Summary
Hemodialysis vascular access dysfunction is a major source of morbidity for patients with ESRD. Development of effective approaches to prevent and treat vascular access failure requires an understanding of the underlying mechanisms, suitable models for preclinical testing, systems for targeted delivery of interventions to maximize efficacy and minimize toxicity, and rigorous clinical trials that use appropriate outcome measures. This article reviews the substantial progress and ongoing challenges in developing novel treatments for arteriovenous vascular access failure and focuses on localized rather than systemic interventions.
Vascular access dysfunction is a major source of morbidity for patients treated with maintenance hemodialysis, and the need for novel treatment approaches for hemodialysis arteriovenous (AV) access failure is well recognized. Two recent randomized, placebo-controlled trials found some benefits on AV graft (AVG) patency of orally administered agents, dipryridamole plus aspirin (1) and fish oil (2), and an ongoing randomized clinical trial is evaluating short-term use of orally administered sirolimus on the patency of AVGs and AV fistulas (AVFs) after clinically indicated angioplasty (Sirolimus Use in Angioplasty for Vascular Access Extension (SAVE) NCT01595841). However, enthusiasm for systemic administration of agents to address vascular access dysfunction is somewhat limited because of off-target effects and toxicities. Recent advances that are setting the stage for the successful development of novel, locally administered interventions for vascular access dysfunction include elucidation of underlying mechanisms (see Lee et al. [3] and Remuzzi et al. [4] in this issue of CJASN), availability of animal models for evaluating treatment candidates, advances in targeted delivery of pharmacologic and biologic agents, progress in establishing outcome measures for clinical trials, and interest by the biopharmaceutical industry.
Animal Models of AV Access
Animal models have been developed to study the pathogenesis of vascular access failure and test therapeutic approaches (Table 1). These models provide information about integrated, whole-body responses to hemodynamic and other factors that occur after placement of an AVG or creation of an AVF. They also provide dose–response and safety information that can guide human studies of new therapeutic agents. However, animal models are expensive and have important limitations that are described below.
Table 1.
Animal models of hemodialysis access dysfunction
| Animal/Access | Location/Configuration | Advantages | Limitations |
| Mouse | |||
| AVF | End of CA to side of JV | Transgenic animals available; numerous commercially available assay tools; inexpensivea | Blood flow parameters (wall shear stress and velocity) different than humana; geometry not used in humans (vein anastomosed to side of artery in human) |
| AVF | Side of aorta to side of vena cava | Easy to perform | Induces congestive heart failure |
| AVF | End of JV to side of CA | Similar geometry to humans | Technically challenging to perform |
| AVG | 2-mm microvascular catheter anastomosed between CA and JV | Easy to perform | Catheter material not the same as graft material used in humans |
| Rat | |||
| AVF | End of CA to side of JV | Relatively inexpensive; relatively easy to perform | Geometry not used in humans (vein anastomosed to side of artery in humans) |
| AVF | End of EPV to side of FA | Similar geometry to humans | Technically challenging to perform |
| AVF | End of lateral vein to side of ventral artery in tail | Easy access to vessels for perivascular application of drug | Technically challenging to perform; scar tissue formation can compress AVF |
| AVF | End of FA to side of FV | Relatively easy to perform | Geometry not used in human |
| Dog | |||
| AVF | End of FV to side of FA | Similar geometry to humans; blood flow parameters similar to humans, particularly in the peripherally placed access locations | Slow development of NH; companion animals; measures may be needed to prevent animal’s access to wounda |
| AVG | Graft anastomosed between FA and FV | ||
| AVG | Graft anastomosed between CA and JV | ||
| Sheep | |||
| AVF | Side FA to side of FV | Geometry similar to that used in humans. Blood flow parameters similar to that in humans, particularly in the peripherally placed access locations. | Expensive; cumbersome to handle; genome not completely sequenced; limited assay tools |
| AVG | Graft anastomosed between JV and CA | ||
| Swine | |||
| AVF | End of FV to side of FA | Similar geometry to humans; blood flow parameters similar to humans, particularly in the peripherally placed access locations; animal cannot access wound. | Expensive; cumbersome to handle; fewer commercially available; limited assay tools; genome not completely sequenced; with FA-FV, edema in limb can occur and restrict mobility; with CA-JV, bilateral access can result in cerebral ischemia and stroke |
| AVF | End of JV to side of CA | ||
| AVF | Side of FV to side of FA | ||
| AVG | Graft anastomosed between JV and CA | ||
| Nonhuman primates | |||
| AVG | Side of aorta to side of vena cava | Blood flow parameters similar to humans, particularly in peripherally placed access locations; phylogenetically close to humans | Very expensive and cumbersome to handle; highly sentient |
| AVG | Axial artery to brachial vein |
These advantages or limitations apply to all models using this animal. AVF, arteriovenous fistula; CA, carotid artery; JV, jugular vein; AVG, arteriovenous graft; EPV, epigastric vein; FA, femoral artery; FV, femoral vein; NH, neointimal hyperplasia.
AVFs have been created in mice by anastomosing the end of the carotid artery to the side of the jugular vein (5). This configuration differs from the configuration of human AVFs, which are usually created through anastomoses between the end of a vein and the side of an artery. Recently, a mouse AVF was created by anastomosing the end of the external jugular vein and the side of the common carotid artery (6). Although creating an AVF with this configuration is more technically challenging, the resulting hemodynamics more closely approximate the hemodynamics of AVFs used for dialysis. Advantages of using mouse models include low cost compared with large animals, the availability of assay reagents, and the ability to study the influence of gene expression on access function through the use of available or relatively easily-created transgenic animals (7–11). Additionally, because mouse models of kidney failure are well established, mouse AVFs provide the important opportunity to study AV access treatments in the presence of uremia. Indeed, AVFs created in uremic mice have more pronounced neointimal hyperplasia, inflammation (12), and endothelial barrier dysfunction compared with AVFs created in the setting of preserved kidney function (13). Because of the small size of mouse vessels, creation of an AVF requires substantial surgical skill, and wall shear stress and blood velocities can be orders of magnitude greater than in human AV accesses. These hemodynamic characteristics likely contribute to the accelerated pace of neointimal hyperplasia in this model (8). It is less technically challenging to create an AVF in a rat compared with a mouse, but as with the mouse, the AV access blood flow parameters differ from those parameters in humans (14–16).
To better replicate human hemodynamics, AV access models have been developed using larger animals, such as dogs, sheep, swine, and nonhuman primates. Some of these models use peripheral locations, but others use central vascular locations, where the suture sites are less likely to be disturbed by the animal. However, this benefit is counteracted by higher blood flow rates in the central arteries and the possibility of lower resistance in the central veins compared with the peripheral locations that are usually used in humans. The development of a neointima is typically slower in dogs compared with other animals (17), suggesting that the dog AV access provides a better representation of the human AV access; however, this finding also means that longer studies are required to evaluate effects of interventions that target intimal hyperplasia.
The vascular system of swine is similar in size and flow parameters to that of humans. As in humans, neointimal hyperplasia in the pig develops predominantly at the vein–graft anastomosis; however, the process is more rapid, typically evident within 4–6 weeks (18–23). Misra et al. (24–26) have developed a porcine model of renal insufficiency that provides a more relevant background for evaluating treatment effects and the pathophysiology of AVG and AVF, but use of this model is costly and technically challenging.
Baboons have been used to study effects of hemodynamics and antithrombotic therapy on neointima development in AVGs (27,28) and to evaluate bioengineered grafts (29). However, baboon purchase and care is expensive, and there is significant public opposition to the use of nonhuman primates in research.
Novel Therapies
This review focuses on therapies to prevent or treat AV access failure that are delivered locally through endovascular approaches or perivascular administration (Table 2).
Table 2.
Localized approaches for AV access dysfunction
| Approach | Comments |
| Endovascular approaches | |
| PTA | Restores patency of thrombosed AVGs and AVFs |
| May enhance maturation of AVFs | |
| Role in preventing access thrombosis unclear | |
| Stents: bare metal and drug-eluting | Newer metallic alloys, such as nitinol, may be more efficacious than older stainless steel stents; stents can fracture, move, and restrict later access revisions; efficacy of drug-eluting stents in the hemodialysis access setting has not been evaluated by a clinical trial |
| Drug-eluting balloon angioplasty (Table 3) | No placement of permanent scaffold |
| Stent grafts | |
| Nonbiodegradable stent grafts | Appear to be efficacious; stent grafts can fracture or move and restrict later access revisions |
| Bioresorbable stent grafts | Can deliver pharmacologic agents and provide temporary intravascular support; have not been assessed in dialysis access |
| VIABAHN endoprosthesis stent graft coated with bioactive heparin | Heparin-induced thrombocytopenia is possible but unlikely; clinical trial underway to assess efficacy after PTA in AVG |
| Cutting balloon angioplasty | Sharp atherotomes embedded in the balloon score the stenotic lesion longitudinally to create more controlled disruption of the lesion |
| Cryotherapy | |
| Brachytherapy | |
| Gene therapy: intraluminal application, naked plasmid DNA vectors, adenoviral vectors | Possible immune rejection and tumorigenesis |
| Microinfusion catheter | Delivers therapeutic agent into the vessel wall and pervascular space through microneedles; avoids systemic exposure |
| Perivascular approaches | |
| Transfection of the adventitial layer with genes encoding antiproliferative proteins | Avoids disturbance of the endothelium |
| Introduction of endothelial cells that provide beneficial vasoactive molecules using a gelatin-based cell-seeded sponge | Uses allogeneic cells |
| Application of autologous adipose tissue as drug depot for glitazone drug and biofactory for adiponectin | Uses autologous cells |
| Recombinant human type I pancreatic elastase | |
| Application of drug-laden biodegradable polymer at time of access creation and/or by ultrasound-guided injection after creation | Paclitaxel, dipyridamole, and sirolimus have been delivered; polymers can induce foreign body reactions |
| Far-infrared treatment | Electromagnetic therapy that induces vasodilation and vasoprotective gene expression |
| Novel grafts or engineered vessels | |
| Grafts | |
| Heparin-bonded synthetic graft (Propaten graft, Gore) for AVG | Heparin-induced thrombocytopenia is possible but unlikely |
| Optiflow (Bioconnect Systems, Inc.) synthetic anastomotic implant | Configuration is an AVF but uses synthetic graft material for anastomosis creation Initial studies in 10 hemodialysis patients showed 100% technical success and 90% patency at 6 wks (96) |
| Tissue-engineered blood vessel | |
| Lifeline (Cytograft Tissue Engineering, Inc.) | Engineered vessel constructed from autologous fibroblasts and endothelial cells isolated from dermal and superficial vein biopsies; 6- to 9-mo vessel production time before implant |
| Decellularized porcine carotid arteries populated with sheep endothelial cells and preconditioned for flow | Withstood repeat needling three times a week for 6 mo in a sheep AVG model; 5-wk vessel production time |
| Polymer scaffold seeded with human allogeneic cells collected from cadaver donors and then decellularized for off-the-shelf use | Clinical trials underway in Europe and the United States |
PTA, percutaneous transluminal angioplasty.
Endovascular Approaches
Percutaneous Balloon Angioplasty.
The recommended approach to treating stenosis in either AVGs or AVFs is percutaneous transluminal angioplasty (PTA) (30). Although PTA can restore patency and function of accesses with thrombosis, the benefits are less clear for accesses that are stenosed but still patent (31–36). Balloon-induced injury may stimulate more aggressive neointimal hyperplasia by damaging endothelial cells and stimulating smooth muscle cell proliferation.
Drug-eluting balloons compress a stenotic lesion and target an antiproliferative drug to the lesion (Table 3) (37,38). Katsanos et al. (39) compared the effects of PTA with a paclitaxel-eluting balloon or a conventional balloon on stenotic lesions in AVGs and AVFs. At 6 months, the primary patency rate was 70% in the paclitaxel-eluting balloon group compared with 25% in the conventional PTA group. Drug-eluting balloons have a number of benefits over drug-eluting stents: (1) they provide uniform delivery of drug to the entire stenotic lesion, whereas delivery from stents is not homogeneous, (2) they do not require placement of a scaffold that could later preclude other endovascular interventions, and (3) they can be used to treat complex lesions and longer vessel segments. Drug-eluting balloons do not have regulatory approval in the United States.
Table 3.
Drug delivery balloon technology
| Name | Principle | Manufacturer |
| Paccocath | Paclitaxel embedded in hydrophilic iopromide coating | Bayer (Bavaria Medizin Technologie, Oberpfaffenhofen, Germany) |
| SeQuentPlease | Paclitaxel embedded in hydrophilic iopromide coating | B. Braun Melsungen AG (Melsungen, Germany) |
| Coroflex DEBlue | Combination of a paclitaxel-eluting balloon and a bare metal stent | B. Braun Melsungen AG |
| DIOR PTCA | Paclitaxel-coated microporous balloon | Eurocor (Bonn, Germany) |
| MAGICAL | Paclitaxel-coated balloon combined with a bare metal stent | Eurocor |
| Elutex | Balloon is coated with a hydrogel; on expansion, paclitaxel is released from balloon to gel; gel is retained on vessel wall for prolonged drug delivery and decreased washout | Aachen Resonance (Aachen, Germany) |
| GENIE | Delivers antiproliferative drug (e.g., paclitaxel) in liquid form | Acrostak Corp. (Winterthur, Switzerland) |
| IN.PACT Amphirion | A wrapped balloon combines urea and paclitaxel molecules to increase paclitaxel solubility | Medtronic Endovascular (Minneapolis, MN) |
| IN.PACT Admirala | A folding balloon structure delivers paclitaxel | Medtronic Endovascular (Minneapolis, MN) |
| Advance PTX | Balloon coated with paclitaxel | Cook Medical (Bloomington, IN) |
| Lutonix DCB | Paclitaxel and proprietary carrier coated on balloon | Lutonix Inc (New Hope, MN) |
None of these devices is currently approved for use in the United States. Modified from ref 37.
A clinical trial in Singapore (Prospective Randomized Trial Comparing DEB Versus Conventional PTA for the Treatment of Hemodialysis AVF or AVG Stenoses; NCT01544907) is underway to assess the efficacy of this drug-eluting balloon versus conventional percutaneous transluminal angioplasty for treatment of arteriovenous graft and arteriovenous fistula stenosis.
Stents.
Because early trials showed that the unassisted patency rate of AVGs did not differ with stenting than with PTA alone (40–42), stent placement was recommended only in the setting of elastic stenoses or PTA failure. However, a recent prospective study of 61 patients with thrombosed AVG reported significantly higher patency rates after placement of a nitinol stent compared with PTA alone (43). Also, improved patency of stenosed AVGs with placement of nitinol stents compared with PTA alone has been reported (44,45). Using a porcine AVG model, sirolimus-eluting stents showed greater patency and flow rates at 4 weeks compared with bare metal stents or unstented AVG (46).
The 12-month interim results of a 2-year study (Post-Approval Study for the FLAIR Endovascular Stent Graft; NCT00677235) assessing the primary AVG patency rates after treatment of stenotic lesions with stent grafts (covered stents) or PTA alone indicate that the stent graft is associated with significantly greater primary patency rates (24.1% versus 10.3%; P=0.005) (published in abstract form only). A previous 6-month trial comparison between stent grafts and PTA alone also reported better patency rates with the stent grafts (47). However, in the 6-month report, the graft thrombosis rates were not significantly different than the rates after PTA alone, and graft survival rates were not reported. A phase III randomized open-label clinical trial (GORE VIABAHN Endoprosthesis Versus Percutaneous Transluminal Angioplasty (PTA) to Revise AV Grafts in Hemodialysis; NCT00737672) is underway to test the safety and efficacy of a stent graft with a heparin bioactive surface (PROPATEN graft; Gore, Inc., Flagstaff, AZ) in AVG. The study will compare primary patency rates with the stent grafts with PTA alone. Another randomized clinical trial is testing the safety and efficacy of a stent graft to treat in-stent restenosis of bare metal stents in the venous outflow of AVFs and AVGs and will be concluded in 2014 (FLUENCY PLUS Endovascular Stent Graf for In-Stent Restenosis; NCT01257438). Unfortunately, stent grafts and stents can limit the ability to perform other access revisions, and they can fracture or move. Polymer or metallic alloy bioresorbable stents provide a temporary support for the vessel wall but degrade over time, mitigating such factors. In a clinical trial of single de novo coronary lesions treated with an everolimus-eluting bioresorbable polymer stent (48), the stents were shown to be safe; no restenoses or thromboses were reported, and the stented arteries were observed to retain vasomotor responses (49). Use of biodegradable stents for hemodialysis vascular access has not been reported.
Cutting Balloon Angioplasty.
The use of cutting balloons for treatment of high-pressure balloon-resistant stenotic lesions in AVGs and AVFs has been described in clinical reports and small studies (50–54). A multicenter randomized clinical trial of 340 patients with venous outflow stenoses reported equivalent 6-month patency rates between PTA alone and cutting balloon angioplasty, but the cutting balloon approach experienced a greater number of device-related complications, including venous rupture and dissections (55). Another randomized clinical trial is currently recruiting to assess the primary 1-year patency rate after treatment of vascular access stenotic lesions with cutting balloon angioplasty or PTA (Cutting Balloon Versus Non-Cutting Balloon for the Treatment of Venous Stenosis in the Fistulas of Hemodialyzed Patients; NCT01321866).
Cryotherapy.
Another endovascular approach is cryotherapy, in which a balloon catheter (PolarCath; Boston Scientific) inflated with nitrous oxide gas compresses the neointima and cools the vessel wall to −5 to −10°C. Huijbregts et al. (56) compared preventive cryoplasty with conventional PTA on the vein–graft anastomosis in a porcine bilateral AVG model. At 4 weeks, a nonsignificant decrease in the intimal/media ratio was observed in the cryoplasty-treated vein–graft anastomoses compared with the PTA-treated grafts. However, outcomes from clinical studies have been mixed. In one study, cryoplasty of neointimal hyperplasia in the AVGs of five patients increased the time to restenosis or thrombosis from 3 weeks to greater than 16 weeks (57). Another study tested cryoplasty in 18 patients with preexisting AVG neointimal hyperplasia and noted that, although the procedure was safe, there were low anatomic success rates; also, patients reported that the procedure was more painful than PTA (58).
Brachytherapy.
Endovascular brachytherapy is the delivery of ionizing radiation to the luminal surface of the vessel. This procedure is considered safe and technically feasible, but outcomes of animal and clinical studies have been varied. El Sharouni et al. (59) reported that brachytherapy (12 Gy) administered 48 hours after AVG placement in patients did not inhibit stenosis development. In a multicenter trial, 30 patients receiving AVG were randomized to receive brachytherapy. At 1 year, the stenosis rate in the brachytherapy group was 56% compared with 37% in the control group (60). A multicenter clinical trial (Beta Radiation for Treatment of Arterial-Venous Graft Outflow) recruited 25 patients with a single stenosis at the vein–graft anastomosis of AVG for randomization to either radiation (18.4 Gy) after PTA or sham intervention after PTA alone (61). At 6 months, target lesion primary patency was significantly improved in the brachytherapy group (41.6% versus 0%; PTA alone); but 1-year thrombosis and patency rates were the same.
Endovascular Gene Delivery.
Efficient gene transfection of the vein wall after intraluminal delivery of naked plasmid DNA in a rat model of AVF was recently reported (15). Reporter gene expression was detected in the endothelium, adventitia, and media of the fistula vein at least 21 days after injection, with no evidence of inflammation. The AVFs were injected with plasmid DNA coding for the gene endothelial nitric oxide synthase (pl-eNOS) with the expectation that transfected AVF would have less neointima development, because endothelial nitric oxide inhibits cell migration and induces vasodilation. However, neointimal hyperplasia was markedly increased in the pl-eNOS–transfected AVFs, possibly because extraordinarily high levels of eNOS expression resulted in the production of excessive amounts of reactive nitrogen species and oxidative stress. The gene for β-adrenergic receptor kinase C terminus has been delivered to the jugular vein segment of AVG in swine (62). Significant inhibition of neointimal hyperplasia at the vein–graft anastomosis at 4 weeks occurred compared with mock-transfected AVG. However, a 100% stenosis rate was observed in the mock-transfected AVG that is much higher than stenosis rates reported by others in that swine model (63,64).
Microinfusion Catheters.
Microinfusion catheters are a novel means to deliver drugs or proteins from the lumen to the vessel wall and perivascular space. These catheters (Mercator Medsystems, San Leandro, CA) are similar to an angioplasty balloon, except that, on inflation, a small needle punctures the vessel wall and delivers the therapeutic agent. This approach has been approved by the US Food and Drug Administration for the delivery of drugs that have been previously approved for vascular injection.
Perivascular Approaches
Application of biologic or pharmacological agents to the adventitial layer of the blood vessel is appealing given that (1) the adventitial and medial layers are thought to be the source of many of the cells that populate the neointima (20,65–67), (2) application of treatments to the perivascular region can be readily accomplished during vascular access creation and also, long after access surgery through image-guided subcutaneous injection, and (3) denudation of the endothelial layer is avoided.
Biodegradable Polymer Gels.
Drugs, genes, or cells can be delivered to the perivascular region by means of solutions or biodegradable films or gels. A single application of a biodegradable polymer gel containing dipyridamole to the vein–graft anastomosis in a porcine model of AVG achieved tissue drug concentrations in the therapeutic range but did not inhibit neointimal hyperplasia (68). A single application of a polymer gel incorporated with the antiproliferative agent, paclitaxel, did inhibit neointimal hyperplasia in AVG in a dog model (17). Repeat application of a sirolimus-laden polymer gel through ultrasound-guided injection to the vein–graft anastomosis significantly inhibited neointimal hyperplasia at 6 weeks in the swine AVG model (63,69). Perivascular application of expression vectors to alter in vivo vascular gene expression to promote better access outcomes has been reported. C-type natriurietic peptide (CNP) is an endothelial-derived protein that inhibits smooth muscle cell migration and inhibits thrombosis. A solution of recombinant adenovirus-encoding mouse CNP was painted directly onto the adventitial surface of AVG in a porcine model at the time of graft placement (22). Although no decrease in neointimal hyperplasia was observed at 2 weeks, a significant increase in lumen area at the venous anastomosis occurred in the CNP-transfected AVG compared with the bilateral AVG transfected with empty adenovirus vector. Thus, this treatment may promote outward remodeling, which would be anticipated to be beneficial.
Biodegradable Polymer Wraps.
Biodegradable polymer wraps have been used to deliver antiproliferative drugs to the perivascular region of hemodialysis access vessels in both animal models (70) and patients. In animal studies, perivascular wraps that elute paclitaxel were beneficial in inhibiting neointimal hyperplasia in AVGs (71). The paclitaxel wrap combination (Vascular Wrap; Angiotech) was shown to be beneficial in preventing neointimal hyperplasia in peripheral arterial bypass grafts in a European cohort. However, a clinical trial in the United States assessing the use of this approach to inhibit neointimal hyperplasia in AVGs was terminated early because of an increase in graft infections (NCT00448708). Collagen-based wraps have been used to deliver sirolimus in animal models (72) and replication-deficient adenoviral vector expressing the gene for vascular endothelial growth factor–D in humans (Adenovirus Vascular Endothelial Growth Factor Therapy in Vascular Access-Novel Trinam AGainst Control Evidence; NCT00895479).
Cell-Based Delivery.
Seeding endothelial cells to AVGs and AVFs through perivascular administration at the time of access creation has been developed based on the presumption that endothelial cells will provide beneficial vasoactive substances to the cells of the vessel wall (73–75). Nugent et al. (76) cultured porcine aortic allogeneic endothelial cells in a gelatin-based sponge that was then wrapped around the side-to-side anastomosis of femoral artery and femoral vein in pigs. At 2 months, the neointimal hyperplasia index for the endothelial cell-treated AVFs was 68% lower than in controls treated with the gelatin-based sponge alone. A randomized, double-blinded, phase I/II trial investigated the safety of this approach in the AVG and AVF of patients (77). Sponges seeded with allogeneic endothelial cells collected from cadaver aortas (Vascugel) were wrapped around the anastomoses and outflow of the newly created AVG or applied to the anastomosis region and vein outflow of AVF. At 24 weeks, there were also no differences in primary or assisted primary patency rates; however, post hoc analysis of the data suggested that Vascugel treatment was associated with enlargement of the fistula vein in the subgroup of diabetic patients (78). A potential problem with this approach is immunosensitization resulting from the use of allogeneic cells, which is a particular concern for patients who are planning to undergo kidney transplantation. A multicenter phase II clinical trial is currently recruiting patients to assess the efficacy and safety of Vascugel in AVF maturation (NCT01806545). Patients for whom organ transplantation is anticipated are excluded from participation because of the possibility of immunosensitization.
Laborious methods for ex vivo seeding of synthetic grafts with endothelial cells have been developed in an effort to create nonthrombogenic graft surfaces that diminish neointimal hyperplasia. Successful in vivo endothelialization of a synthetic AVG has been accomplished in a porcine model by coating the graft surface with anti-CD34 antibodies. However, an increase in neointimal hyperplasia was observed, and it was postulated that the captured progenitor cells developed into proliferating nonendothelial cells and/or that the captured cells released proliferative growth factors to stimulate cell proliferation/migration from the native cell wall (79,80).
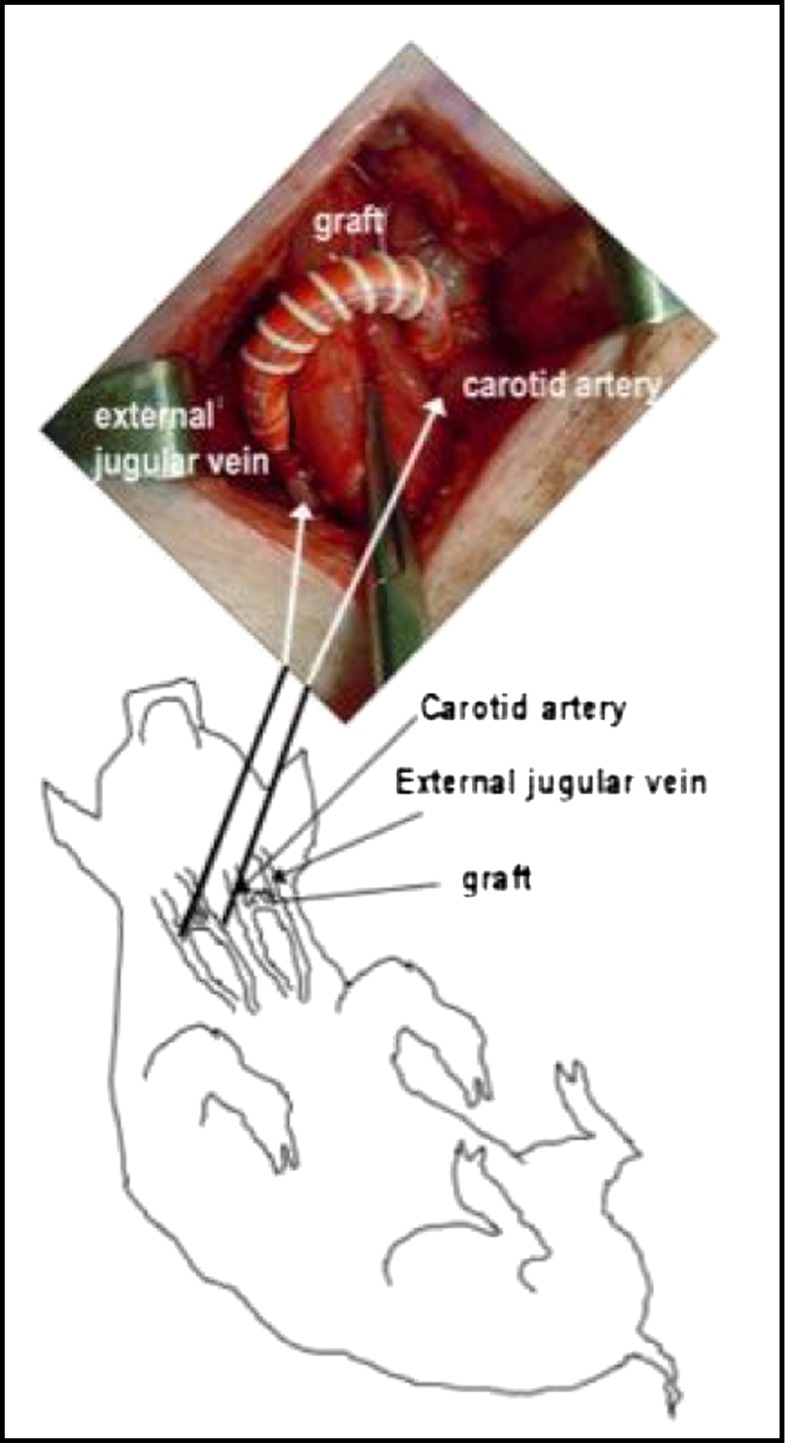
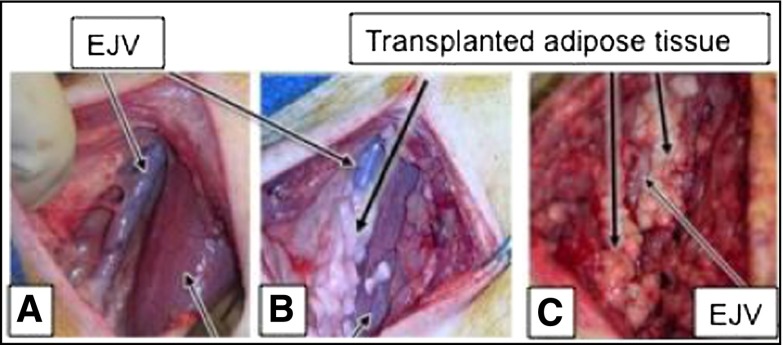
Another cell-based approach is to apply autologous subcutaneous adipose tissue transplants to the perivascular surface of accesses at time of creation. The adipose tissue is mixed with insulin-sensitizing drugs (pioglitazone or rosiglitazone) before transplantation to the perivascular surface of the access veins. Thiazolidinediones induce adipose tissue production of adiponectin, a protein that has many vasculoprotective effects, including inhibition of smooth muscle cell proliferation and inflammation. The adipose/glitazone mixture is proposed to serve as a biofactory for the production of adiponectin, which will then interact with the cells of the vessel wall. The adipose tissue also provides controlled release of thiazolidinedione, which inhibits inflammation and smooth muscle cell proliferation and promotes the release of nitric oxide. The ability of this approach to inhibit AVG stenosis is being assessed in a porcine AVG model in the laboratory by author C.M.T. (Figures 1 and 2).
Figure 1.
Bilateral arteriovenous grafts placed between the common carotid arteries and external jugular veins in swine. Modified from ref 63, with permission.
Figure 2.
Autologous adipose tissue transplants for drug delivery and protein production. (A) External jugular vein (EJV) in swine before adipose tissue transplantation. (B) EJV directly after adipose tissue placement. (C) Adipose tissue grafted perivascular to EJV at 6 days post-transplantation.
Topical Administration of Recombinant Elastase.
Topical administration of recombinant human elastase is being studied in clinical trials of newly created AVFs and AVGs. The hypothesis is that degradation of elastin in the vessel wall will allow greater early dilation after access creation, resulting in favorable hemodynamics. Additionally, it is postulated that elastin fragments have chemotactic properties that could inhibit migration of myofibroblasts from the adventitial to the intima and thereby, inhibit neointimal hyperplasia. An early phase placebo-controlled, dose-escalation trial of human type I pancreatic elastase (NCT01305824) applied to the adventitial surface of the newly created AVFs found that the treatment was well tolerated (81). Larger trials of human type I pancreatic elastase for AVFs (NCT01305824) and AVGs (NCT01001351) have recently been completed, and the results are forthcoming.
Far-Infrared Electromagetic Radiation.
The use of far-infrared electromagnetic radiation is not, strictly speaking, a perivascular approach, because it is applied to the external surface of the arm containing an AV access. This treatment involves the application of electromagnetic radiation (wavelengths of 3–25 μm) to the skin surface overlying the vascular access. The electromagnetic radiation, which travels 2–3 cm below the skin surface, is hypothesized to be beneficial for the vasculature by inducing vasodilation, decreasing oxidative stress (82), inhibiting cell proliferation (83), and inducing protective enzymes, such as heme oxygenase-1 (84). The emitting device is positioned approximately 25 cm above the arm for 30–60 minutes per treatment. A randomized trial of far-infrared therapy administered three times per week for 1 year to patients with new AVFs was recently published (85). Although there were no differences in vein diameter between the intervention and control groups at any time period evaluated, volumetric flow rates were greater in the intervention group at 1, 3, and 12 months (P=0.001), and the intervention group experienced a higher rate of AVF clinical maturation (P=0.008), which was defined as the ability of the AVF to support dialysis within the 12-month follow-up period. Limitations of the trial include its single-center, nonblinded design and enrollment of patients of a single ethnicity.
Novel Grafts and Engineered Vessels
Significant efforts have been directed to developing better materials for AVGs. Conduits derived from native products, such as bovine carotid arteries or ureter, decellularized native vessels, and a patient’s own transplanted veins, have been used with varying outcomes (86). Bioengineering approaches have been used to create autologous grafts from dermal fibroblasts and endothelial cells obtained through tissue biopsy and expanded in tissue culture (87,88). Clinical trials are currently underway in Europe and South America. A drawback of this approach is a prolonged production time of 6–9 months. Another approach used harvested porcine carotids arteries that were depopulated and reseeded with sheep endothelial progenitor cells captured from peripheral blood (89). The repopulated conduits were placed in a sheep AVG model, and after 1 month, they were punctured three times per week. The conduits withstood repeat puncture, but like standard polytetrafluoroethylene grafts, they failed because of neointimal hyperplasia.
Dahl et al. (90) reported the development of a synthetic polymer scaffold incorporated with allogeneic human vascular cells from cadaver donors. After culturing for 8–10 weeks, the cells deposit an extracellular matrix onto the scaffold, and the grafts are decellularized to remove immunogenic proteins. The grafts were tested in a baboon model of arteriovenous access, with seven of eight grafts remaining patent during the observation periods of 1, 3, or 6 months. Very recently, a conduit created using this method was placed in a patient with ESRD as part of a United States–based phase I trial with 20 patients (91). Clinical trials are also underway in Europe.
Outcome Measures for Clinical Trials
Adequate evaluation of the efficacy of the novel interventions described above requires properly designed clinical trials with well-developed outcome measures. Establishing outcome measures for vascular access interventions is in the early stages but progressing. Recent efforts by groups comprised of surgeons, interventional radiologists, and nephrologists to generate standardized terminology and definitions for access function and complications should promote harmonization across studies that will be important for developing outcome measures as well as comparing results across trials (30,92,93). In the absence of well validated surrogate end points, regulatory approval of a new treatment usually requires demonstration of benefit on clinical outcomes. For vascular access interventions, clinical outcomes often relate to usability of the access. Although seemingly straightforward, establishing criteria for access use outcomes has challenges. For AVFs, clinical use outcomes for trials evaluating interventions designed to promote maturation might differ from outcomes for interventions designed to promote long-term access function. Because the time period between access creation and its initial use can vary substantially, especially if the vascular access is created before the initiation of maintenance dialysis, outcomes that incorporate time to access failure can be problematic, particularly if access use, through cannulation trauma or deleterious hemodynamics, contributes to access failure. Excluding from trial participation the subset of patients who are not yet receiving dialysis treatment at the time of access creation should partially mitigate this problem, but it also reduces generalizability of the findings and would likely substantially delay recruitment. An alternative is to begin the time-to-failure clock at initial use of the access; however, there is not an obvious method for assigning failure time for accesses for which use is never attempted.
For interventions targeting AVF maturation failure, it is not clear how to incorporate maturation-enhancing procedures into the access use outcome. The need for a procedure may suggest maturation failure; however, if the AVF ultimately was used for dialysis, an alternative perspective is that maturation was successful. Because performance of a maturation-enhancing procedure is often driven by subjective assessments by treating clinicians, classifying AVFs that had procedures as maturation failures might place excessive reliance on clinical decisions to define the outcome. These issues are also relevant to AVG trials because prophylactic repair procedures are driven by a variety of factors that can be difficult to standardize.
An additional challenge is that access use outcomes can be affected by multiple factors that are remote from the direct effects of the intervention. For example, an outcome based on ability to use a new AVF for dialysis could be affected by clinical decisions about when to initiate use of the AVF or the skill of the individuals performing AVF cannulation—variables that cannot be controlled in a clinical trial and do not reflect the effects of the intervention. If clinical use of an AVF is an outcome for maturation-enhancing interventions, what duration of use should be considered sufficient for defining the AVF as having successful maturation? Should a fistula be required to be usable within a specific time period after surgical creation to be categorized as having successful maturation? If so, what is the appropriate time period?
The cost of conducting clinical trials is an important barrier to developing new treatments for vascular access dysfunction. Surrogate outcomes, although typically not adequate for pivotal clinical trials, can facilitate shorter, less-expensive, early phase evaluations of interventions. Ultrasonographic characteristics, such as blood flow rate and vessel diameter within the first few months after AVF creation, have been proposed as surrogate outcomes for evaluating AVF maturating-enhancing interventions. Although clinical practice guidelines recommend the use of ultrasound for early clinical assessment of AVF maturation (94), the suitability of ultrasonographic parameters as surrogate outcomes for clinical trials is not known. In the National Institutes of Health–sponsored Hemodialysis Fistula Maturation (HFM) Study, a prospective, multicenter, observational study that is currently underway, serial ultrasounds are being performed at uniform time points after AVF creation (95). The rigorous evaluation by the HFM Study of the utility of ultrasounds for predicting AVF maturation should provide important information about the appropriateness of using ultrasonographic characteristics as surrogate outcomes in clinical trials.
Unique Advantages and Challenges for Trials in Vascular Access
From a clinical trial perspective, there are several advantages of studying interventions for vascular access failure. The frequency of access failure (i.e., high event rate) reduces the necessary sample size and/or duration of follow-up needed to detect an effect of the intervention. The ability to follow trial participants in outpatient dialysis settings can reduce the burden to participants of repeated study visits (at least for visits that do not require specialized evaluations, such as ultrasound or other imaging). The superficial location of AV access allows for relatively easy assessment of certain surrogate or intermediate outcomes, and it also allows for repeated administration of localized therapies after its activity diminishes. The potential applicability of therapies for vascular access failure to other conditions, such as peripheral arterial disease or coronary artery disease, provides efficiencies that are advantageous and of interest to the biopharmaceutical industry.
A challenge for vascular access trials is the technical expertise necessary for administration of many of the novel interventions currently under development. Additionally, the underappreciation by many patients and clinicians of the clinical significance of vascular access failure can make it difficult to enroll participants in trials of novel treatments that have uncertain risks. For example, it might be more difficult to enroll patients in first-in-man studies that evaluate cells or viral vectors to deliver the intervention if the target is vascular access failure rather than heart failure or cancer. Efforts to increase awareness of the clinical importance of vascular access failure might reduce this challenge.
Development of effective approaches to prevent and treat vascular access failure is clearly challenging. Agents that seem to work in animals often do not work in the clinical setting, and efficacy of interventions for coronary artery disease or peripheral arterial disease does not necessarily translate into benefit in the context of an artery–vein connection or conduit rather than an artery–artery conduit, or in the setting of uremia. Immunogenicity of cell-based therapies might interfere with future kidney transplantation, the use of stents or stent grafts can limit future surgical revisions, and the perception that vascular access failure is not a highly significant clinical event can limit acceptance of novel experimental treatments. However, the high interest level by academic investigators in multiple disciplines, the growing interest and commitments from the biotechnology and pharmaceutical industries, and the broad array of therapeutic agents and delivery systems that are currently in preclinical and clinical phases of investigation provide strong indications that effective novel interventions in the near future are a distinct reality.
Disclosures
L.M.D. is a member of the Clinical Advisory Board of Proteon Therapeutics and has received research funding from Proteon Therapeutics, the manufacturer of human type I pancreatic elastase, which is discussed in the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, Dember LM, Himmelfarb J, Gassman JJ, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Fenves AZ, Kaufman JS, Cotton JR, Jr., Martin KJ, McNeil JW, Rahman A, Lawson JH, Whiting JF, Hu B, Meyers CM, Kusek JW, Feldman HI; DAC Study Group: Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med 360: 2191–2201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lok CE, Moist L, Hemmelgarn BR, Tonelli M, Vazquez MA, Dorval M, Oliver M, Donnelly S, Allon M, Stanley K; Fish Oil Inhibition of Stenosis in Hemodialysis Grafts (FISH) Study Group: Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: A randomized controlled trial. JAMA 307: 1809–1816, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee T: Novel paradigms for dialysis vascular access: Downstream vascular biology—is there a final common pathway? Clin J Am Soc Nephrol 8: 2194–2201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remuzzi A, Ene-Iordache B: Novel paradigms for dialysis vascular access: Upstream hemodynamics and vascular remodeling in dialysis access stenosis Clin J Am Soc Nephrol 8: 2186–2193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castier Y, Lehoux S, Hu Y, Foteinos G, Tedgui A, Xu Q: Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. Kidney Int 70: 315–320, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Wong CY, de Vries MR, Wang Y, van der Vorst JR, Vahrmeijer AL, van Zonneveld AJ, Roy-Chaudhury P, Rabelink TJ, Quax PH, Rotmans JI: Vascular remodeling and intimal hyperplasia in a novel murine model of arteriovenous fistula failure [published online ahead of print May 15, 2013]. J Vasc Surg, 10.1016/j.jvs.2013.02.242 [DOI] [PubMed] [Google Scholar]
- 7.Abeles D, Kwei S, Stavrakis G, Zhang Y, Wang ET, García-Cardeña G: Gene expression changes evoked in a venous segment exposed to arterial flow. J Vasc Surg 44: 863–870, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kang L, Yamada S, Hernandez MC, Croatt AJ, Grande JP, Juncos JP, Vercellotti GM, Hebbel RP, Katusic ZS, Terzic A, Nath KA: Regional and systemic hemodynamic responses following the creation of a murine arteriovenous fistula. Am J Physiol Renal Physiol 301: F845–F851, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang L, Grande JP, Farrugia G, Croatt AJ, Katusic ZS, Nath KA: Functioning of an arteriovenous fistula requires heme oxygenase-2. Am J Physiol Renal Physiol 305: F545–F552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders WG, Morisseau C, Hammock BD, Cheung AK, Terry CM: Soluble epoxide hydrolase expression in a porcine model of arteriovenous graft stenosis and anti-inflammatory effects of a soluble epoxide hydrolase inhibitor. Am J Physiol Cell Physiol 303: C278–C290, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA: Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int 74: 47–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokubo T, Ishikawa N, Uchida H, Chasnoff SE, Xie X, Mathew S, Hruska KA, Choi ET: CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol 20: 1236–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang A, Wang Y, Han G, Truong L, Cheng J: Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol 304: F1413–F1420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croatt AJ, Grande JP, Hernandez MC, Ackerman AW, Katusic ZS, Nath KA: Characterization of a model of an arteriovenous fistula in the rat: The effect of L-NAME. Am J Pathol 176: 2530–2541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Globerman AS, Chaouat M, Shlomai Z, Galun E, Zeira E, Zamir G: Efficient transgene expression from naked DNA delivered into an arterio-venous fistula model for kidney dialysis. J Gene Med 13: 611–621, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Lin T, Horsfield C, Robson MG: Arteriovenous fistula in the rat tail: A new model of hemodialysis access dysfunction. Kidney Int 74: 528–531, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Masaki T, Rathi R, Zentner G, Leypoldt JK, Mohammad SF, Burns GL, Li L, Zhuplatov S, Chirananthavat T, Kim SJ, Kern S, Holman J, Kim SW, Cheung AK: Inhibition of neointimal hyperplasia in vascular grafts by sustained perivascular delivery of paclitaxel. Kidney Int 66: 2061–2069, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kelly BS, Narayana A, Heffelfinger SC, Denman D, Miller MA, Elson H, Armstrong J, Karle W, Nanayakkara N, Roy-Chaudhury P: External beam radiation attenuates venous neointimal hyperplasia in a pig model of arteriovenous polytetrafluoroethylene (PTFE) graft stenosis. Int J Radiat Oncol Biol Phys 54: 263–269, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Terry CM, Kim SE, Li L, Goodrich KC, Hadley JR, Blumenthal DK, Parker DL, Cheung AK: Longitudinal assessment of hyperplasia using magnetic resonance imaging without contrast in a porcine arteriovenous graft model. Acad Radiol 16: 96–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Terry CM, Blumenthal DK, Kuji T, Masaki T, Kwan BC, Zhuplatov I, Leypoldt JK, Cheung AK: Cellular and morphological changes during neointimal hyperplasia development in a porcine arteriovenous graft model. Nephrol Dial Transplant 22: 3139–3146, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kuji T, Masaki T, Li L, Cheung AK: Expression of C-reactive protein in myointimal hyperplasia in a porcine arteriovenous graft model. Nephrol Dial Transplant 22: 2469–2475, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rotmans JI, Verhagen HJ, Velema E, de Kleijn DP, van den Heuvel M, Kastelein JJ, Pasterkamp G, Stroes ES: Local overexpression of C-type natriuretic peptide ameliorates vascular adaptation of porcine hemodialysis grafts. Kidney Int 65: 1897–1905, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Rotmans JI, Velema E, Verhagen HJ, Blankensteijn JD, Kastelein JJ, de Kleijn DP, Yo M, Pasterkamp G, Stroes ES: Rapid, arteriovenous graft failure due to intimal hyperplasia: A porcine, bilateral, carotid arteriovenous graft model. J Surg Res 113: 161–171, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Misra S, Gordon JD, Fu AA, Glockner JF, Chade AR, Mandrekar J, Lerman L, Mukhopadhyay D: The porcine remnant kidney model of chronic renal insufficiency. J Surg Res 135: 370–379, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Misra S, Fu AA, Puggioni A, Glockner JF, Rajan DK, McKusick MA, Bjarnason H, Mukhopadhyay D: Increased expression of hypoxia-inducible factor-1 alpha in venous stenosis of arteriovenous polytetrafluoroethylene grafts in a chronic renal insufficiency porcine model. J Vasc Interv Radiol 19: 260–265, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Misra S, Misra KD, Glockner JF: Vascular endothelial growth factor-A, matrix metalloproteinase-1, and macrophage migration inhibition factor changes in the porcine remnant kidney model: Evaluation by magnetic resonance imaging. J Vasc Interv Radiol 21: 1071–1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraiss LW, Geary RL, Mattsson EJ, Vergel S, Au YP, Clowes AW: Acute reductions in blood flow and shear stress induce platelet-derived growth factor-A expression in baboon prosthetic grafts. Circ Res 79: 45–53, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Lundell A, Kelly AB, Anderson J, Marijianowski M, Rade JJ, Hanson SR, Harker LA: Reduction in vascular lesion formation by hirudin secreted from retrovirus-transduced confluent endothelial cells on vascular grafts in baboons. Circulation 100: 2018–2024, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Prichard HL, Manson RJ, DiBernardo L, Niklason LE, Lawson JH, Dahl SL: An early study on the mechanisms that allow tissue-engineered vascular grafts to resist intimal hyperplasia. J Cardiovasc Transl Res 4: 674–682, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vascular Access 2006 Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006 [DOI] [PubMed]
- 31.Lumsden AB, MacDonald MJ, Kikeri D, Cotsonis GA, Harker LA, Martin LG: Prophylactic balloon angioplasty fails to prolong the patency of expanded polytetrafluoroethylene arteriovenous grafts: Results of a prospective randomized study. J Vasc Surg 26: 382–390, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Ram SJ, Work J, Caldito GC, Eason JM, Pervez A, Paulson WD: A randomized controlled trial of blood flow and stenosis surveillance of hemodialysis grafts. Kidney Int 64: 272–280, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Moist LM, Churchill DN, House AA, Millward SF, Elliott JE, Kribs SW, DeYoung WJ, Blythe L, Stitt LW, Lindsay RM: Regular monitoring of access flow compared with monitoring of venous pressure fails to improve graft survival. J Am Soc Nephrol 14: 2645–2653, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Dember LM, Holmberg EF, Kaufman JS: Randomized controlled trial of prophylactic repair of hemodialysis arteriovenous graft stenosis. Kidney Int 66: 390–398, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Robbin ML, Oser RF, Lee JY, Heudebert GR, Mennemeyer ST, Allon M: Randomized comparison of ultrasound surveillance and clinical monitoring on arteriovenous graft outcomes. Kidney Int 69: 730–735, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Work J: Role of access surveillance and preemptive intervention. Semin Vasc Surg 24: 137–142, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Waksman R, Pakala R: Drug-eluting balloon: The comeback kid? Circ Cardiovasc Interv 2: 352–358, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Indermuehle A, Bahl R, Lansky AJ, Froehlich GM, Knapp G, Timmis A, Meier P: Drug-eluting balloon angioplasty for in-stent restenosis: A systematic review and meta-analysis of randomised controlled trials. Heart 99: 327–333, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Katsanos K, Karnabatidis D, Kitrou P, Spiliopoulos S, Christeas N, Siablis D: Paclitaxel-coated balloon angioplasty vs. plain balloon dilation for the treatment of failing dialysis access: 6-month interim results from a prospective randomized controlled trial. J Endovasc Ther 19: 263–272, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Quinn SF, Schuman ES, Demlow TA, Standage BA, Ragsdale JW, Green GS, Sheley RC: Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: Intermediate results. J Vasc Interv Radiol 6: 851–855, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Beathard GA: Gianturco self-expanding stent in the treatment of stenosis in dialysis access grafts. Kidney Int 43: 872–877, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Hoffer EK, Sultan S, Herskowitz MM, Daniels ID, Sclafani SJ: Prospective randomized trial of a metallic intravascular stent in hemodialysis graft maintenance. J Vasc Interv Radiol 8: 965–973, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Kakisis JD, Avgerinos E, Giannakopoulos T, Moulakakis K, Papapetrou A, Liapis CD: Balloon angioplasty vs nitinol stent placement in the treatment of venous anastomotic stenoses of hemodialysis grafts after surgical thrombectomy. J Vasc Surg 55: 472–478, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Vogel PM, Parise C: Comparison of SMART stent placement for arteriovenous graft salvage versus successful graft PTA. J Vasc Interv Radiol 16: 1619–1626, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Chan MR, Bedi S, Sanchez RJ, Young HN, Becker YT, Kellerman PS, Yevzlin AS: Stent placement versus angioplasty improves patency of arteriovenous grafts and blood flow of arteriovenous fistulae. Clin J Am Soc Nephrol 3: 699–705, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotmans JI, Pattynama PM, Verhagen HJ, Hino I, Velema E, Pasterkamp G, Stroes ES: Sirolimus-eluting stents to abolish intimal hyperplasia and improve flow in porcine arteriovenous grafts: A 4-week follow-up study. Circulation 111: 1537–1542, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Haskal ZJ, Trerotola S, Dolmatch B, Schuman E, Altman S, Mietling S, Berman S, McLennan G, Trimmer C, Ross J, Vesely T: Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med 362: 494–503, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Seedial SM, Ghosh S, Saunders RS, Suwanabol PA, Shi X, Liu B, Kent KC: Local drug delivery to prevent restenosis. J Vasc Surg 57: 1403–1414, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hébert K, Veldhof S, Webster M, Thuesen L, Dudek D: A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373: 897–910, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Barath P, Fishbein MC, Vari S, Forrester JS: Cutting balloon: A novel approach to percutaneous angioplasty. Am J Cardiol 68: 1249–1252, 1991 [DOI] [PubMed] [Google Scholar]
- 51.Wu CC, Lin MC, Pu SY, Tsai KC, Wen SC: Comparison of cutting balloon versus high-pressure balloon angioplasty for resistant venous stenoses of native hemodialysis fistulas. J Vasc Interv Radiol 19: 877–883, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Bhat R, McBride K, Chakraverty S, Vikram R, Severn A: Primary cutting balloon angioplasty for treatment of venous stenoses in native hemodialysis fistulas: Long-term results from three centers. Cardiovasc Intervent Radiol 30: 1166–1170, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Vorwerk D, Adam G, Müller-Leisse C, Guenther RW: Hemodialysis fistulas and grafts: Use of cutting balloons to dilate venous stenoses. Radiology 201: 864–867, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Guiu B, Loffroy R, Ben Salem D, Cercueil JP, Aho S, Mousson C, Krausé D: Angioplasty of long venous stenoses in hemodialysis access: At last an indication for cutting balloon? J Vasc Interv Radiol 18: 994–1000, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Vesely TM, Siegel JB: Use of the peripheral cutting balloon to treat hemodialysis-related stenoses. J Vasc Interv Radiol 16: 1593–1603, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Huijbregts HJ, de Borst GJ, Veldhuis WB, Verhagen HJ, Velema E, Pasterkamp G, Moll FL, Blankestijn PJ, Hoefer IE: Cryoplasty of the venous anastomosis for prevention of intimal hyperplasia in a validated porcine arteriovenous graft model. Eur J Vasc Endovasc Surg 39: 620–626, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Rifkin BS, Brewster UC, Aruny JE, Perazella MA: Percutaneous balloon cryoplasty: A new therapy for rapidly recurrent anastomotic venous stenoses of hemodialysis grafts? Am J Kidney Dis 45: e27–e32, 2005 [DOI] [PubMed]
- 58.Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, Wecksler AT, Hammock BD: Synthesis and structure-activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem 54: 3037–3050, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Sharouni SY, Smits HF, Wüst AF, Battermann JJ, Blankestijn PJ: Endovascular brachytherapy in arteriovenous grafts for haemodialysis does not prevent development of stenosis. Radiother Oncol 49: 199–200, 1998 [DOI] [PubMed] [Google Scholar]
- 60.van Tongeren RB, Levendag PC, Coen VL, Schmitz PI, Gescher FM, Vernhout RM, Wittens CH, Bruijninckx CM: External beam radiation therapy to prevent anastomotic intimal hyperplasia in prosthetic arteriovenous fistulas: Results of a randomized trial. Radiother Oncol 69: 73–77, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Misra S, Bonan R, Pflederer T, Roy-Chaudhury P; BRAVO I Investigators: BRAVO I: A pilot study of vascular brachytherapy in polytetrafluoroethylene dialysis access grafts. Kidney Int 70: 2006–2013, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Luo Z, Akita GY, Date T, Treleaven C, Vincent KA, Woodcock D, Cheng SH, Gregory RJ, Jiang C: Adenovirus-mediated expression of beta-adrenergic receptor kinase C-terminus reduces intimal hyperplasia and luminal stenosis of arteriovenous polytetrafluoroethylene grafts in pigs. Circulation 111: 1679–1684, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Terry CM, Li L, Li H, Zhuplatov I, Blumenthal DK, Kim SE, Owen SC, Kholmovski EG, Fowers KD, Rathi R, Cheung AK: In vivo evaluation of the delivery and efficacy of a sirolimus-laden polymer gel for inhibition of hyperplasia in a porcine model of arteriovenous hemodialysis graft stenosis. J Control Release 160: 459–467, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rotmans JI, Stroes ES, Pasterkamp G: Letter regarding article by Luo et al, “Adenovirus-mediated expression of beta-adrenergic receptor kinase C-terminus reduces intimal hyperplasia and luminal stenosis of arteriovenous polytetrafluoroethylene grafts in pigs.” Circulation 112: e153, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D, Zalewski A: Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 94: 1655–1664, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN: Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation 93: 2178–2187, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Tomas JJ, Stark VE, Kim JL, Wolff RA, Hullett DA, Warner TF, Hoch JR: Beta-galactosidase-tagged adventitial myofibroblasts tracked to the neointima in healing rat vein grafts. J Vasc Res 40: 266–275, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Kuji T, Masaki T, Goteti K, Li L, Zhuplatov S, Terry CM, Zhu W, Leypoldt JK, Rathi R, Blumenthal DK, Kern SE, Cheung AK: Efficacy of local dipyridamole therapy in a porcine model of arteriovenous graft stenosis. Kidney Int 69: 2179–2185, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Owen SC, Li H, Sanders WG, Cheung AK, Terry CM: Correlation of tissue drug concentrations with in vivo magnetic resonance images of polymer drug depot around arteriovenous graft. J Control Release 146: 23–30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanders WG, Hogrebe PC, Grainger DW, Cheung AK, Terry CM: A biodegradable perivascular wrap for controlled, local and directed drug delivery. J Control Release 161: 81–89, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly B, Melhem M, Zhang J, Kasting G, Li J, Krishnamoorthy M, Heffelfinger S, Rudich S, Desai P, Roy-Chaudhury P: Perivascular paclitaxel wraps block arteriovenous graft stenosis in a pig model. Nephrol Dial Transplant 21: 2425–2431, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Paulson WD, Kipshidze N, Kipiani K, Beridze N, DeVita MV, Shenoy S, Iyer SS: Safety and efficacy of local periadventitial delivery of sirolimus for improving hemodialysis graft patency: First human experience with a sirolimus-eluting collagen membrane (Coll-R). Nephrol Dial Transplant 27: 1219–1224, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Nathan A, Nugent MA, Edelman ER: Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci U S A 92: 8130–8134, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nugent HM, Rogers C, Edelman ER: Endothelial implants inhibit intimal hyperplasia after porcine angioplasty. Circ Res 84: 384–391, 1999 [DOI] [PubMed] [Google Scholar]
- 75.Nugent HM, Edelman ER: Endothelial implants provide long-term control of vascular repair in a porcine model of arterial injury. J Surg Res 99: 228–234, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Nugent HM, Groothuis A, Seifert P, Guerraro JL, Nedelman M, Mohanakumar T, Edelman ER: Perivascular endothelial implants inhibit intimal hyperplasia in a model of arteriovenous fistulae: A safety and efficacy study in the pig. J Vasc Res 39: 524–533, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Conte MS, Nugent HM, Gaccione P, Guleria I, Roy-Chaudhury P, Lawson JH: Multicenter phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: The V-HEALTH study. J Vasc Surg 50: 1359–1368, 2009 [DOI] [PubMed]
- 78.Conte MS, Nugent HM, Gaccione P, Roy-Chaudhury P, Lawson JH: Influence of diabetes and perivascular allogeneic endothelial cell implants on arteriovenous fistula remodeling. J Vasc Surg 54: 1383–1389, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Rotmans JI, Heyligers JM, Verhagen HJ, Velema E, Nagtegaal MM, de Kleijn DP, de Groot FG, Stroes ES, Pasterkamp G: In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation 112: 12–18, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Rotmans JI, Heyligers JM, Stroes ES, Pasterkamp G: Endothelial progenitor cell-seeded grafts: Rash and risky. Can J Cardiol 22: 1113–1116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peden EK, Leeser DB, Dixon BS, El-Khatib MT, Roy-Chaudhury P, Lawson JH, Menard MT, Dember LM, Glickman MH, Gustafson PN, Blair AT, Magill M, Franano FN, Burke SK: A multi-center, dose-escalation study of human type I pancreatic elastase (PRT-201) administered after arteriovenous fistula creation. J Vasc Access 14: 143–151, 2013 [DOI] [PMC free article] [PubMed]
- 82.Masuda A, Miyata M, Kihara T, Minagoe S, Tei C: Repeated sauna therapy reduces urinary 8-epi-prostaglandin F(2alpha). Jpn Heart J 45: 297–303, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Kipshidze N, Nikolaychik V, Muckerheidi M, Keelan MH, Chekanov V, Maternowski M, Chawla P, Hernandez I, Iyer S, Dangas G, Sahota H, Leon MB, Roubin G, Moses JW: Effect of short pulsed nonablative infrared laser irradiation on vascular cells in vitro and neointimal hyperplasia in a rabbit balloon injury model. Circulation 104: 1850–1855, 2001 [DOI] [PubMed] [Google Scholar]
- 84.Lin CC, Liu XM, Peyton K, Wang H, Yang WC, Lin SJ, Durante W: Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol 28: 739–745, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin CC, Yang WC, Chen MC, Liu WS, Yang CY, Lee PC: Effect of far infrared therapy on arteriovenous fistula maturation: An open-label randomized controlled trial. Am J Kidney Dis 62: 304–311, 2013 [DOI] [PubMed]
- 86.Dahl SL, Blum JL, Niklason LE: Bioengineered vascular grafts: Can we make them off-the-shelf? Trends Cardiovasc Med 21: 83–89, 2011 [DOI] [PubMed] [Google Scholar]
- 87.L’Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L, McAllister T: Technology insight: The evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med 4: 389–395, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Peck MK, Dusserre N, Zagalski K, Garrido SA, Wystrychowski W, Glickman MH, Chronos NA, Cierpka L, L’Heureux N, McAllister TN: New biological solutions for hemodialysis access. J Vasc Access 12: 185–192, 2011 [DOI] [PubMed] [Google Scholar]
- 89.Tillman BW, Yazdani SK, Neff LP, Corriere MA, Christ GJ, Soker S, Atala A, Geary RL, Yoo JJ: Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J Vasc Surg 56: 783–793, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begelman KG, Niklason LE: Readily available tissue-engineered vascular grafts. Sci Transl Med 3: 68ra9–192, 2011 [DOI] [PubMed]
- 91.Surgeons at Duke University Hospital Implant Bioengineered Vein. Available at: http://www.dukehealth.org/health_library/news/surgeons-at-duke-university-hospital-implant-bioengineered-vein Accessed June 6, 2013
- 92.Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M, Jr, Miller A, Scher L, Trerotola S, Gregory RT, Rutherford RB, Kent KC: Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 35: 603–610, 2002 [DOI] [PubMed] [Google Scholar]
- 93.Murad MH, Swiglo BA, Sidawy AN, Ascher E, Montori VM: Methodology for clinical practice guidelines for the management of arteriovenous access. J Vasc Surg 48[Suppl]: 26S–30S, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Lee T, Mokrzycki M, Moist L, Maya I, Vazquez M, Lok CE; North American Vascular Access Consortium: Standardized definitions for hemodialysis vascular access. Semin Dial 24: 515–524, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dember LM, Imrey PB, Beck GJ, Cheung AK, Himmelfarb J, Huber TS, Kusek JW, Roy-Chaudhury P, Vazquez MA, Alpers CE, Robbin ML, Vita JA, Greene T, Gassman JJ, Feldman HI; Hemodialysis Fistula Maturation Study Group: Objectives and design of the Hemodialysis Fistula Maturation Study [published online ahead of print August 27, 2013]. Am J Kidney Dis, 10.1053/j.ajkd.2013.06.024 [DOI] [PMC free article] [PubMed]
- 96.Manson RJ, Ebner A, Gallo S, Chemla E, Mantell M, Deaton D, Roy-Chaudhury P: Arteriovenous fistula creation using the Optiflow vascular anastomosis device: A first in man pilot study. Semin Dial 26: 97–99, 2013 [DOI] [PubMed] [Google Scholar]