients in this manner may be one method to begin developing clinically relevant metrics to assess the overall quality of CKD-MBD care in hemodialysis patients.
Disclosures
G.A.B serves as an advisor and consultant for Amgen, Inc. and a Medical Director for DaVita, Inc. R.D.K. and K.A.L. are employees and stockholders of Amgen, Inc. W.W. and M.D.D. are employees of Outcomes Insights, Inc., which receives research and consulting funding from Amgen, Inc. This research was funded by Amgen, Inc.
Supplementary Material
Acknowledgments
We appreciate the work of the National Institutes of Health (NIH) and the US Renal Data System (USRDS) Coordinating Center in developing and maintaining the USRDS and DaVita, Inc. for enabling the merging of the datasets.
The opinions of the authors do not reflect the opinions of DaVita, Inc., the NIH, or the USRDS.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04260413/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Danese MD, Belozeroff V, Smirnakis K, Rothman KJ: Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol 3: 1423–1429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Schisterman EF, Cole SR, Platt RW: Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 20: 488–495, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Molnar MZ, Zaritsky JJ, Sim JJ, Streja E, Kovesdy CP, Salusky I, Kalantar-Zadeh K: Correlates of parathyroid hormone concentration in hemodialysis patients. Nephrol Dial Transplant 28: 1516–1525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Greenland S, Pearl J, Robins JM: Causal diagrams for epidemiologic research. Epidemiology 10: 37–48, 1999 [PubMed] [Google Scholar]
- 13.US Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 14.Glynn RJ, Gagne JJ, Schneeweiss S: Role of disease risk scores in comparative effectiveness research with emerging therapies. Pharmacoepidemiol Drug Saf 21[Suppl 2]: 138–147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 16.St Peter WL, Li Q, Liu J, Persky M, Nieman K, Arko C, Block GA: Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 4: 354–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Renal Data System: USRDS 2012 Annual Data Report Appendix: Analytical Methods, ESRD, 2012. Available at: http://www.usrds.org/2012/pdf/v2_z_appendix_12.pdf Accessed December 5, 2012
- 18.Centers for Medicare & Medicaid Services (CMS) HHS : Medicare program; end-stage renal disease prospective payment system, quality incentive program, and bad debt reductions for all Medicare providers. Final rule. Fed Regist 77: 67450–67531, 2012 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.