Summary
Background and objectives
Sequential echocardiography is routinely performed in patients with ESRD listed for transplantation. The benefit of this labor- and time-intensive measure, however, remains unclear. Thus, this study elucidated the various obtained routine echocardiography parameters that best predicted mortality and graft survival after renal transplantation.
Design, setting, participants, & measurements
This study investigated 553 first renal transplant recipients listed in the Austrian Dialysis and Transplant Registry between 1992 and 2011 who had echocardiographic analysis at transplantation and survived at least 1 year. Cox proportional hazards models with the purposeful selection algorithms for covariables were used to identify predictors of mortality and graft loss. A Fine and Gray model was used to evaluate cause-specific death.
Results
During a median follow-up of 7.14 years, 81 patients died, and 59 patients experienced graft loss after the first year. The Kaplan–Meier analysis showed that 85% of patients with a left atrial diameter below the median of 53 mm were alive 10 years after transplantation, whereas only 70% of those patients with a left atrial diameter equal to or above the median had survived (P<0.001). In the multivariable model, left atrial diameter (per millimeter) independently predicted overall mortality (hazard ratio, 1.06; 95% confidence interval, 1.03 to 1.08; P<0.001) and cause-specific cardiac death (hazard ratio, 1.04; 95% confidence interval, 1.00 to 1.08; P=0.04). Functional graft loss was predicted by the right atrial diameter (hazard ratio, 1.04; 95% confidence interval, 1.02 to 1.07; P=0.001).
Conclusion
The left atrial diameter determined at transplantation predicted overall and cardiac mortality. Patients with widely enlarged left atria exhibit a considerably reduced life expectancy. It remains to be determined, however, whether renal transplantation is futile in these patients.
Introduction
Renal transplantation is the preferred therapy for ESRD, because it increases life expectancy and quality of life compared with other forms of renal replacement therapies. Although the annual rate of cardiac death of dialysis patients decreases from roughly 5% to 2% after successful transplantation, it remains the main cause of mortality (1). Prominent risk factors in patients on dialysis are left ventricular hypertrophy, left ventricular dysfunction, and left atrial enlargement (2–4). It has been shown that structural and functional improvements of echocardiographic parameters occur after transplantation, but it remains elusive whether baseline echocardiographic measures remain predictors of mortality (5–7).
We, therefore, elucidated whether echocardiographic parameters obtained at transplantation remain predictive of mortality and potentially, graft survival after kidney transplantation. We made use of the Austrian Dialysis and Transplantation Registry, which holds complete entries of all patients with ESRD in Austria since 1980 (8).
Materials and Methods
Patients
We studied all patients registered in the Austrian Dialysis and Transplant Database (OEDTR) who received their first renal allograft between January 1, 1992 and December 31, 2011 (9). All patients who were potentially eligible for renal allograft wait-listing underwent a baseline echocardiographic evaluation with annual follow-up while being waitlisted. Thus, the baseline echocardiography for the present analysis could be within 1 year of transplantation. Only echocardiographic investigations that were performed on a dialysis-free day were considered to allow for comparisons between patients and avoid bias caused by fluid overload. A list of variables reported in this registry may be found elsewhere (8). For the multivariate analysis, we used clinical laboratory parameters 1 year after transplantation.
Echocardiography
Standard two-dimensional echocardiographic and M-mode pictures were performed by a cardiologist using either a Vivid i or Vivid 7 Cardiovascular Ultrasound System (GE Medical Systems, Fairfield, CT).
The following echocardiographic parameters were used: length of left atrial diameter (LA2D), left ventricular diameter (LV2D), length of right atrial diameter (RA2D), right ventricular diameter, intraventricular septum diameter, mean gradient over the aortic valve, maximal velocity, left ventricular end systolic diameter, left ventricular end diastolic diameter, maximal velocity through tricuspid valve, left ventricular function (LVF), and mitral insufficiency. Left ventricular hypertrophy (LVH) was defined as suggested by the 2007 European Society of Hypertension/European Society of Cardiology Guidelines (10).
Outcomes
The main outcome parameter was mortality—with and without censored graft loss. Because all patients are followed routinely in the transplant centers in Austria and entered annually in the OEDTR, only 17 patients were lost to follow-up since 1992, and outcomes are known for each subject. These 17 patients were not included in the analysis. Functional graft loss was also considered and defined as the need for retransplantation or permanent return to dialysis. We also evaluated the risk of cardiac death compared with other causes of death.
Statistical Analyses
Continuous demographic variables were compared between the groups with a t or Mann–Whitney U test, and categorical data were analyzed by a chi-squared test. For patient survival analysis, we performed unadjusted Kaplan–Meier analysis for visualization of data and two multivariable Cox models. The first Cox model counted all causes of death as an event, and the second Cox model only counted cardiac death as an event. We also analyzed a competing risk model according to the work by Fine and Gray (11), where all causes of death not being cardiac death were counted as a competing event. Furthermore, we used the purposeful selection algorithm to select covariables. This algorithm, which was published by Bursac et al. (12), adds significant variables from a univariate model as well as variables that modify the parameter estimate of any other variable by more than 30%. To investigate any nonlinearity, we applied a restricted cubic spline model with four knots, which were placed at the 5th, 35th, 65th, and 95th percentiles of the analyzed variable. The SAS macro used for this analysis was a slightly modified version of %RCS, which was programmed by Heinzl et al. (13).
For the evaluation of GFR decline, a multivariable linear regression model was applied. GFR between year 1 of first transplantation and graft loss or death, whichever occurred first, was computed with the Modification of Diet in Renal Disease Equation (14). All possible estimates of GFR during the first transplant were used for analysis.
A median of 7.2% of the data was missing in 29 variables. Analyses were carried out in complete cases only and all patients after multiple imputation with five generated datasets. Because results were materially identical, only the Cox models with imputed values are presented.
A P value<0.05 was considered statistically significant. For all statistical analysis, SAS 9.3 for Windows (Cary, NC) was used.
Results
Demographics
Of 2959 patients registered in the OEDTR with their first allograft in the investigated time period, 553 patients underwent echocardiographic investigation within 1 year before transplantation. The clinical and demographic parameters and outcomes were not different between the studied cohort and the patients without timely echocardiographic evaluation (Supplemental Table 1). Demographic and clinical variables relevant for the outcomes after renal transplantation and the results of the echocardiographic evaluation stratified by the LA2D median (>53 mm) are provided in Table 1.
Table 1.
Demographic data of patients at time of transplantation stratified by left atrial diameter median (53 mm) and echocardiographic parameters at time of transplantation
| Variable | LA2D≤53 mm | LA2D>53 mm | P Value |
|---|---|---|---|
| Demographic data of patients at time of transplantation | |||
| Number of patients | 266 | 287 | na |
| Donor age (yr) | 46 (14) | 45 (17) | 0.29 |
| Donor last creatinine (mg/dl) | 1.0 (0.4) | 1.1 (0.4) | 0.51 |
| Recipient sex (women) | 132 (49.6%) | 98 (34.1%) | <0.001 |
| Renal diagnosis | 0.009 | ||
| GN | 75 (28.2%) | 51 (17.8%) | |
| Vascular | 19 (7.1%) | 28 (9.8%) | |
| Diabetes | 28 (10.5%) | 48 (16.7%) | |
| Other | 144 (54.1%) | 160 (55.7%) | |
| Immunsuppression | 0.88 | ||
| Standard | 106 (39.8%) | 116 (40.4%) | |
| Steroid-free | 10 (3.8%) | 13 (4.5%) | |
| Other | 150 (56.4%) | 158 (55.1%) | |
| Cerebrovascular disease | 14 (5.2%) | 13 (4.5%) | 0.18 |
| Periphervascular disease | 19 (7.1%) | 53 (18.5%) | 0.002 |
| Coronary heart disease | 22 (8.3%) | 59 (20.6%) | 0.01 |
| Diabetes mellitus T2 | 36 (13.5%) | 63 (22.0%) | 0.01 |
| Erythropoietin-stimulating agent use | 54 (20.3%) | 82 (28.5%) | 0.02 |
| Donor type | 0.006 | ||
| Deceased | 231 (86.8%) | 269 (93.7%) | |
| Living | 35 (13.2%) | 18 (6.3%) | |
| Cytomegalovirus recipient (positive) | 79 (29.7%) | 132 (46.0%) | 0.21 |
| Cytomegalovirus donor (positive) | 122 (45.9%) | 117 (40.8%) | 0.02 |
| MMA | 1 (0, 1) | 1 (0, 1) | 0.63 |
| MMB | 1 (1, 1) | 1 (0, 1) | 0.32 |
| MMDR | 1 (0, 1) | 1 (0, 1) | 0.65 |
| Sum of HLA mismatch | 3 (2, 3) | 3 (2, 3) | 0.99 |
| Panel reactive antibody at TX (%) | 0 (0, 0) | 0 (0, 4) | 0.001 |
| Maximal panel reactive antibody (%) | 4 (0, 12) | 4 (0, 10) | 0.46 |
| Mean arterial pressure (mmHg) | 101 (24) | 103 (22) | 0.37 |
| Atrial fibrillation | 14 (5.3%) | 28 (9.8%) | 0.05 |
| Age at transplantation (yr) | 49 (13) | 54 (13) | <0.001 |
| Year of transplantation | 2001 (1997, 2004) | 1997 (1994, 2002) | <0.001 |
| Vintage of dialysis (yr) | 1.9 (0.8, 3.2) | 1.8 (0.9, 3.2) | 0.92 |
| ALAT (units/L) | 11 (7, 17) | 10 (7, 14) | 0.12 |
| ASAT (units/L) | 11(7, 20) | 9 (6, 15) | 0.006 |
| Albumin (g/L) | 36 (13, 42) | 41 (37, 45) | <0.001 |
| BUN (mg/dl) | 46 (18) | 46 (19) | 0.99 |
| C-reactive protein (mg/dl) | 0.7 (0.0, 1.6) | 0.7 (0.0, 2.1) | 0.98 |
| Cholesterol (mg/dl) | 169 (57) | 155 (44) | 0.01 |
| Creatinine (mg/dl) | 6.8 (2.8) | 7.2 (2.9) | 0.14 |
| Iron (µg/dl) | 61 (35) | 55 (32) | 0.16 |
| Ferritin (ng/ml) | 209 (80, 357) | 209 (121, 330) | 0.56 |
| Hemoglobin (g/dl) | 11.2 (1.6) | 11.4 (1.7) | 0.13 |
| Hematocrit (%) | 33.7 (5.1) | 35 (5) | 0.02 |
| Potassium (mmol/L) | 4.7 (0.8) | 4.8 (0.7) | 0.62 |
| Microalbuminuria (mg/24 h) | 0 (0, 190) | 0 (0, 64) | 0.002 |
| Sodium (mmol/L) | 139 (3) | 139 (4) | 0.19 |
| Serum transferrin (mg/dl) | 180 (49) | 172 (45) | 0.33 |
| Transferrin saturation (%) | 25 (18, 32) | 24 (17, 35) | 0.74 |
| Echocardiographic parameters at time of transplantation | |||
| Mean gradient over the aortic valve (mmHg) | 12 (9) | 20 (14) | 0.001 |
| Maximal velocity through aortic valve (m/s) | 7.47 (12.10) | 6.43 (12.01) | 0.64 |
| Left ventricular end diastolic diameter (mm) | 48 (6) | 53 (7) | <0.001 |
| Left ventricular end systolic diameter (mm) | 29 (6) | 33 (8) | <0.001 |
| Left ventricular function (<50%) | 3 | 21 | <0.001 |
| Maximal velocity through the tricuspid valve (m/s) | 2.68 (0.30) | 3.01 (0.43) | <0.001 |
| Mitral insufficiency | 7 | 35 | <0.001 |
| Intraventricular septum diameter (mm) | 14 (3) | 16 (3) | 0.004 |
| LA2D (mm) | 44 (6) | 61 (6) | <0.001 |
| Left ventricular diameter (mm) | 47 (6) | 51 (7) | <0.001 |
| Right atrial diameter (mm) | 43 (6) | 57 (7) | <0.001 |
| Right ventricular diameter (mm) | 29 (4) | 34 (5) | <0.001 |
The standard immunosuppression regimen is steroid + mycophenolat + calcineurin inhibitor. Values represent number (percentage), mean (SD), or median (25th, 75th percentile). LA2D, left atrial diameter; na, not applicable; MMA, HLA mismatch on locus A; MMB, locus B; MMDR, locus DR; TX, transplantation; ALAT, alanine aminotransferase; ASAT, apartate aminotransferase.
Mortality
Univariable Analysis.
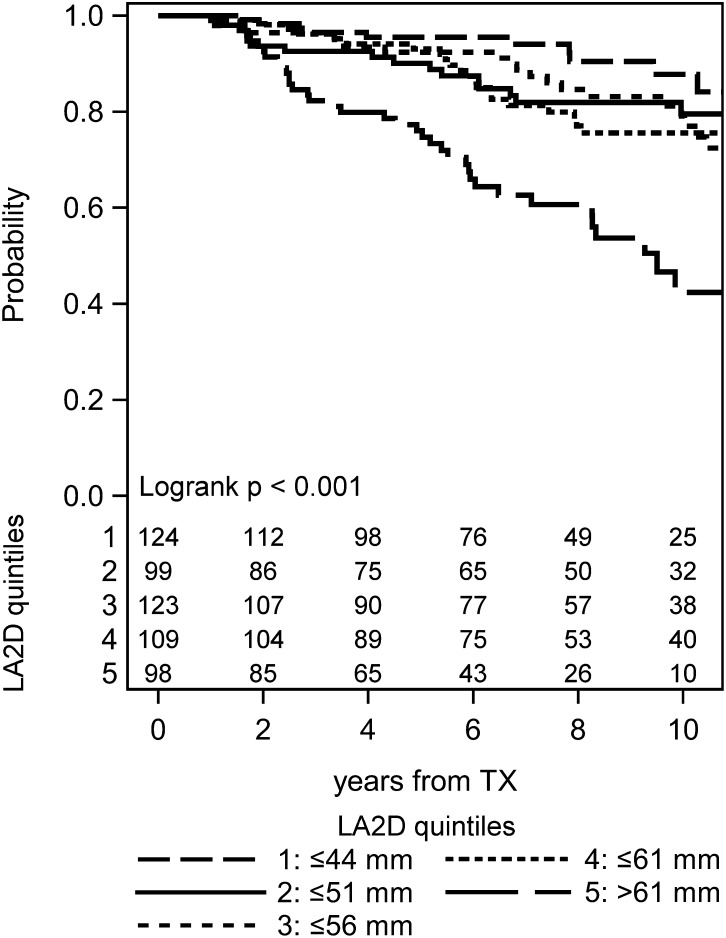
The Kaplan–Meier analysis of the population stratified by the median or quintiles of LA2D showed a significantly higher mortality in patients with enlarged left atria (Figure 1 and Supplemental Figure 1). At 10 years, 33.6% of patients in the upper LA2D stratum had died, whereas only 16.3% of patients in the lower stratum died by that time (log rank P<0.001). Cox models of univariate analyses are presented in Supplemental Table 2. Additionally, mortality with censored graft loss changed the results only marginally (Supplemental Figure 1A).
Figure 1.
Kaplan–Meier plot with numbers of subjects at risk for mortality stratified by quintiles of left atrial diameter (LA2D). TX, transplantation.
Patients in the top quintile of LA2D (>61 mm) exhibited an annual death rate of roughly 6%, leaving only one half of these patients alive at 9 years after engraftment.
Cox Models.
The purposeful selection algorithm revealed the following parameters as confounders: LA2D, right ventricular diameter, hemoglobin, peripheral vascular disease, and immunosuppression. The hazard ratio (HR) for LA2D per millimeter was 1.06 (95% confidence interval [95% CI], 1.03 to 1.08; P<0.001) (Table 2). Additionally, we included atrial fibrillation as a known predictor of mortality according to the work by Lentine et al. (15). A spline model did not show nonlinearity for LA2D (Supplemental Figure 2). In a cause-specific model, LA2D remained significant (HR, 1.05; 95% CI, 1.01 to 1.10; P=0.01), which was also confirmed in the competing risk model (Fine and Gray model; HR, 1.04; 95% CI, 1.00 to 1.08; P=0.04).
Table 2.
Predictors of mortality and functional graft loss
| Parameter | Hazard Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Outcome mortality (n=106) | ||||
| Left atrial diameter (mm) | 1.06 | 1.03 | 1.08 | <0.001 |
| Right ventricular diameter (mm) | 0.95 | 0.90 | 1.01 | 0.12 |
| Hemoglobin (g/dl) | 0.80 | 0.70 | 0.90 | <0.001 |
| Periphervascular disease (yes versus no) | 4.60 | 2.20 | 9.60 | <0.001 |
| Immunosuppression (any versus standard) | 1.45 | 0.69 | 3.04 | 0.32 |
| Immunosuppression (azathioprine versus mycophenolat) | 1.41 | 0.73 | 2.72 | 0.30 |
| Calcineurin inhibitor use (yes versus no) | 1.43 | 0.52 | 3.92 | 0.46 |
| Atrial fibrillation (yes versus no) | 1.50 | 0.75 | 2.99 | 0.25 |
| Outcome functional graft loss (n=119) | ||||
| Right atrial diameter (mm) | 1.04 | 1.02 | 1.07 | 0.001 |
| Hemoglobin (g/dl) | 0.75 | 0.67 | 0.84 | <0.001 |
| Cerebrovascular disease (yes versus no) | 2.52 | 0.61 | 10.36 | 0.16 |
| Periphervascular disease (yes versus no) | 2.29 | 0.97 | 5.41 | 0.06 |
| Age at transplantation (yr) | 0.98 | 0.96 | 1.00 | 0.02 |
| Donor age (yr) | 1.02 | 1.00 | 1.03 | 0.01 |
| Immunosuppression (any versus standard) | 3.37 | 1.97 | 5.76 | <0.001 |
| Immunosuppression (steroid-free versus standard) | 3.91 | 1.25 | 12.19 | 0.02 |
| Calcineurin inhibitor use (yes versus no) | 1.88 | 0.91 | 3.89 | 0.09 |
| Coronary heart disease (yes versus no) | 0.60 | 0.18 | 1.99 | 0.34 |
| Atrial fibrillation (yes versus no) | 1.95 | 0.81 | 4.70 | 0.13 |
| Year of transplantation | 0.96 | 0.90 | 1.02 | 0.18 |
The standard immunosuppression regimen is steroid + mycophenolat + calcineurin inhibitor. Variables were determined by purposeful selection algorithm.
Causes of Death.
Cardiac death occurred in 15 (6%) of 266 patients in the group with LA2D below median and 35 (12%) of 287 patients in the group with LA2D above the median (P=0.13) (Table 3).
Table 3.
Causes of death
| Cause of Death | LA2D≤53 mm (n=266) | LA2D>53 mm (n=287) |
|---|---|---|
| Cardiovascular | 15 (5.6%) | 35 (12.2%) |
| Infectious disease | 3 (1.1%) | 19 (6.6%) |
| Malignancy | 5 (1.9%) | 4 (1.4%) |
| Other | 7 (2.6%) | 18 (6.3%) |
| Total | 30 | 76 |
Values represent number (percentage; 100% corresponds to the number of patients in the respective group). LA2D, left atrial diameter.
Functional Graft Loss.
For functional graft loss, LA2D was not selected by the purposeful selection algorithm. The variables selected were RA2D, level of serum hemoglobin, vascular disease, coronary heart disease, age at transplantation, donor age, year of transplantation, and immunosuppression. In this model, we also included atrial fibrillation for the same reason as stated above. In this combination of variables, RA2D was significantly associated with functional graft loss (HR, 1.04; 95% CI, 1.02 to 1.07; P=0.001) (Table 2).
Slope of GFR.
The median GFR loss was 4.33 ml/min per 1.73 m2 (95% CI, −5.00 to −3.66) per year in the LA2D stratum above median (>53 mm) and 2.35 ml/min per 1.73 m2 (95% CI, −3.10 to −1.61) per year in the lower LA2D stratum (P<0.001).
Discussion
In this study, we showed that LA2D is significantly associated with mortality in renal transplant patients. In our data, LV2D as well as LVH were not associated with mortality. However, these parameters are frequently reported as risk factors in dialysis and renal transplant patients (2,3,16).
LVH and LVF were not associated with mortality, likely because patients with severely reduced LVF were not waitlisted; thus, all patients in the present transplant cohort had a relatively well maintained LVF as indicated by the fact that only 24 patients exhibited an LVF below 50%. Accordingly, other cohort studies of patients with only mildly to moderately reduced LVF did not find an association of this parameter with mortality (17). It is noteworthy that a recent retrospective study by Stallworthy et al. (18) showed an association of LVF with mortality. However, Stallworthy et al. (18) had no measurements of LA2D, and therefore, this parameter was not included in their analysis.
LVH is induced by factors such as anemia and hypertension (19). We found through the purposeful selection algorithm that hemoglobin was, indeed, a risk factor, and thus, it got included into the Cox model for mortality.
In the Kaplan–Meier plot, we showed significantly increased mortality in patients with higher LA2D when dichotomized by the median. This finding is in concordance with the results found by Patel et al. (4) in dialysis patients with LVH. Patel et al. (4) divided the measured left atrial volume into quartiles and found higher quartiles with higher mortality. However, in a general population of 5888 persons evaluated in the cardiovascular health study, LA2D showed no significance when adjusted for risk factors, because only very few patients showed enlarged left atria (20).
LA2D interacts with comorbidities, such as atrial fibrillation, congestive heart failure, and mitral insufficiency (21). However, in our dataset, only 42 patients had atrial fibrillation, and LA2D was not significantly different between the groups (51.6 versus 54.6 mm, P=0.12). However, in a considerably larger dataset, Lentine et al. (15) showed a clear association with mortality.
For functional graft survival, we found a significant association with RA2D. A study performed by Mielniczuk et al. (22) showed that the consequence of higher right atrial pressure is reduced renal function. Another recently published study evaluated several echocardiographic parameters in a population with pulmonary hypertension (23). The group with the larger right atrial area suffered from significantly reduced renal function, although it has to be mentioned that this result might not be a causal relationship (23).
Likewise, the hemoglobin level reached significance for functional graft loss. We showed in a previous study that hemoglobin is associated with mortality and functional graft loss (24). In that analysis, hemoglobin variability was significant. However, for the analyses reported here, we had no repeated measurements of echocardiographic parameters and therefore, analyzed no longitudinal trajectories of any parameter.
Slope of GFR decline was predicted by LA2D. Recently, Chen et al. (25) reported that LA2D was predictive of GFR decline in patients with chronic native kidney disease stages CKD 3–5. Chen et al. (25) also found the LVF to be associated with the GFR loss, but it is of note that the patients in this cohort had an excellent ejection fraction, with a median LVF of almost 70%.
The lack of serial measurements is a limitation of our study. Consequently, we could not analyze any influence of changes in LA2D or any other parameters. However, compared with other studies, there is a discrepancy in the evolution of the parameters over time, especially in LVH. The study conducted by Patel et al. (26) compared results of cardiac magnetic resonance of 25 transplanted and 25 nontransplanted patients. Patel et al. (26) did not found any difference between the two groups. However, two studies performed by Iqbal et al. (27,28) showed improvements in cardiac parameters during the first year after renal transplantation. Most of the time, left atrial volume is considered the preferred parameter over LA2D. However, left atrial volume was not available in all echocardiographic analyses (29).
Additional limitations include residual confounding, which is inherent to observational studies, as well as limited external validity because of predominantly Caucasian patients in our region. In conclusion, our data suggest that the functional graft survival could be predicted by the RA2D. The diameter of the left atrium was associated with mortality in renal transplant patients, whereas the LV2D did not predict mortality. Given the almost 50% mortality rate in the first 5 years in patients with an LA2D in the highest quintile (i.e., above 61 mm) and the lack of donor organs, wait-listing such candidates may lead to futility of allografts. It remains to be determined, however, if transplantation of patients with enlarged atria is beneficial given the increased mortality risk in these patients who are on dialysis.
Disclosures
None.
Supplementary Material
Acknowledgments
We are indebted to the administrators and all contributors of the Austrian Dialysis and Transplant Registry and the echocardiography database who are listed in the Annual Data Report (www.nephro.at/oedr2011).
This study was supported by Austrian Science Fund Grant FWF P-21436 (to R.O.)
Footnotes
A.K. and G.G. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04300413/-/DCSupplemental.
References
- 1.Briggs JD: Causes of death after renal transplantation. Nephrol Dial Transplant 16: 1545–1549, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Silberberg JS, Barre PE, Prichard SS, Sniderman AD: Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 36: 286–290, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP: Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Patel RK, Jardine AG, Mark PB, Cunningham AF, Steedman T, Powell JR, McQuarrie EP, Stevens KK, Dargie HJ, Jardine AG: Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis 55: 1088–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Montanaro D, Gropuzzo M, Tulissi P, Vallone C, Boscutti G, Mioni R, Risaliti A, Baccarani U, Adani GL, Sainz M, Lorenzin D, Bresadola F, Mioni G: Effects of successful renal transplantation on left ventricular mass. Transplant Proc 37: 2485–2487, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Dudziak M, Debska-Slizień A, Rutkowski B: Cardiovascular effects of successful renal transplantation: A 30-month study on left ventricular morphology, systolic and diastolic functions. Transplant Proc 37: 1039–1043, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Heinze G, Mitterbauer C, Regele H, Kramar R, Winkelmayer WC, Curhan GC, Oberbauer R: Angiotensin-converting enzyme inhibitor or angiotensin II type 1 receptor antagonist therapy is associated with prolonged patient and graft survival after renal transplantation. J Am Soc Nephrol 17: 889–899, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Kramar R, Oberbauer R: Austrian Dialysis and Transplantation Registry (OEDTR), Annual Report 2010, 2011. Available at: www.nephro.at/oedr2010 Accessed August 22, 2013 [Google Scholar]
- 10.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B, Management of Arterial Hypertension of the European Society of Hypertension. European Society of Cardiology : 2007 guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 25: 1105–1187, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fine J, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 12.Bursac Z, Gauss CH, Williams DK, Hosmer DW: Purposeful selection of variables in logistic regression. Source Code Biol Med 3: 17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzl H, Kaider A: Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed 54: 201–208, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Lentine KL, Schnitzler MA, Abbott KC, Li L, Xiao H, Burroughs TE, Takemoto SK, Willoughby LM, Gavard JA, Brennan DC: Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation. Clin J Am Soc Nephrol 1: 288–296, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Covic A, Mardare NG, Ardeleanu S, Prisada O, Gusbeth-Tatomir P, Goldsmith DJ: Serial echocardiographic changes in patients on hemodialysis: An evaluation of guideline implementation. J Nephrol 19: 783–793, 2006 [PubMed] [Google Scholar]
- 17.Cygankiewicz I, Zareba W, Vazquez R, Bayes-Genis A, Pascual D, Macaya C, Almendral J, Fiol M, Bardaji A, Gonzalez-Juanatey JR, Nieto V, Valdes M, Cinca J, de Luna AB, MUSIC Investigators : Risk stratification of mortality in patients with heart failure and left ventricular ejection fraction >35%. Am J Cardiol 103: 1003–1010, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Stallworthy EJ, Pilmore HL, Webster MW, Sidhu KK, Curry EM, Brown P, Scaria A: Do echocardiographic parameters predict mortality in patients with end-stage renal disease? Transplantation 95: 1225–1232, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Ibernon M, Moreso F, Ruiz-Majoral A, Sarrias X, Sarrias M, Grinyó JM, Serón D: Contribution of anemia and hypertension to left ventricular hypertrophy during the initial 2 years after renal transplantation. Transplant Proc 43: 2199–2204, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J: M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol 87: 1051–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Khoo CW, Krishnamoorthy S, Lim HS, Lip GY: Assessment of left atrial volume: A focus on echocardiographic methods and clinical implications. Clin Res Cardiol 100: 97–105, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Mielniczuk LM, Chandy G, Stewart D, Contreras-Dominguez V, Haddad H, Pugliese C, Davies RA: Worsening renal function and prognosis in pulmonary hypertension patients hospitalized for right heart failure. Congest Heart Fail 18: 151–157, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Rottlaender D, Motloch LJ, Schmidt D, Reda S, Larbig R, Wolny M, Dumitrescu D, Rosenkranz S, Erdmann E, Hoppe UC: Clinical impact of atrial fibrillation in patients with pulmonary hypertension. PLoS One 7: e33902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kainz A, Wilflingseder J, Függer R, Kramar R, Oberbauer R: Hemoglobin variability after renal transplantation is associated with mortality. Transpl Int 25: 323–327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen SC, Su HM, Hung CC, Chang JM, Liu WC, Tsai JC, Lin MY, Hwang SJ, Chen HC: Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 2750–2758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel RK, Mark PB, Johnston N, McGregor E, Dargie HJ, Jardine AG: Renal transplantation is not associated with regression of left ventricular hypertrophy: A magnetic resonance study. Clin J Am Soc Nephrol 3: 1807–1811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iqbal M, Banerjee S, Alam MR, Islam S, Rahman H, Rashid HU: Clinical and echocardiographic evaluation of renal allograft recipients in the first year after transplantation. Transplant Proc 35: 271–272, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Iqbal MM, Banerjee SK, Rahman MH, Rashid HU: Cardiac functional and morphologic changes of renal allograft recipients in the early posttransplant period. Transplant Proc 38: 3527–3529, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB: Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: The Strong Heart Study (SHS). Am Heart J 151: 412–418, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.