Summary
Protein energy wasting is common in patients with CKD and ESRD and is associated with adverse clinical outcomes, such as increased rates of hospitalization and death, in these patients. A multitude of factors can affect the nutritional and metabolic status of patients with CKD, including decreased dietary nutrient intake, catabolic effects of renal replacement therapy, systemic inflammation, metabolic and hormonal derangements, and comorbid conditions (such as diabetes and depression). Unique aspects of CKD also confound reliable assessment of nutritional status, further complicating management of this comorbid condition. In patients in whom preventive measures and oral dietary intake from regular meals cannot help them maintain adequate nutritional status, nutritional supplementation, administered orally, enterally, or parenterally, is effective in replenishing protein and energy stores. The advantages of oral nutritional supplements include proven efficacy, safety, and compliance. Anabolic steroids and exercise, with nutritional supplementation or alone, improve protein stores and represent potential additional approaches for the treatment of PEW. There are several emerging novel therapies, such as appetite stimulants, anti-inflammatory interventions, and anabolic agents.
Case Presentation
First Encounter
A 74-year-old woman was admitted to the hospital with nausea and vomiting for several weeks, which she had attributed to a gastrointestinal viral disease. She reported no fever, chills, diarrhea, or abdominal pain. She was known to have had CKD for >10 years, attributed to diabetic kidney disease. Her kidney function has been deteriorating slowly over the past few years. For the past few months, she reported poor appetite, some taste changes, sleepiness, and decreased physical activity. She had lost 7 kg in the last 6 months. She had become less able to care for herself and was having difficulty maintaining her regular activities.
In addition to type 2 diabetes mellitus for 15 years and CKD, she has hypertension, hypercholesterolemia, and osteoarthritis. She is retired. Her husband died 9 months ago. Her son and grandchildren visit once a month. Medications include metoprolol tartrate, lisinopril, furosemide, atorvastatin, sodium bicarbonate tablets, calcitriol, and glyburide.
Physical examination on admission showed weight of 68.5 kg, body mass index of 28.5 kg/m2, BP of 115/65 mmHg, and heart rate of 65 beats/min. She was in no apparent distress. There was mild to moderate muscle and fat wasting. Her skin and mucous membranes were dry. Lungs were clear and her cardiac examination was unremarkable. Her abdomen was soft and nondistended. She had 1+ edema of the feet and ankles. Neurologic examination was unremarkable. Her admission laboratory findings are depicted in Table 1, showing that her serum creatinine had risen compared with 6 months earlier.
Table 1.
Selected laboratory values for different encounters with the patient
| Variable | 6 mo before First Encounter | First Encounter | Discharge | Second Encounter | Third Encounter |
| TCO2 (mEq/L) | 24 | 21 | 24 | 17 | 24 |
| BUN (mg/dl) | 36 | 52 | 36 | 42 | 24 |
| Creatinine (mg/dl) | 2.5 | 3.6 | 3.1 | 4.7 | 4.5 |
| Glucose (mg/dl) | 145 | 265 | 135 | 185 | 165 |
| Albumin (g/dl) | 3.4 | 3.2 | 3.3 | 3.1 | 2.9 |
| Calcium (mg/dl) | 8.2 | 8.4 | 9.1 | 8.6 | 9.3 |
| Phosphorus (mg/dl) | 4.0 | 5.2 | 4.2 | 5.1 | 5.3 |
| Hemoglobin A1c | 9.2 | 9.4 | ND | ND | ND |
| Urine spot ACR | 3.5 | 2.4 | ND | 2.0 | ND |
| Hemoglobin (g/dl) | 11.2 | 11.8 | 10.8 | 9.8 | 9.4 |
| Estimated GFR (ml/min per 1.73 m2) | 19 | 12 | 15 | 9 | ND |
tCO2, total serum bicarbonate; ND, not done; ACR, albumin-to-creatinine ratio.
During this hospitalization, she was treated with intravenous fluids and her kidney function improved. Diabetic gastroparesis was diagnosed, and she began receiving metoclopramide. She was evaluated by a dietitian, with the dietary assessment revealing daily intake of 1200 kcal, 40 g (0.6 g/kg) protein, 80 mmol potassium, and 800 mg phosphorus. She was discharged home in stable condition with appointments to see her primary care physician in 2 weeks and a nephrologist in 1 month. She missed both appointments because she could not get to their offices.
Second Encounter
Four months after discharge, the patient was brought to the emergency department with failure to thrive and shortness of breath on exertion. Her laboratory findings are listed in Table 1. On the basis of her clinical presentation and laboratory findings, she was initiated on maintenance hemodialysis (HD) via a newly placed cuffed HD catheter. She was discharged home after three HD sessions and was referred to a dialysis clinic for continuation of her outpatient maintenance HD.
Third Encounter
The patient was again admitted to the hospital 5 months after starting maintenance HD with low-grade fever and chills. She stated that she did not tolerate meals well (usually less than half consumed) and had a very poor appetite. She felt sick toward the end of her HD treatments, usually after the third hour, and often signed off early because of cramps. Her dialysis prescription was 4 hours with a biocompatible high-flux membrane, 350 ml/min blood flow, 800 ml/min dialysate flow, and Kt/V of 1.6. She was dialyzed with ultrapure dialysate and 2 mEq/L potassium and 2.5 mg/dl calcium dialysate concentrations. She continued to be dialyzed through the dialysis catheter. An arteriovenous fistula (AVF) of the upper left arm had been created 3 months earlier but was not being used. Her urine output had dropped to less than 200 ml/d.
Her physical exam on admission revealed weight of 59 kg, body mass index of 24.6 kg/m2, BP of 110/60 mmHg, and heart rate of 95 beats/min. Her skin and mucous membranes were dry. She had decreased lung sounds at the bases bilaterally. Cardiac examination was unremarkable other than sinus tachycardia. Her abdomen was soft and nondistended. She had trace edema of the feet and ankles. A bruit was audible at the AVF site without any palpable veins. Her catheter exit site was clean. Admission laboratory findings are depicted in Table 1. Blood cultures were positive for gram-positive cocci in clusters. The HD catheter was removed and she was started on intravenous antibiotics. She became afebrile and a new cuffed HD catheter was placed. She was discharged to a skilled nursing facility.
Case Discussion
This case illustrates a common scenario in which a patient with CKD progressed to ESRD, was initiated on maintenance HD, and endured several complications during this transition and the initial 6 months of ESRD, including a subtle but clinically important progressive deterioration of her nutritional status. CKD leads to a state of metabolic and nutritional derangements, more aptly called protein energy wasting (PEW) (1,2). PEW is closely associated with major adverse clinical outcomes, such as increased rates of hospitalization and death, in patients with ESRD (3,4).
Screening and Assessment of Nutritional Status in CKD
A clinically meaningful assessment of nutritional status should be able to identify and risk-stratify patients with PEW, distinguishing the causes and consequences of both PEW and the underlying disease states, and to determine whether there is potential benefit from nutritional interventions (5). No single nutritional marker can adequately phenotype this comorbid state, and a comprehensive assessment of protein and energy nutritional status requires several different measurements (6).
Table 2 lists screening and assessment tools that can be used to identify patients at risk for or with PEW. Although several considerations must be accounted for given the unique situation of patients with CKD upon screening and assessing their nutritional status (Table 3), most of these tests are easy to perform, readily available, and inexpensive. Screening variables can be collected routinely in clinical practice by any health professional and mostly provide a trigger to conduct more extensive assessment, to confirm or establish the diagnosis and determine best course of treatment, if needed. Any of these tests is adequate to initiate a more thorough work-up. On the other hand, a thorough nutritional assessment that provides comprehensive information to make a nutritional diagnosis and aids in intervention and monitoring plan should be performed by qualified individuals, preferably trained dietitians. These tests should also be used for guiding nutritional therapies once initiated.
Table 2.
Suggested strategies to screen and assess nutritional status in advanced CKD
| Variable | Threshold for Detailed Assessment/Intervention | Relevant to Case? |
| Screening | ||
| Body weight | Continuous decline or <85% IBW | Yes |
| Dietary nutrient intake | ||
| DEI (kcal/kg IBW/d) | <25 | Yes |
| DPI (g/kg IBW/d) | <0.8 | Yes |
| Serum albumin (g/dl) | <4.0 | Yes |
| Serum creatinine | Relatively low value | Yes but subtle |
| MST | >2 | Yes |
| Assessment | ||
| Serum prealbumin (mg/dl) | <28 | ND |
| hsCRP (mg/dl) | >10 | ND |
| Anthropometrics | Deviation from norms | ND |
| SGA | B or C (moderately or severely malnourished) | ND (presumed score B or C) |
| MIS | >5 | ND |
| Diagnosis (2 of the 4) | ||
| Serum chemistry | ||
| Albumin (g/dl) | <3.8 | Yes |
| Prealbumin (mg/dl) | <28a | ND |
| Cholesterol (mg/dl) | <100 | ND |
| Body mass | ||
| BMI (kg/m2) | <23 | No |
| Weight loss | >5% over 3 mo or 10% over 6 mo | Yes |
| Total body fat (%) | <10 | ND |
| Muscle mass | ||
| Muscle wasting | >5% over 3 mo or 10% over 6 mo | ND |
| Reduced MAMC | >10% reduction compared with norms | ND |
| Creatinine appearance (g/kg IBW) | <1 | ND |
| Dietary intake | ||
| Low DPI (g/kg IBW per d) | <0.8 | Yes |
| Low DEI (kcal/kg IBW per d) | <25 | Yes |
IBW, ideal body weight; DEI, dietary energy intake; DPI, dietary protein intake; MST, Malnutrition Screening Tool; hsCRP, high-sensitivity C-reactive protein; SGA, subjective global assessment; MIS: malnutrition inflammation score; MAMC, mid-arm muscle circumference; ND, not done.
Influenced by kidney function.
Table 3.
Factors that affect interpretation of nutritional markers in CKD
| Fluid status: altered body composition and biochemical markers |
| Systemic inflammation: increased (hsCRP) or decreased (albumin, prealbumin, cholesterol) acute phase protein synthesis |
| Proteinuria: major determinant of serum albumin levels |
| Residual renal function: some biochemical markers, such as prealbumin, are cleared by the kidneys |
hsCRP, high-sensitivity C-reactive protein.
The illustrated case provides insight into the clinical steps that can be taken to prevent some of the nutritional complications associated with CKD. The most striking finding that indicated development of PEW in this patient is the progressive weight loss combined with a lower than expected dietary protein and calorie intake. Serum creatinine concentration, while a marker of kidney function, is also markedly influenced by muscle mass. The relatively low serum creatinine in a patient such as this with low muscle mass complicates the assessment of residual kidney function and can delay timely initiation of maintenance HD. The patient also has low serum albumin and obvious loss of subcutaneous fat tissue; even in the absence of a formal nutritional assessment, it is obvious that she has passed the risk status and has PEW, even defined by the most stringent criteria (7).
Prevention of PEW: A Cause-Specific Approach
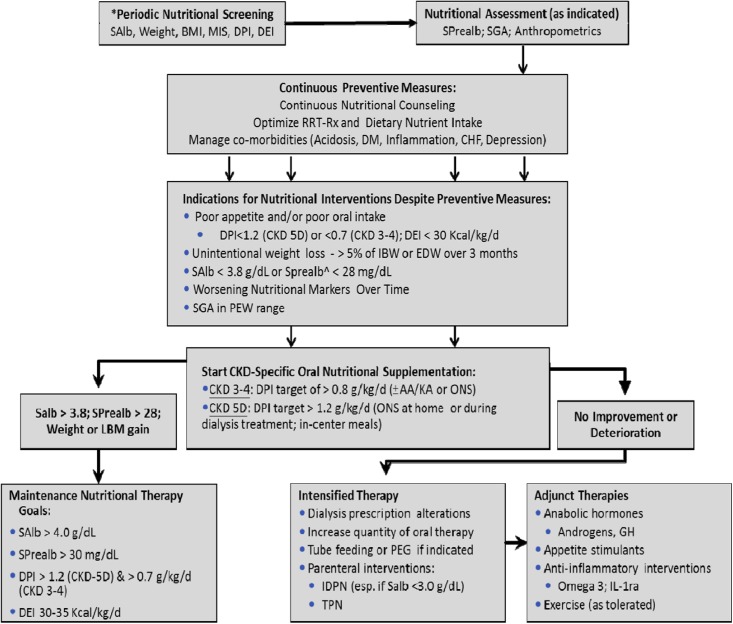
Many factors affect nutritional and metabolic status in patients with CKD, leading to multiple adverse consequences (8). Accordingly, prevention and treatment of PEW of CKD should involve an integrated approach to reduce protein and energy depletion, avoid further losses, and replenish already wasted stores (Figure 1). The illustrated case exemplifies several factors that can be identified and prevented before development of PEW, including but not limited to decreased dietary nutrient intake, catabolic effects of renal replacement therapy, systemic inflammation, and comorbid conditions, such as diabetes mellitus and depression.
Figure 1.
Algorithm for nutritional management and support in patients with CKD. *Minimum every 3 months, monthly screening recommended. ^Only for patients with ESRD who do not have residual renal function. AA, amino acid; BMI, body mass index; CHF, congestive heart failure; DEI, dietary energy intake; DM, diabetes mellitus; DPI, dietary protein intake; EDW, estimated dry weight; GH, growth hormone; IDPN, intradialytic parenteral nutrition; IL-1ra, IL-1 receptor antagonist; KA, ketoanalogs; LBM, lean body mass; MIS, malnutrition-inflammation score; ONS, oral nutritional supplement; PEG, percutaneous endoscopic gastrostomy; PEW, protein energy wasting; RRT-Rx, renal replacement therapy prescription; SAlb, serum albumin (measured by bromocresol green); SGA, subjective global assessment; SPrealb, serum prealbumin; TPN, total parenteral nutrition. Reprinted from reference 52, with permission.
Dietary Nutrient Intake in Patients with CKD.
A frequent and important cause of PEW in patients with advanced CKD is inadequate dietary protein and energy intake relative to their needs (9,10), which is primarily due to uremic anorexia. Anorexia has long been considered the hallmark of advanced CKD. Patients with CKD spontaneously restrict their dietary protein intake often to levels <0.6 g/kg per day when estimated GFR falls to <15 ml/min (11). Anorexia in CKD may develop because of retention of uremic toxins (12), intercurrent illness and inflammation (3,13), comorbid illness that affects gastrointestinal function, depression, and poor socioeconomic situations (14,15). In clinically stable patients with stage 3–5 CKD not undergoing dialysis, daily dietary protein and energy intake of 0.6–0.8 g/kg ideal body weight and 30–35 kcal/kg ideal body weight, respectively, are sufficient to preserve protein stores (16–18). However, these levels should be increased when hypermetabolic conditions, such as acute illness and hospitalizations, occur. Another important implication of anorexia in advanced CKD is its use as an indication for initiation of maintenance dialysis. Data available from a randomized clinical trial (RCT) indicate that patient symptoms (19), including anorexia accompanied with significant weight loss, is often used as an indication for initiation of maintenance dialysis, which can be readily applicable to the present case.
Renal Replacement Therapy as a Catabolic Stimulus.
Provision of an adequate dialysis dose has long been considered as a cornerstone among measures to prevent and treat PEW in maintenance dialysis patients to avoid uremic anorexia and maintain optimal dietary nutrient intake, with a minimum dose of dialysis generally recommended (20–22). Data from RCTs in patients receiving maintenance HD (HEMO [Hemodialysis] study) (23) and peritoneal dialysis (PD; ADEMEX [ADEquacy of PD in MEXico] trial) (24) indicate that what is currently considered adequate dialysis in various guidelines is sufficient to preserve nutritional status, although the HEMO study showed that maintenance HD patients lose weight over time regardless of “adequate” dialysis dose (23). Increasing dialysis dose beyond these targets does not further improve the nutritional status. Further, most nutritional measures did not differ among patients exposed to high-flux compared with low-flux HD membranes (25). The Frequent Hemodialysis Networks trial found no appreciable difference in nutritional markers between patients randomly assigned to in-center HD six times per week versus standard in-center HD three times per week (26).
In patients with ESRD receiving maintenance dialysis, there are additional protein catabolic processes, such as the unavoidable loss of amino acids (6–8 g per HD session) and albumin into the dialysate and the inflammatory stimulus associated with the dialysis procedure or other components of ESRD (i.e., HD catheters) (27). An important caveat is the need to increase dietary protein intake targets once the patient begins undergoing maintenance dialysis, especially in those who were instructed to reduce protein intake to slow progression of CKD. Accordingly, minimum daily protein and energy requirements for maintenance HD and PD patients are 1.2–1.3 g/kg ideal body weight and 30–35 kcal/kg ideal body weight, respectively. The energy intake should be adjusted according to physical activity levels. Furthermore, it is important that at least 50% of the protein intake should be of high biologic value. An important consideration regarding strategies to improve dietary protein intake in patients with ESRD is the potential increase in the intake of several potentially harmful elements, especially phosphorus (28). Dietary recommendations to improve protein intake should take into account the phosphorus content of the specific protein sources (i.e., vegetarian diets tend to be low in phosphorus) and other phosphorus sources, such as additives and preservatives in processed food (29,30).
Systemic Inflammation.
Inflammation is a major driving force for many uremic complications, including PEW. Systemic levels of proinflammatory cytokines are elevated in CKD and ESRD (31) and are thought to play an integral role in the muscle catabolism of patients with ESRD. For example, elevated levels of IL-6 are associated with increased muscle proteolysis, an effect that can be blocked with administration of IL-6 receptor antibodies (32). IL-1 and TNF-α cause anorexia through effects on the satiety center in the central nervous system (33).
The initial step in treatment of inflammation should be elimination of etiologic factors, such as central venous HD catheters, which is directly applicable to the present case (34). Because the dialysis procedure per se might stimulate the immune system, proinflammatory effects of dialysis membranes and fluids should also be taken into account. Many uremic toxins are known to be proinflammatory (35) and are inadequately removed with standard dialysis methods; alternative removal techniques, such as strategies to modify intestinal generation or absorption, may have a role in this regard (36). Appropriate management of fluid status might improve systemic inflammation in patients with ESRD because volume overload leads to immunoactivation and increased cytokine production via bacterial or endotoxin translocation (37).
Comorbid Conditions in CKD.
Patients with CKD secondary to diabetes mellitus have a higher incidence of PEW than those without diabetes (38). The degree of insulin resistance and/or insulin deprivation seems to play the most critical role in this process (39–42). Patients with CKD also often have protein depletion because of gastrointestinal disturbances (e.g., diabetic gastroparesis, nausea and vomiting, bacterial overgrowth in the gut, pancreatic insufficiency, and impaired intestinal protein absorption). Polypharmacy can worsen these gastrointestinal problems. Appropriate management of these disturbances, along with an emphasis on oral health, especially in the elderly, is critical to maintain optimal oral nutrient intake. Uncontrolled hyperparathyroidism and cardiac cachexia are associated with systemic inflammation and increased energy expenditure, so their appropriate management is also necessary to prevent PEW (43,44). Depressive symptoms, which are common in patients with CKD and ESRD and were quite evident in the illustrated case, are linked to fatigue, lack of appetite (45), and weight loss. Early recognition and treatment are important components in the prevention of PEW (46).
Metabolic Acidosis.
Metabolic acidosis is associated with increased muscle protein catabolism and promotes PEW in patients with advanced CKD (47). Metabolic acidosis stimulates oxidation of essential amino acids and further raises protein requirements for maintenance HD patients. Oral bicarbonate supplementation can improve nutritional status in patients with CKD (48,49). In an RCT of 134 patients with stage 4 CKD in whom serum bicarbonate was increased to 24 mmol/L, dietary protein and energy intake, mid-arm muscle circumference, and serum albumin improved and progression of CKD was slowed over 2 years compared with patients with a serum bicarbonate level of 20 mmol/L. Accordingly, a steady-state serum bicarbonate level should be ≥24 mmol/L in patients with CKD not yet undergoing maintenance HD and patients receiving PD. Recent epidemiologic data indicate adverse outcomes with high predialysis serum bicarbonate levels in maintenance HD patients, with a target of 22–24 mmol/L in these patients to avoid alkalosis after HD (50,51).
Treatment of PEW: An Integrated Approach
Oral and Enteral Nutritional Supplementation.
In certain patients with CKD and ESRD, preventive measures are unable to diminish loss of protein and energy stores. As a result, nutritional supplementation may become appropriate (Figure 1). Oral supplementation should be given two to three times a day, preferably 1 hour after main meals and/or during dialysis for maintenance HD patients. This can provide an additional 7–10 kcal/kg of energy and 0.3–0.4 g/kg of protein per day. This requires a minimum spontaneous dietary intake of 20 kcal/kg energy and 0.4–0.8 g/kg protein per day in order to meet the recommended dietary energy and protein intake targets.
The efficacy of oral supplementation has been studied in multiple settings (reviewed by Ikizler et al. [52]). The beneficial nutritional effects of these supplements range from improvements in serum biomarkers, such as albumin, prealbumin, and transferrin, to gains in different body compartments, such as weight and lean body mass. The effects are evident as early as within a month and are generally sustained and associated with improvements in quality of life and physical functioning. In several studies, improvements in hospitalizations and death were reported, but none had the statistical power to appropriately assess these outcomes. For patients who are unable to tolerate nutritional supplementation by mouth, nasogastric tubes, percutaneous endoscopic gastroscopy, or jejunostomy tubes can be considered (17).
Two recent large-scale observational studies reported significant survival benefit in hypoalbuminemic maintenance HD patients receiving nutritional supplementation compared with matched controls (53,54). In a retrospective cohort study of 4289 matched pairs, death rates were 30.9% versus 37.3% in the treated versus untreated groups, respectively (53). In a prospective observational study of oral nutritional supplementation (ONS) performed as part of a disease management plan in maintenance HD patients, ONS use was associated with higher serum albumin and lower hospitalization rate at 1 year, without reduction in mortality risk, compared with patients who did not receive ONS (54). The limitations of these studies include their retrospective design, convenience sampling, and residual confounding from unmeasured variables.
Intradialytic Parenteral Nutrition.
Parenteral provision of nutrients during HD (intradialytic parenteral nutrition [IDPN]) has been shown in many studies, including RCTs, to be a safe and convenient approach for individuals who cannot tolerate oral or enteral supplements, including those with overt PEW, although many studies had significant design flaws (55,56). In the largest nutritional intervention study in maintenance HD patients with PEW (FINE [French Intradialytic Nutrition Evaluation] study) (57), similar improvements of nutritional measures were observed in both the IDPN and oral nutritional supplementation groups, without differences in rates of hospitalization or death. These various studies have shown that there is a direct correlation between response to nutritional supplementation and the severity of PEW and the amount of nutrients received, with diabetic patients showing a reduced response to nutritional support, and that an inflammatory status does not significantly affect the response to nutritional support. The high cost of IDPN and regulatory concerns remain the greatest barriers for use of IDPN, which should be reserved for patients in whom oral or enteral supplements are not feasible. Other studies using amino acids in dialysate (AAD) as a nutritional intervention in PD patients with PEW have provided conflicting results (58,59). Overall, AAD remains a viable option in PD patients with PEW who cannot tolerate or are not suitable for oral and other enteral supplements. AAD is not currently available in the United States, although some compounding pharmacies prepare solutions by injection of amino acid concentrate into glucose-based dialysate for compassionate use.
Anabolic Hormones.
Recombinant human growth hormone (rhGH), an approved treatment of short stature in pediatric patients with CKD (60), leads to improved growth, confirming that rhGH could overcome GH resistance associated with CKD. In adults with CKD, resistance to native GH may be responsible for the premature decline in body composition (61). In a large multicenter RCT, C-reactive protein, homocysteine, HDL cholesterol, and transferrin levels significantly improved in hypoalbuminemic patients receiving maintenance HD (62). Unfortunately, this large RCT was prematurely terminated because of slow recruitment and thus could not assess the effects of rhGH on hospitalization or death.
Testosterone deficiency is also very common in male patients undergoing maintenance HD (63) and is associated with increased mortality risk. Several RCTs performed in maintenance HD patients showed significant benefits of nandrolone decanoate treatment in both anthropometric and biochemical measures, including body weight; body mass index; skinfold; mid-arm muscle circumference; and serum levels of total protein, prealbumin, and transferrin. No consistent effect of nandrolone decanoate was demonstrated on physical functioning in several studies, and high-dose nandrolone decanoate (100 mg/week) was intolerable in women because of its virilizing effects (64,65). Anabolic steroids can be used for preventing sarcopenia, albeit under close supervision with use limited to 6 months.
Exercise.
Abnormalities in muscle function, exercise performance, and physical activity begin in the early stages of CKD and progress dramatically as ESRD develops (66). In ESRD, there are metabolic and structural muscle abnormalities with reductions in oxidative capacity and type 1 fibers with associated decrease in muscle endurance (67,68). Although several studies have examined the effects of cardiopulmonary fitness training in patients with ESRD (69), few studies have examined the role of exercise training on stimulating muscle growth. Collectively, these studies indicate that beneficial effects of exercise, such as improvements in muscle quality and quantity, strength, and physical functioning, are not consistently observed in patients with ESRD (70), perhaps because of limitations of methods to assess body composition, inadequate intensity and/or duration of exercise, and lack of understanding of the actual metabolic and morphologic abnormalities related to PEW in advanced CKD.
Case Follow-up
After discharge, the family elected to place the patient in a nursing home. She began receiving renal specific oral nutritional supplement, two cans a day taken between main meals. She also received one of the supplements during dialysis. The renal dietitian performed biweekly food recalls and instructed the patient and nursing home staff on increased dietary protein and calorie intake. The patient also began to take an antidepressant. She subsequently resumed more of her social activities. A procedure was performed to ligate accessory veins at the site of the AVF, with improved fistula blood flow within a few weeks and removal of the tunnelled HD catheter. The patient’s weight stabilized and her serum albumin increased to 3.7 g/dl over the subsequent 3 months.
Summary and Recommendations
Because of its metabolic and functional importance in whole-body homeostasis, preservation of muscle mass is the ultimate goal in the management of PEW in patients with CKD. In patients with CKD and ESRD, in whom many catabolic signals dominate, it is critical to maintain a dietary protein and energy intake relative to needs. Treatment of concurrent conditions that contribute to catabolism, such as metabolic acidosis, insulin resistance, and systemic inflammation, is of paramount importance for the prevention of PEW. When supplemental nutrition is indicated, it is crucial to take into account all the determinants of body and muscle mass, including protein and energy content, exercise, anabolic hormones, antioxidants, anti-inflammatory nutrients and drugs, and other specific nutrients. Finally, it is important to assess the effect of nutritional supplements on nutritional measures; unfortunately, cost-effectiveness of these interventions and effects on hospitalization and mortality remain to be determined, with an absence of large, adequately powered RCTs demonstrating benefits of nutritional interventions on morbidity and mortality.
Question 1 (Dr. R. Hakim): What is the clinically recommended approach for nutritional assessment in patients with CKD?
Answer: In patients with stage 3–5 CKD not on maintenance dialysis, nutritional screening should include assessments of serum albumin, weight loss, and a malnutrition screening tool at every outpatient clinic visit. For those receiving in-center maintenance HD, this should be performed monthly. In patients deemed to be at risk for PEW, anthropometric measurements, subjective global assessment, or malnutrition-inflammation score should be performed every 6 months, in addition to periodic measurements of serum prealbumin, high-sensitivity C-reactive protein, and cholesterol. A thorough physical examination is critical for appropriate analysis of these findings. In addition to examining absolute values for certain thresholds, trends over time should be considered. For all indirect methods, such as subjective global assessment, repeated measures and technical standardization are extremely important to reduce variability of results. Regardless of the method, it is important to keep in mind that none is perfect and definitive, and the results should always be analyzed in the clinical context of each individual patient.
Question 2 (Dr. E. Siew): What are the role of appetite stimulants and other novel interventions, such as probiotics, in the treatment of PEW?
Answer: Several pharmacologic agents may stimulate appetite, including megestrol acetate, dronabinol, cyproheptadine, melatonin, thalidomide and ghrelin. Most of these drugs have not been studied systematically in maintenance HD patients. In such patients, small uncontrolled studies showed that megestrol acetate can stimulate appetite and induce small increases in serum albumin and weight (71), but large-scale prospective studies are needed. Ghrelin is an orexigenic peptide released primarily from the stomach; it increases appetite and adjusts both short- and long-term energy balance, making it a good candidate for treatment of anorexic patients with ESRD. Two pilot studies suggested improved energy intake during short-term ghrelin administration (72,73). There is also limited but supportive evidence for the effectiveness of pre- and probiotics on reducing plasma levels of some uremic toxins (74).
Question 3 (Dr. J. Berns): You mentioned depression in your discussion of the case. How common is this as a contributing factor to PEW in patients with CKD and ESRD, how should we assess for it, and are there any recommended treatment options?
Answer: Depression and anxiety obviously influence the willingness to eat, and observational reports show that appetite is poorer when patients score worse on their depression tests. A recent study showed strong associations between depression, inflammation, and serum albumin in maintenance dialysis patients (75). It is recommended that patients with ESRD be screened at the initiation of dialysis therapy, within 3–6 months after therapy initiation, and then yearly using depression screening self-report questionnaires, such as the Beck Depression Inventory. At-risk patients should then undergo a structured clinical interview to confirm the diagnosis, with subsequent consideration of treatment with a selective serotonin reuptake inhibitor or serotonergic agonist (76).
Disclosures
T.A.I. is a consultant for or received Honoraria from Abbott Nutrition, Abbott Renal, DSI Renal, Inc. and Baxter Renal.
Acknowledgments
The author would like to thank Dr. Arnie Berns for his thoughtful review of the manuscript. This work is partly sponsored by Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources, K24 DK 62849 from the National Institute of Diabetes and Digestive and Kidney Diseases, and R01 HL070938 from the National Heart, Lung, and Blood Institute.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Ikizler TA, Hakim RM: Nutrition in end-stage renal disease. Kidney Int 50: 343–357, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD: Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 80: 299–307, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 35: S1–140, 2000 [DOI] [PubMed]
- 5.Jeejeebhoy KN: Nutritional assessment. Nutrition 16: 585–590, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Association AD; Council on Practice (COP) Quality Management Committee: Identifying patients at risk: ADA’s definitions for nutrition screening and nutrition assessment. J Am Diet Assoc 94: 838–839, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Pupim LB, Ikizler TA: Assessment and monitoring of uremic malnutrition. J Ren Nutr 14: 6–19, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, Mitch WE, Price SR, Wanner C, Wang AY, ter Wee P, Franch HA: Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 23: 77–90, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Rocco MV, Paranandi L, Burrowes JD, Cockram DB, Dwyer JT, Kusek JW, Leung J, Makoff R, Maroni B, Poole D: Nutritional status in the HEMO Study cohort at baseline. Hemodialysis. Am J Kidney Dis 39: 245–256, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Wang AY, Sanderson J, Sea MM, Wang M, Lam CW, Li PK, Lui SF, Woo J: Important factors other than dialysis adequacy associated with inadequate dietary protein and energy intakes in patients receiving maintenance peritoneal dialysis. Am J Clin Nutr 77: 834–841, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Ikizler TA, Greene JH, Wingard RL, Parker RA, Hakim RM: Spontaneous dietary protein intake during progression of chronic renal failure. J Am Soc Nephrol 6: 1386–1391, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Anderstam B, Mamoun AH, Södersten P, Bergström J: Middle-sized molecule fractions isolated from uremic ultrafiltrate and normal urine inhibit ingestive behavior in the rat. J Am Soc Nephrol 7: 2453–2460, 1996 [DOI] [PubMed] [Google Scholar]
- 13.McCarthy DO: Tumor necrosis factor alpha and interleukin-6 have differential effects on food intake and gastric emptying in fasted rats. Res Nurs Health 23: 222–228, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Wright M, Woodrow G, O’Brien S, King N, Dye L, Blundell J, Brownjohn A, Turney J: Disturbed appetite patterns and nutrient intake in peritoneal dialysis patients. Perit Dial Int 23: 550–556, 2003 [PubMed] [Google Scholar]
- 15.Mamoun AH, Anderstam B, Södersten P, Lindholm B, Bergström J: Influence of peritoneal dialysis solutions with glucose and amino acids on ingestive behavior in rats. Kidney Int 49: 1276–1282, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Lim VS, Kopple JD: Protein metabolism in patients with chronic renal failure: Role of uremia and dialysis. Kidney Int 58: 1–10, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Cano NJ, Aparicio M, Brunori G, Carrero JJ, Cianciaruso B, Fiaccadori E, Lindholm B, Teplan V, Fouque D, Guarnieri G; ESPEN: ESPEN Guidelines on Parenteral Nutrition: Adult renal failure. Clin Nutr 28: 401–414, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kopple JD: National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 37[Suppl 2]: S66–S70, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA; IDEAL Study: A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld PY, Henry RR, Laird NM, Roxe DM: Assessment of nutritional status of the National Cooperative Dialysis Study population. Kidney Int Suppl 23: S80–S88, 1983 [PubMed] [Google Scholar]
- 21.Lindsay R, Spanner E, Heidenheim P, LeFebure J, Hodsman A, Baird J, Allison M: Which comes first, Kt/V or PCR—chicken or egg? Kidney Int 42[Suppl 38]: S32–S37, 1992 [PubMed] [Google Scholar]
- 22.Bergström J, Lindholm B: Nutrition and adequacy of dialysis. How do hemodialysis and CAPD compare? Kidney Int Suppl 40[Suppl 40]: S39–S50, 1993 [PubMed] [Google Scholar]
- 23.Rocco MV, Dwyer JT, Larive B, Greene T, Cockram DB, Chumlea WC, Kusek JW, Leung J, Burrowes JD, McLeroy SL, Poole D, Uhlin L; HEMO Study Group: The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2321–2334, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S; Mexican Nephrology Collaborative Study Group: Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R; Membrane Permeability Outcome (MPO) Study Group: Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS; FHN Trial Group: In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikizler TA, Flakoll PJ, Parker RA, Hakim RM: Amino acid and albumin losses during hemodialysis. Kidney Int 46: 830–837, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Kovesdy CP, Shinaberger CS, Kalantar-Zadeh K: Epidemiology of dietary nutrient intake in ESRD. Semin Dial 23: 353–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, Noori N, Hirschberg R, Benner D, Nissenson AR, Kopple JD: Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 519–530, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA: Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int 65: 2371–2379, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Nishimoto N, Kishimoto T: Interleukin 6: From bench to bedside. Nat Clin Pract Rheumatol 2: 619–626, 2006 [DOI] [PubMed] [Google Scholar]
- 33.DeBoer MD, Scarlett JM, Levasseur PR, Grant WF, Marks DL: Administration of IL-1β to the 4th ventricle causes anorexia that is blocked by agouti-related peptide and that coincides with activation of tyrosine-hydroxylase neurons in the nucleus of the solitary tract. Peptides 30: 210–218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein SL, Ikizler TA, Zappitelli M, Silverstein DM, Ayus JC: Non-infected hemodialysis catheters are associated with increased inflammation compared to arteriovenous fistulas. Kidney Int 76: 1063–1069, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A; European Uremic Toxin Work Group: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanholder R, Schepers E, Pletinck A, Neirynck N, Glorieux G: An update on protein-bound uremic retention solutes. J Ren Nutr 22: 90–94, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD: Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 353: 1838–1842, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Cano NJ, Roth H, Aparicio M, Azar R, Canaud B, Chauveau P, Combe C, Fouque D, Laville M, Leverve XM; French Study Group for Nutrition in Dialysis (FSG-ND): Malnutrition in hemodialysis diabetic patients: Evaluation and prognostic influence. Kidney Int 62: 593–601, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Deger SM, Sundell MB, Siew ED, Egbert P, Ellis CD, Sha F, Ikizler TA, Hung AM: Insulin resistance and protein metabolism in chronic hemodialysis patients. J Ren Nutr 23: e59–e66, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siew ED, Ikizler TA: Determinants of insulin resistance and its effects on protein metabolism in patients with advanced chronic kidney disease. Contrib Nephrol 161: 138–144, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Pupim LB, Flakoll PJ, Majchrzak KM, Aftab Guy DL, Stenvinkel P, Ikizler TA: Increased muscle protein breakdown in chronic hemodialysis patients with type 2 diabetes mellitus. Kidney Int 68: 1857–1865, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA: Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int 71: 146–152, 2007 [DOI] [PubMed] [Google Scholar]
- 43.von Haehling S, Lainscak M, Springer J, Anker SD: Cardiac cachexia: A systematic overview. Pharmacol Ther 121: 227–252, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Cuppari L, de Carvalho AB, Avesani CM, Kamimura MA, Dos Santos Lobão RR, Draibe SA: Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol 15: 2933–2939, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Carrero JJ: Identification of patients with eating disorders: Clinical and biochemical signs of appetite loss in dialysis patients. J Ren Nutr 19: 10–15, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Nagler EV, Webster AC, Vanholder R, Zoccali C: Antidepressants for depression in stage 3-5 chronic kidney disease: A systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrol Dial Transplant 27: 3736–3745, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE: The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest 97: 1447–1453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein A, Moorhouse J, Iles-Smith H, Baker F, Johnstone J, James G, Troughton J, Bircher G, Walls J: Role of an improvement in acid-base status and nutrition in CAPD patients. Kidney Int 52: 1089–1095, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K: Association between serum bicarbonate and death in hemodialysis patients: Is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol 1: 70–78, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Tentori F, Karaboyas A, Robinson BM, Morgenstern H, Zhang J, Sen A, Ikizler TA, Rayner H, Fissell RB, Vanholder R, Tomo T, Port FK: Association of Dialysate Bicarbonate Concentration With Mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS) [published online ahead of print May 24, 2013]. Am J Kidney Dis doi: 10.1053/j.ajkd.2013.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, Kuhlmann MK, Stenvinkel P, Terwee P, Teta D, Wang AY, Wanner C: Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism [published online ahead of print May 24, 2013]. Kidney Int doi: 10.1038/ki.2013.147 [DOI] [PubMed] [Google Scholar]
- 53.Lacson E, Jr, Wang W, Zebrowski B, Wingard R, Hakim RM: Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: a quality improvement report. Am J Kidney Dis 60: 591–600, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Cheu C, Pearson J, Dahlerus C, Lantz B, Chowdhury T, Sauer PF, Farrell RE, Port FK, Ramirez SPB: Association between oral nutritional supplementation and clinical outcomes among patients with ESRD. Clin J Am Soc Nephrol 8: 100–107, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chertow GM, Ling J, Lew NL, Lazarus JM, Lowrie EG: The association of intradialytic parenteral nutrition administration with survival in hemodialysis patients. Am J Kidney Dis 24: 912–920, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Mortelmans AK, Duym P, Vandenbroucke J, De Smet R, Dhondt A, Lesaffer G, Verwimp H, Vanholder R: Intradialytic parenteral nutrition in malnourished hemodialysis patients: A prospective long-term study. JPEN J Parenter Enteral Nutr 23: 90–95, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Cano NJ, Fouque D, Roth H, Aparicio M, Azar R, Canaud B, Chauveau P, Combe C, Laville M, Leverve XM; French Study Group for Nutrition in Dialysis: Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: A 2-year multicenter, prospective, randomized study. J Am Soc Nephrol 18: 2583–2591, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Jones M, Hagen T, Boyle CA, Vonesh E, Hamburger R, Charytan C, Sandroni S, Bernard D, Piraino B, Schreiber M, Gehr T, Fein P, Friedlander M, Burkart J, Ross D, Zimmerman S, Swartz R, Knight T, Kraus A, Jr, McDonald L, Hartnett M, Weaver M, Martis L, Moran J: Treatment of malnutrition with 1.1% amino acid peritoneal dialysis solution: Results of a multicenter outpatient study. Am J Kidney Dis 32: 761–769, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Tjiong HL, van den Berg JW, Wattimena JL, Rietveld T, van Dijk LJ, van der Wiel AM, van Egmond AM, Fieren MW, Swart R: Dialysate as food: Combined amino acid and glucose dialysate improves protein anabolism in renal failure patients on automated peritoneal dialysis. J Am Soc Nephrol 16: 1486–1493, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Mehls O, Wühl E, Tönshoff B, Schaefer F, Nissel R, Haffner D: Growth hormone treatment in short children with chronic kidney disease. Acta Paediatr 97: 1159–1164, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Feldt-Rasmussen B, Lange M, Sulowicz W, Gafter U, Lai KN, Wiedemann J, Christiansen JS, El Nahas M; APCD Study Group: Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol 18: 2161–2171, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Kopple JD, Cheung AK, Christiansen JS, Djurhuus CB, El Nahas M, Feldt-Rasmussen B, Mitch WE, Wanner C, Göthberg M, Ikizler TA: OPPORTUNITY: A large-scale randomized clinical trial of growth hormone in hemodialysis patients. Nephrol Dial Transplant 26: 4095–4103, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Carrero JJ, Stenvinkel P: The vulnerable man: Impact of testosterone deficiency on the uraemic phenotype. Nephrol Dial Transplant 27: 4030–4041, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Macdonald JH, Marcora SM, Jibani MM, Kumwenda MJ, Ahmed W, Lemmey AB: Nandrolone decanoate as anabolic therapy in chronic kidney disease: A randomized phase II dose-finding study. Nephron Clin Pract 106: c125–c135, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Johansen KL, Mulligan K, Schambelan M: Anabolic effects of nandrolone decanoate in patients receiving dialysis: A randomized controlled trial. JAMA 281: 1275–1281, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Leikis MJ, McKenna MJ, Petersen AC, Kent AB, Murphy KT, Leppik JA, Gong X, McMahon LP: Exercise performance falls over time in patients with chronic kidney disease despite maintenance of hemoglobin concentration. Clin J Am Soc Nephrol 1: 488–495, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Ikizler TA, Himmelfarb J: Muscle wasting in kidney disease: Let’s get physical. J Am Soc Nephrol 17: 2097–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Kopple JD, Storer T, Casburi R: Impaired exercise capacity and exercise training in maintenance hemodialysis patients. J Ren Nutr 15: 44–48, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Painter P, Johansen KL: Improving physical functioning: Time to be a part of routine care. Am J Kidney Dis 48: 167–170, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Ikizler TA: Exercise as an anabolic intervention in patients with end-stage renal disease. J Ren Nutr 21: 52–56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeh S-S, Marandi M, Thode HC, Jr, Levine DM, Parker T, Dixon T, Schuster MW: Report of a pilot, double-blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr 20: 52–62, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Ashby DR, Ford HE, Wynne KJ, Wren AM, Murphy KG, Busbridge M, Brown EA, Taube DH, Ghatei MA, Tam FW, Bloom SR, Choi P: Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int 76: 199–206, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, Brown EA, Bloom SR, Choi P: Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: A randomized, placebo-controlled trial. J Am Soc Nephrol 16: 2111–2118, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Rossi M, Klein K, Johnson DW, Campbell KL: Pre-, pro-, and synbiotics: Do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol 2012: 673631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hung K-C, Wu C-C, Chen H-S, Ma W-Y, Tseng C-F, Yang L-K, Hsieh H-L, Lu K-C: Serum IL-6, albumin and co-morbidities are closely correlated with symptoms of depression in patients on maintenance haemodialysis. Nephrol Dial Transplant 26: 658–664, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Hedayati SS, Yalamanchili V, Finkelstein FO: A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int 81: 247–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]