Abstract
Objective
Surgical management of colon cancer for patients with Lynch Syndrome who carry a mismatch repair gene mutation is controversial. The decision to remove more or less of the colon involves the consideration of a relatively high risk of metachronous colorectal cancer (CRC) with the impact of more extensive surgery. Our aim was to estimate and compare the risks of metachronous CRC for Lynch Syndrome patients undergoing either segmental or extensive (subtotal or total) resection for first colon cancer.
Design
Risk of metachronous CRC was estimated for 382 MMR gene mutation carriers (172 MLH1, 167 MSH2, 23 MSH6 and 20 PMS2) from the Colon Cancer Family Registry, who had surgery for their first colon cancer using retrospective cohort analysis. Age-dependent cumulative risks of metachronous CRC were calculated using the Kaplan-Meier method. Risk factors for metachronous CRC were assessed by a Cox proportional hazards regression.
Results
None of 50 subjects who had extensive colectomy was diagnosed with metachronous CRC (incidence rate 0.0; 95%CI 0.0–7.2 per 1000 person-years). Of 332 subjects who had segmental resections, 74 (22%) were diagnosed with metachronous CRC (incidence rate 23.6; 95%CI 18.8–29.7 per 1000 person-years). For those who had segmental resections, incidence was statistically higher than for those who had extensive surgery (P <0.001). Cumulative risk of metachronous CRC was 16% (95%CI 10–25%) at 10 years, 41% (95%CI 30–52%) at 20 years and 62% (95%CI 50–77%) at 30 years after segmental colectomy. Risk of metachronous CRC reduced by 31% (95%CI 12–46%; P 0.002) for every 10 cm of bowel removed.
Conclusions
Lynch Syndrome patients with first colon cancer treated with more extensive colonic resection have a lower risk of metachronous CRC compared with those receiving less extensive surgery. This finding will better inform decision-making regarding the extent of primary surgical resection.
Keywords: Lynch Syndrome, metachronous colorectal cancer, colorectal surgery
Introduction
Lynch Syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC)[1], refers to colorectal (CRC) and other cancers diagnosed in carriers of germline mutations in DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6 and PMS2.[2] Approximately 1 in 3,000 of the general population carry a mutation in a MMR gene[3]; and these MMR gene mutation carriers are at substantially increased risk of CRC. Cumulative risk of CRC to age 70 years has been estimated to vary from 40% to 70% depending on the ascertainment of the population studied, the sex of the carriers and the MMR gene mutated[4, 5, 6, 7]. To date, there is no consensus on the optimal extent of colonic resection to be performed when Lynch Syndrome patients present for curative surgical management of a first primary colon cancer.
On the one hand, the relatively high risk of metachronous CRC, i.e. primary CRC diagnosed more than 12 months after the first diagnosis of primary colon cancer, (16% after 10 years)[8] supports a more aggressive primary surgical approach involving the removal of all, or at least most, of the colon after diagnosis. The functional consequence of an increase in bowel frequency and possible negative impact on quality of life might be balanced against the reduction in risk of metachronous CRC afforded by more extensive surgery, particularly if the person is aged less than 60 years at the time of surgery.[9] On the other hand, surveillance of the remaining colon and rectum will be required after most surgery (except total proctocolectomy) and the inconvenience of yearly colonoscopy (with the requirement for standard bowel preparation rather than enema preparation) may be offset by the better functional outcome after segmental surgery. This clinical equipoise is reflected in world surgical opinion.[10, 11]
To guide surgical decision-making the risk of metachronous CRC needs to be known for Lynch Syndrome patients who have had extensive resection and for patients who have had segmental resection. A randomized controlled trial to compare metachronous CRC risk between segmental and extensive colectomy would be ideal but has not been feasible. An observational study comparing metachronous CRC incidence after either curative segmental or extensive resection for colon cancer in Lynch Syndrome can provide data to address this question but the studies reported to date have generally been small. In this study, we aimed to estimate the risk of metachronous CRC for MMR gene mutation carriers following segmental or extensive surgery for their first colon cancer, and to determine if metachronous CRC risk differs by the characteristics of first colon cancer.
Patients and Methods
Study Sample
The study sample comprised carriers of pathogenic mutations (see below) in one of the MMR genes MLH1, MSH2, MSH6 and PMS2 who had a surgical resection for first colon cancer and had been recruited and genetically characterized by the Colon Cancer Family Registry (Colon CFR).
Details of recruitment methods have been described previously.[12] Briefly, probands were recruited between 1997 and 2007, and ascertained from family cancer clinics in Australia (Melbourne, Adelaide, Perth, Brisbane, Sydney), New Zealand (Auckland) and the USA (Mayo Clinic, Rochester, Minnesota and Cleveland Clinic) or from population-based cancer registries in the USA (Puget Sound, Washington; the State of Minnesota; Los Angeles, California; Arizona; Colorado; New Hampshire; North Carolina; and Hawaii), Australia (Victoria) and Canada (Ontario).
Probands were asked for permission to contact their relatives to seek their enrolment in the Colon CFR. The subjects in this study included the probands and their affected participating relatives found to be MMR gene mutation carriers. Written informed consent was obtained from all study participants, and the study protocol was approved by the institutional review board at each Colon CFR recruitment site.
Data Collection
Information on demographics, personal characteristics, personal and family history of cancer, cancer screening and surgery were obtained at enrolment from probands and all participating relatives. Reported cancer diagnoses and age at diagnosis were confirmed, where possible using pathology reports, medical records, cancer registry reports and/or death certificates. Blood samples and tumour tissues were collected for genetic testing. Approximately five years after recruitment, attempts were made to re-interview participants to update demographic information, personal characteristics, personal and family history of cancer, cancer surveillance and surgery. The present study was based on all available enrolment and follow-up data.
The following details of the first diagnosis of CRC and colorectal surgery for MMR gene mutation carriers were extracted from the pathology reports: date of surgery, type of surgery (total proctocolectomy, total colectomy, subtotal colectomy, right or left hemicolectomy, sigmoid colectomy, anterior resection, segmental colectomy, polypectomy, other type, unknown), length of bowel removed (millimetres), site of tumour (right colon, left colon, splenic flexure, rectosigmoid, rectum, unknown), maximum dimension of tumour (millimetres), T- and N-stage, histological grade (well, moderate, poor differentiation, other), and synchronous tumour (present, absent). Patients with first primary rectal cancer were excluded from this study because operative strategies are based primarily on oncologic clearance and to a lesser extent functional consequences, and not on extensiveness of resection per se.
Mutation Testing
Testing for MLH1, MSH2, MSH6 and PMS2 mutations was performed for all probands ascertained from family cancer clinics and for all probands from population-based ascertainment who had a colorectal tumour displaying evidence of impaired MMR function as evidenced by either MSI, or by lack of MMR protein expression by immunohistochemistry. Mutation testing was performed by Sanger sequencing or denaturing high pressure liquid chromatography (dHPLC), followed by confirmatory DNA sequencing. Large insertion and deletion mutations were detected by Multiplex Ligation Dependent Probe Amplification (MLPA) according to the manufacturer’s instructions (MRC Holland, Amsterdam, The Netherlands).[6, 12] All participants who provided a blood sample, and who were relatives of probands with a pathogenic mutation, underwent predictive testing for the specific mutation identified in the proband.
Of the 501 MMR gene mutation carriers who had colon resection for the first primary colon cancer, a surgical pathology report was available for 385 (77%). Of these 385, we excluded three who had a total proctocolectomy with end ileostomy or ileoanal anastomosis (pouch) as they had no subsequent risk of metachronous CRC. The remaining 382 were included in the analyses.
Definitions
A ‘pathogenic mutation’ was defined as a variant that was predicted to result in a stop codon, a large insertion or deletion, or a missense mutation previously reported to be pathogenic. A ‘metachronous CRC’ was defined as a primary colon or rectal cancer diagnosed more than 12 months after the first diagnosis of primary colon cancer. Total colectomy with ileorectal anastomosis and subtotal colectomy with ileosigmoid anastomosis were defined as ‘extensive colon resection’. Right or left hemicolectomy, sigmoid colectomy, anterior resection, and transverse colectomy were defined as ‘segmental colon resection’. T- and N-staging of the cancer was categorized according to the American Joint Committee on Cancer (AJCC) staging.[13]
Statistical Analysis
Incidence rates of metachronous CRC diagnoses per 1,000 person-years and 95% confidence intervals (CIs) were estimated separately for the carriers of a mutation in any MMR gene who had segmental colon resection and for the carriers who had extensive colon resection for their first colon cancer. The primary time scale for risk started from the time of first colon surgery and ended at the time of diagnosis of metachronous CRC, or second colorectal surgery (either prophylactic or other reasons), or last follow-up or death, whichever came first.
The relationship between the risk of metachronous CRC and the length of bowel removed during the resection of the first colon cancer was estimated by Cox regression by fitting the length of bowel removed as a continuous variable. The proportional hazards assumption was tested by examining the relationship between the scaled Schoenfeld residuals and survival time. We accommodated age in the model by splitting participant’s follow-up time into 5-year age brackets and including a separate risk parameter in the model for each age bracket. This is preferable to simply adjusting for age at surgery for first colon cancer since the risk of metachronous CRC depends on age as well as time since surgery and several participants will contribute follow-up time to more than one 5-year age bracket. To control for other potential confounding factors, we also adjusted for sex, site of first colon cancer (right colon, left colon, rectosigmoid), the specific MMR gene mutated, country/region of recruitment (Australasia, Canada, the USA), characteristics of first colon cancer including the maximum dimension of tumour (tertile groups), AJCC stage (Stage I, II and III), the histology grade (well, moderate, poor, mucinous), synchronous tumour (absent, present), and height of individuals. We applied the Huber-White robust variance correction by clustering on family membership to allow for any correlation of risk between family members [14, 15].
Kaplan-Meier hazard estimation method was used to estimate the age-dependent cumulative risk of metachronous CRC to 10, 20 and 30 years following segmental colon resection for first colon cancer.
Frequency of colonoscopy or sigmoidoscopy after colon surgery for first colon cancer, but before the diagnosis of metachronous CRC, was estimated from the self-reported questionnaire data. The endoscopic examination within one year before the age of first colon cancer or metachronous CRC was excluded as we assumed it being a diagnostic test for CRC rather than a screening test. The frequency of colonoscopy or sigmoidoscopy was assumed to be distributed uniformly in the period between first and last age of endoscopy. The average interval between colonoscopies or sigmoidoscopies after extensive colon resection was compared with that for segmental colon resection using Student’s t-test.
All statistical tests were two-sided and, P <0.05 was assumed as statistically significant. All statistical analyses were performed using Stata 10.0 [16].
Results
The study cohort comprised 382 carriers (187 females) of MMR gene mutations (172 in MLH1, 167 in MSH2, 23 in MSH6, and 20 in PMS2) who had a colon resection for their first colon cancer, contributing a total of 3,545 person-years since first colon cancer (mean follow-up 9 years, standard deviation, SD 7 years, range 1 – 40 years). The mean follow-up was 8 (SD 6, range 1 – 30) years for those who had extensive surgery and 9 (SD 8, range 1 – 40) years for those who had segmental surgery. The mean age at diagnosis of first colon cancer was 46 (SD 11) years, ranging from 20 to 74 years. Of all carriers, 192 (50%) were recruited in Australia or New Zealand, 118 (31%) in the USA, and 72 (19%) in Canada. The baseline characteristics of study subjects were summarized in Table 1.
Table 1.
Baseline characteristics of subjects included in the study
| Partial Surgery | Extensive Surgery | |||
|---|---|---|---|---|
| No metachronous cancer | Metachronous cancer | Total | ||
|
|
|
|||
| Number (%) | Number (%) | Number (%) | Number (%) | |
| Total number | 258 | 74 | 332 | 50 |
| Sex | ||||
| female | 122 (47) | 42 (57) | 164 (49) | 23 (46) |
| Country | ||||
| Australasia | 135 (52) | 32 (43) | 167 (50) | 25 (50) |
| Canada | 41 (16) | 21 (28) | 62 (19) | 10 (20) |
| USA | 80 (32) | 21 (28) | 103 (31) | 15 (30) |
| MMR gene mutated | ||||
| MLH1 | 116 (45) | 33 (45) | 149 (45) | 136 (44) |
| MSH2 | 104 (40) | 38 (51) | 142 (43) | 127 (42) |
| MSH6 | 19 (7) | 3 (4) | 22 (6) | 20 (7) |
| PMS2 | 19 (7) | 0 (0) | 19 (6) | 20 (7) |
| First diagnosis of colon cancer | ||||
| Age at diagnosis (year), mean (SD) | 46 (11) | 44 (10) | 46 (11) | 45 (10) |
| Site of tumour | ||||
| Right colon* | 194 (76) | 47 (64) | 241 (73) | 28 (58) |
| Left colon** | 50 (20) | 23 (32) | 73 (22) | 17 (35) |
| Rectosigmoid | 11 (4) | 3 (4) | 14 (4) | 3 (6) |
| Unknown | 3 | 1 | 4 | 2 |
| AJCC stage of tumour# | ||||
| I | 63 (27) | 11 (18) | 74 (25) | 19 (40) |
| II (A, B, C) | 119 (50) | 32 (52) | 151 (51) | 14 (29) |
| III (A, B, C) | 54 (23) | 19 (30) | 73 (24) | 15 (31) |
| Unknown | 22 | 12 | 34 | 2 |
| Histological grade | ||||
| Well | 21 (9) | 7 (12) | 28 (9) | 1 (2) |
| Moderate | 151 (62) | 32 (54) | 183 (61) | 32 (67) |
| Poor | 60 (25) | 16 (27) | 76 (25) | 14 (29) |
| Mucinous | 9 (4) | 4 (7) | 13 (4) | 1 (2) |
| Unknown | 17 | 15 | 32 | 2 |
| Synchronous tumour | ||||
| Absent | 234 (95) | 65 (94) | 299 (95) | 37 (76) |
| Present | 12 (5) | 4 (6) | 16(5) | 12 (24) |
| Unknown | 12 | 5 | 17 | 1 |
| Type of surgery | ||||
| Extensive resection | - | - | - | 50 |
| Total colectomy† | - | - | - | 34 (68) |
| Subtotal colectomy‡ | - | - | - | 16 (32) |
| Segmental resection | 258 | 74 | 332 | - |
| Right hemicolectomy | 180 (70) | 43 (58) | 223 (67) | - |
| Left hemicolectomy | 22 (9) | 17 (23) | 39 (12) | - |
| Anterior resection | 11 (4) | 0 (0) | 11 (3) | - |
| Sigmoid colectomy | 19 (7) | 8 (11) | 27 (8) | - |
| Transverse colectomy | 26 (10) | 6 (8) | 32 (10) | - |
| Length of bowel removed (cm), mean (SD) | 27.3 (15.7) | 21.7 (8.4) | 26.1 (14.6) | 71.4 (20.9) |
Right colon included caecum, ascending colon, hepatic flexure and transverse colon
Left colon included splenic flexure, descending colon and sigmoid colon
Metastasis status was unavailable; and any of this stage could be Stage IV.
Total colectomy and ileorectal anastomosis
Subtotal colectomy and ileosigmoid anastomosis
Of the 50 cases (13% of all cases studied) who had extensive colon resection for their first colon cancer, none were diagnosed with metachronous CRC over 414 person-years of follow-up (mean 8 (SD 6) years); incidence rate 0.0, one-sided 95% CI 0.0–7.2 per 1000 person-years (Table 2).
Table 2.
Incidence rate (per 1000 person-years) of metachronous colorectal cancer following segmental or extensive colon resection for first colon cancer
| Extent of colon resection | Total number | Total years of observation | Metachronous CRC n (%) | Rate per 1000 person-years (95%CI) |
|---|---|---|---|---|
| Extensive | 50 | 414 | 0 (0%) | 0 (0–7.21)* |
| Segmental | 332 | 3,131 | 74 (22%) | 23.64 (18.82–29.68) |
| Sex | ||||
| Male | 168 | 1,448 | 32 (19%) | 22.10 (15.63–31.25) |
| Female | 164 | 1,683 | 42 (26%) | 24.96 (18.44–33.77) |
| Age at first colon cancer (year) | ||||
| < 40 | 95 | 1,146 | 24 (25%) | 20.94 (14.04–31.25) |
| 40 – 49 | 127 | 1,222 | 32 (25%) | 28.52 (20.17–40.33) |
| ≥50 | 110 | 863 | 18 (16%) | 20.86 (13.14–33.11) |
| MMR gene mutated | ||||
| MLH1 | 149 | 1,426 | 33 (22%) | 23.14 (16.45–32.55) |
| MSH2 | 142 | 1,410 | 38 (27%) | 26.95 (19.61–37.04) |
| MSH6 | 22 | 191 | 3 (14%) | 15.71 (5.07–48.70) |
| PMS2 | 19 | 104 | 0 (0%) | 0 (0–28.39)* |
| Country | ||||
| Australasia | 167 | 1,803 | 32 (19%) | 17.75 (12.55–25.10) |
| Canada | 62 | 441 | 21 (34%) | 49.89 (31.05–73.04) |
| USA | 103 | 887 | 21 (21%) | 25.93 (15.44–36.31) |
one-sided 95% CI
Of the 332 cases (87% of all cases studied) who had segmental colon resection for their first colon cancer, 74 (22%) were diagnosed with metachronous CRC over 3,131 person-years of follow-up (mean 9 (SD 8) years); incidence rate 23.6, 95%CI 18.8–29.7 per 1000 person-years. This incidence rate was statistically higher that that for cases who had extensive surgery (P <001). There was no evidence for the incidence rate differing substantially by sex, MMR gene mutated or age at first colon cancer diagnosis (Table 2). Cumulative risk of metachronous CRC was 16% (95%CI 10–25%) at 10 years, 41% (95%CI 30–52%) at 20 years and 62% (95%CI 50–77%) at 30 years after segmental colectomy for a first colon cancer (Figure 1).
Figure 1.
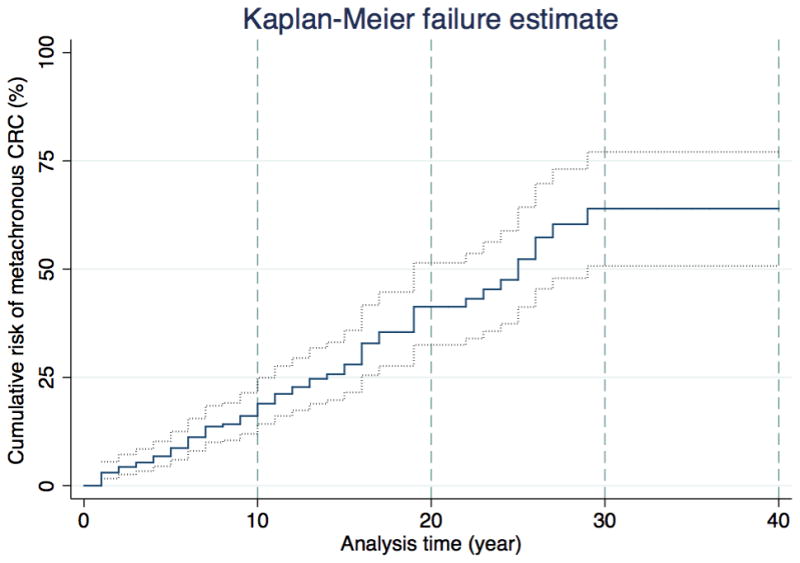
Kaplan-Meier hazards estimation curve for the risk of metachronous CRC following segmental colon resection for the first diagnosis of colon cancer
The mean length of bowel removed was 71.4 (SD 20.9) cm for carriers who had extensive resection and 26.1 (SD 14.6) cm for carriers who had segmental resection. The mean length of bowel removed for carriers with segmental resection differed by country; 28.0 (SD 16.2) cm for Australasia, 25.1 (SD 13.5) cm for the USA and 23.5 (SD 13.5) cm for Canada. Given the adjusted hazard ratio per 10 cm bowel removed was 0.69 (95%CI 0.54–0.88) after adjusting for sex, the MMR gene mutated, country of recruitment and the characteristics of first colon cancer (Table 3), this equates to an average 31% reduction in metachronous risk for every 10 cm removed. For example, a patient who had 30 cm colon resection would have 31% lower metachronous CRC risk compared to a case with 20 cm colon resection. Note, this applies only to the subjects undergoing segmental surgery. As this is a percentage reduction in risk, the total reduction in risk for 20 cm removal will be 52% and for 30 cm will be 67% on average. Risk of metachronous CRC was not associated with the characteristics of first colon cancer (age at diagnosis, site, maximum dimension, AJCC stage, histological grade, and the presence of synchronous tumour).
Table 3.
Hazard ratio of metachronous colon cancer following segmental colon resection for first colon cancer
| Univariate | Multivariate^ | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Sex | ||||
| Male | 1.00 (Referent) | 1.00 (Referent) | ||
| Female | 1.08 (0.68–1.72) | 0.74 | 1.68 (0.75–3.75) | 0.21 |
| Age (years) | ||||
| < 40 | 1.00 (Referent) | 1.00 (Referent) | ||
| 40 – 49 | 1.40 (0.82–2.38) | 0.69† | 1.29 (0.49–3.41) | 0.43† |
| ≥50 | 1.09 (0.59–2.03) | 1.42 (0.60–3.35) | ||
| MMR gene mutated | ||||
| MLH1 | 1.00 (Referent) | 1.00 (Referent) | ||
| MSH2 | 1.19 (0.75–1.90) | 0.46 | 1.21 (0.62–2.35) | 0.58 |
| MSH6 | 0.63 (0.21–2.24) | 0.53 | 0.84 (0.22–3.24) | 0.80 |
| PMS2 | - | - | ||
| Country of recruitment | ||||
| Australasia | 1.00 (Referent) | 1.00 (Referent) | ||
| Canada | 2.73 (1.57–4.75) | <0.001 | 5.00 (2.04–12.29) | <0.001 |
| USA | 1.40 (0.80–2.43) | 0.24 | 2.71 (1.21–6.06) | 0.02 |
| Length of bowel removed (per 10 cm) | 0.72 (0.56 – 0.93) | 0.01 | 0.69 (0.54–0.88) | 0.002 |
| Characteristics of first colon cancer | ||||
| Site of tumour in colon | ||||
| Right colon* | 1.00 (Referent) | 1.00 (Referent) | ||
| Left colon** | 1.46 (0.89–2.41) | 0.14 | 1.46 (0.61–3.50) | 0.40 |
| Rectosigmoid | 1.50 (0.47–4.86) | 0.50 | 2.02 (0.32–12.83) | 0.46 |
| Maximum dimension of tumour | ||||
| Tertile 1 (10 – 40 mm) | 1.00 (Referent) | 1.00 (Referent) | ||
| Tertile 2 (41 – 60 mm) | 0.75 (0.38 – 1.46) | 0.99† | 0.62 (0.24–1.64) | 0.59† |
| Tertile 3 (61 – 140 mm) | 1.00 (0.52 – 1.95) | 0.78 (0.32–1.90) | ||
| AJCC Stage of Tumor | ||||
| I | 1.00 (Referent) | 1.00 (Referent) | ||
| II (A, B, C) | 1.36 (0.68–2.70) | 0.08† | 0.94 (0.35–2.55) | 0.48† |
| III (A, B, C) | 1.91 (0.91–4.03) | 1.40 (0.51–3.87) | ||
| Histological grade | ||||
| Well | 1.00 (Referent) | 1.00 (Referent) | ||
| Moderate | 1.04 (0.46–2.36) | 1.92 (0.52–7.06) | ||
| Poor | 0.98 (0.40–2.40) | 0.75† | 1.89 (0.45–7.86) | 0.40† |
| Mucinous | 1.48 (0.43–5.11) | 2.83 (0.32–24.70) | ||
| Synchronous tumour | ||||
| Absent | 1.00 (Referent) | 1.00 (Referent) | ||
| Present | 1.56 (0.57–4.30) | 0.39 | 1.28 (0.40–4.07) | 0.67 |
P trend: calculated from Cox regression models with ordinal variables as continuous measures.
adjusted for variables in the table and height of individuals, with robust variance correction for familial correlation
Right colon included caecum, ascending colon, hepatic flexure and transverse colon
Left colon included descending colon and sigmoid colon
Table 4 shows there was no difference in the frequency of colonoscopy or sigmoidoscopy after colon surgery for first colon cancer between extensive and segmental surgery group (one endoscopic examination per 16 (95%CI 13–20) months after extensive surgery compared to one endoscopic examination per 20 (95%CI 18–21) months after segmental surgery; P 0.2). Importantly, the majority of patients (78%) who developed metachronous CRC were undergoing one to two yearly colonoscopy (Supplementary Table 1). There was no statistical evidence of difference of endoscopic frequency after surgery by country. (data not shown).
Table 4.
Frequency of colonoscopy or sigmoidoscopy after extensive or segmental colon resection for their first colon cancer
| Colonoscopy or Sigmoidoscopy# | Extent of resection
|
|
|---|---|---|
| Extensive no (%) | Segmental no (%) | |
| At least one | 37 (80) | 269 (86) |
| Average frequency* | ||
| every year | 25 (67) | 144 (54) |
| every 2 years | 8 (22) | 55 (20) |
| every 3 years | 1 (3) | 29 (11) |
| every 4 years | 0 (0) | 3 (1) |
| every 5 years | 1 (3) | 5 (2) |
| every 6 years | 0 (0) | 3 (1) |
| unknown frequency | 2 (5) | 30 (11) |
| Mean interval (months) for one examination (95%CI) | 16 (13–20) | 20 (18–21) |
| P 0.16† | ||
| None | 9 (20) | 42 (14) |
| Missing | 4 | 21 |
|
| ||
| Total | 50 | 332 |
extracted from the self-reported questionnaire data
The frequency of the endoscopic examinations was assumed to be distributed uniformly in the period between first and last age of the endoscopic examinations.
Student’s t-test comparing the interval for one colonoscopy or sigmoidoscopy after extensive colon resection with that after segmental colon resection
AJCC stage of 74 metachronous cancers were as follows: 27 (47%) stage-I, 20 (35%) at stage II, and 10 (18%) at stage III. Stages of 17 cases were not known because they were verified from biopsy reports and/or clinical records. Of the ten patients who developed AJCC Stage III metachronous CRC (mean follow-up 12 (SD 10) years), six reported 1–2 yearly lower endoscopy, one reported no endoscopy and for three it was unknown if they underwent surveillance following their first surgery.
At 5 years after surgical resection, 49 (98%) who had extensive colectomy and 327 (98%) who had partial colectomy were alive (P 0.8). At 10 years, 49 (98%) who had extensive colectomy and 322 (97%) who had partial colectomy were alive (P 0.7).
Discussion
This large observational study of 382 MMR gene mutation carriers followed for a mean 9 years confirms a high cumulative risk of metachronous CRC for the 332 carriers treated by segmental resection for their first colon cancer. This occurred despite one to two yearly surveillance by colonoscopy or flexible sigmoidoscopy for the group overall and for those who developed metachronous CRC. Furthermore we found no evidence that the metachronous CRC risk estimated by our study differed by gender, genotype, any clinicopathological characteristic of the index colon cancer, or age at time of surgery. No metachronous cancers were observed in PMS2 mutation carriers; however their numbers were too small to allow conclusions with respect to their risk of metachronous cancer. We found that metachronous CRC risk for carriers who had segmental surgery differed by country, but this was not explained by any differences in endoscopic frequency or length of bowel removed.
It is noteworthy that there were no diagnoses of metachronous CRC during the 414 person-years of follow-up for the 50 MMR gene mutation carriers treated by extensive colon resection for their first colon cancer, and undergoing lower endoscopy at a similar frequency to those treated by segmental resection. These data are somewhat surprising given the 12% risk of rectal cancer at 12 years after ileorectal anastomosis for 71 CRC patients who had met the Amsterdam criteria (a median of 158 months from the primary procedure) reported by Rodriguez-Bigas et al[17]. Nevertheless this study and ours support the case for more extensive surgical resection in patients with Lynch Syndrome when the predominant end point being considered is metachronous CRC risk. Additionally and importantly our study provides a quantification of risk indexed to the remaining length of colon; this informs patients in decision-making by balancing the risk of metachronous cancer with potential functional impairment.
The high risk of CRC for carriers of a MMR gene mutation[4, 5, 6, 7] can be reduced by colonoscopy surveillance[18, 19] and is generally advised at one to two yearly intervals from the age of 20–25 years or from the age of 30 years for MSH6 mutation carriers[10, 11]. A Dutch study[8] of subjects from families carrying MMR gene mutations who had had surgical resection for CRC and were under colonoscopic surveillance reported 10-year cumulative risk of metachronous CRC of approximately 15.7% for 68 treated with segmental resection (mean follow-up 7.1 years) compared with approximately 3.4% for 29 treated with extensive resection (mean follow-up 5 years). Interestingly 10 of the 29 subjects who received an extensive resection for CRC had undergone a segmental resection for a previous CRC. Natarajan et al[20] recently reported data from the Lynch Syndrome registry in Creighton University, comparing metachronous colon cancer, reoperation and survival in MMR gene mutation carriers with total or subtotal colectomy (37/106) with those undergoing limited colectomy (69/106). Over a median follow-up of 12 years, there were differences in metachronous cancer rate (6% vs. 26%), reoperation rate (16% vs. 37%) and death (7% vs. 12%) in favor of the extended operation.
The risk of metachronous CRC for MMR gene mutation carriers has been reported to be higher for MLH1 and MSH2 carriers and for subjects aged over 40 years.[19] In contrast, we found no difference in metachronous CRC risk by genotype although in the cases of MSH6 and PMS2 carriers the numbers were relatively small. But our data concurred with previous studies that found sex, pathological stage of index CRC, or age at cancer onset did not impact on the risk of metachronous CRC. These data support that there is an advantage to the patient in offering extensive colectomy to reduce the metachronous CRC risk; in addition to ongoing bowel surveillance by minimizing at-risk mucosa and the severity of bowel preparation. There was no difference in survival between those receiving either extensive or segmental resection for their first CRC and this likely reflects the early AJCC stage reported in the majority of patients with metachronous CRC for whom we could obtain this information (57 of 74). Although this is reassuring there is no room for complacency because 10 of the 57 metachronous cancers were AJCC stage III despite the fact that at least half of these patients were undergoing 1–2 yearly surveillance colonoscopy.
Current recommendations in the USA, suggest that persons with Lynch Syndrome undergoing surgical resection of a colon cancer should be offered an extensive resection rather than a segmental resection, even though this policy has not previously been proven to be superior to a policy of 1–2 yearly colonoscopic surveillance.[10] Despite this recommendation, the extent of resection performed varies between centers in the USA. For example, the Cleveland Clinic performed total colectomy for 16 of 33 CRC patients from Amsterdam criteria-positive families compared with seven of 60 from clinics elsewhere in the USA.[21] In Europe, on the basis of a decision analysis study[9] and the documented high risk of a metachronous cancer, current guidelines recommend the option of extensive resection be discussed with patients, particularly those under the age of 50 years.[11] Therefore in planning the extent of surgical resection for MMR gene mutation carriers presenting with colon cancer in the non-emergent setting, surgeons need to consider patient preference, patient age, bowel and sphincter function, as well as likely compliance with surveillance and the quality or otherwise of post-operative surveillance endoscopy. The latter has the potential to influence metachronous CRC risk as suggested by one of seventeen patients having a segmental resection at Cleveland Clinic developing CRC compared with 15 of 53 patients elsewhere.[21]
The strengths of our study include its large sample size, long mean follow-up, inclusion of carriers of mismatch repair genes from four countries, and exclusion of cases for which we did not have pathological confirmation of extent of colon surgery. These provide some confidence that our findings reflect current practice and outcomes for these countries; whereas smaller, single centre or country studies may underestimate the size of the problem. In addition our statistical analysis accommodated the change in risk of metachronous CRC due to both age and time since surgery for first colon cancer by allowing two time scales in the Cox regression.
There are some limitations inherent to this study. Only a small percentage of the study patients (13%) had extensive surgery for their first CRC and this may reflect the fact that surgery was performed in the emergency setting or that at the time surgery was planned the diagnosis of HNPCC or MMR gene mutation carrier status was unknown. Unfortunately we do not have information on factors influencing choice of surgery. The colonoscopy data used for this analysis was obtained from questionnaires completed by the participants. We have previously conducted a validation study of reported colonoscopies reported by participants from the Colon CFR (from which carriers for this study were selected). The positive predictive value for colonoscopy resulting in a polypectomy was 81% and negative predictive value was 86% [22]. Another limitation of our study is the absence of data on the quality of surveillance colonoscopy which, if deficient, has the potential to upwardly bias the estimates of metachronous CRC for the segmental resection cases and could contribute to the differing metachronous CRC risk by country. Additionally, we cannot report on the timing of the preceding surveillance colonoscopy for those cases developing a metachronous CRC. However, the majority of metachronous CRC detected in our cohort were early stage. Rectal cancer is relatively uncommon as a first cancer in patients with Lynch Syndrome and we do not address this presentation here, as the surgical choices lie between total proctocolectomy (either restorative or with ileostomy) and anterior resection with colostomy, colorectal or coloanal anastomosis.
Data on bowel function and quality of life measurements were not collected therefore comparisons of bowel function between patient groups with greater or lesser colon resections could not be addressed. Following a subtotal or total colectomy, median stool frequency of four to five times a day has been reported[23] and quality of life including sexual relations, recreation, travel, house work and social activity may be adversely affected by the increased bowel frequency, urgency and looseness of stool. However a small study[24] from the Cleveland Clinic focussed on patients undergoing colectomy and ileorectal anastomosis for colon cancer and matching patients for age and gender with controls undergoing right or left colectomy found an increase in stool frequency from 2 to 4 per day with total colectomy, but there were no differences in quality of life as measured by the SF36 instrument.
The metachronous CRC risk following segmental resection in this study has quantified risk reduction associated with the length of colon removed. By contrast, no metachronous CRC was reported following total/subtotal colectomy. These data will augment results of current prospective studies of quality of life following surgery for CRC in Lynch Syndrome to provide the balanced information that surgeons and patients require for improved decision-making regarding the extent of primary surgical resection.
Supplementary Material
Summary Box.
What is already known about this subject?
Surgical management of colon cancer for patients with Lynch Syndrome who carry a mismatch repair (MMR) gene mutation is controversial.
The decision to remove more or less of the colon involves the consideration of a relatively high risk of metachronous colorectal cancer (CRC) with the impact of more extensive surgery.
What are the new findings?
Metachronous CRC risk following segmental resection reduced by 31% (95%CI 12–46%; P 0.002) for every 10 cm of bowel removed. No metachronous CRC was reported following total or subtotal colectomy.
Risk of metachronous CRC for patients having segmental resection was evident despite the majority undergoing regular surveillance by colonoscopy or flexible sigmoidoscopy.
Metachronous CRC risk did not differ by gender, the mismtach repair gene that was mutated, any clinicopathological characteristic of the first colon cancer, or the patient’s age at time of surgery.
How might it impact on clinical practice in the foreseeable future?
This finding will better inform decision-making regarding the extent of primary surgical resection.
Acknowledgments
Funding
This work was supported by the National Cancer Institute, National Institutes of Health under Request for Application #CA-95-011, and through cooperative agreements with the Australasian Colorectal Cancer Family Registry (UO1 CA097735), the USC Familial Colorectal Neoplasia Collaborative Group (UO1 CA074799), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (UO1 CA074800), Ontario Registry for Studies of Familial Colorectal Cancer (UO1 CA074783), Seattle Colorectal Cancer Family Registry (UO1 CA074794), and The University of Hawaii Colorectal Cancer Family Registry (UO1 CA074806).
We thank all study participants of the Colon Cancer Family Registry and staff for their contributions to this project.
Abbreviations used in this paper
- CRC
colorectal cancer
- MMR
DNA mismatch repair gene
- CI
confidence interval
- HR
hazard ratio
- SD
standard deviation
- Colon CFR
the Colon Cancer Family Registry
Footnotes
Disclaimer
The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Cancer Family Registries, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the Cancer Family Registry. Authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
Disclosures
The authors have no conflict of interest to declare with respect to this manuscript.
Licence for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
References
- 1.Jass JR. Hereditary Non-Polyposis Colorectal Cancer: the rise and fall of a confusing term. World J Gastroenterol. 2006;12:4943–50. doi: 10.3748/wjg.v12.i31.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasen HFA, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 3.Dunlop MG, Farrington SM, Nicholl I, et al. Population carrier frequency of hMSH2 and hMLH1 mutations. Br J Cancer. 2000;83:1643–5. doi: 10.1054/bjoc.2000.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baglietto L, Lindor NM, Dowty JG, et al. Risks of Lynch Syndrome Cancers for MSH6 Mutation Carriers. J Natl Cancer Inst. 2010;102:193–201. doi: 10.1093/jnci/djp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins MA, Baglietto L, Dowty JG, et al. Cancer Risks For Mismatch Repair Gene Mutation Carriers: A Population-Based Early Onset Case-Family Study. Clinical gastroenterology and hepatology. 2006;4:489–98. doi: 10.1016/j.cgh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Senter L, Clendenning M, Sotamaa K, et al. The Clinical Phenotype of Lynch Syndrome Due to Germ-Line PMS2 Mutations. Gastroenterology. 2008;135:419–28. e1. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Wang W, Lee S, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA: the journal of the American Medical Association. 2006;296:1479. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos tot Nederveen Cappel WH. Surveillance for Hereditary Nonpolyposis Colorectal Cancer. Diseases of the Colon & Rectum. 2002;45:1588–94. doi: 10.1007/s10350-004-7244-3. [DOI] [PubMed] [Google Scholar]
- 9.de Vos tot Nederveen Cappel WH, Buskens E, van Duijvendijk P, et al. Decision analysis in the surgical treatment of colorectal cancer due to a mismatch repair gene defect. Gut. 2003;52:1752–5. doi: 10.1136/gut.52.12.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the Care of Individuals With an Inherited Predisposition to Lynch Syndrome: A Systematic Review. JAMA. 2006;296:1507–17. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 11.Vasen HFA, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer) Journal of Medical Genetics. 2007;44:353–62. doi: 10.1136/jmg.2007.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: An International Resource for Studies of the Genetic Epidemiology of Colon Cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC, et al., editors. The AJCC Cancer Staging Manual. New York, NY: Springer; 2009. [Google Scholar]
- 14.Rogers WH. Regression standard errors in clustered samples. Stata Technical Bulletin. 1993;3:19–23. [Google Scholar]
- 15.Williams RL. A Note on Robust Variance Estimation for Cluster-Correlated Data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 16.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 17.Rodriguez-Bigas M, Vasen H, Pekka-Mecklin J, et al. Rectal cancer risk in hereditary nonpolyposis colorectal cancer after abdominal colectomy. International Collaborative Group on HNPCC Annals of surgery. 1997;225:202. doi: 10.1097/00000658-199702000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–34. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 19.Vasen HFA, Abdirahman M, Brohet R, et al. One to 2-Year Surveillance Intervals Reduce Risk of Colorectal Cancer in Families With Lynch Syndrome. Gastroenterology. 2010;138:2300–6. doi: 10.1053/j.gastro.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan N, Watson P, Silva-Lopez E, et al. Comparison of extended colectomy and limited resection in patients with Lynch syndrome. Diseases of the Colon & Rectum. 2010;53:77. doi: 10.1007/DCR.0b013e3181c702de. [DOI] [PubMed] [Google Scholar]
- 21.Van Dalen R, Church J, McGannon E, et al. Patterns of surgery in patients belonging to Amsterdam-positive families. Diseases of the Colon & Rectum. 2003;46:617–20. doi: 10.1007/s10350-004-6619-9. [DOI] [PubMed] [Google Scholar]
- 22.Madlensky L, Daftary D, Burnett T, et al. Accuracy of colorectal polyp self-reports: findings from the colon cancer family registry. Cancer Epidemiology Biomarkers & Prevention. 2007;16:1898. doi: 10.1158/1055-9965.EPI-07-0151. [DOI] [PubMed] [Google Scholar]
- 23.You YN, Chua HK, Nelson H, et al. Segmental vs. extended colectomy: Measurable differences in morbidity, function, and quality of life. Diseases of the Colon & Rectum. 2008;51:1036–43. doi: 10.1007/s10350-008-9325-1. [DOI] [PubMed] [Google Scholar]
- 24.Lynch A, Church J, Lavery I. Quality of life following partial or total colectomy. Relevance to hereditary non-polyposis colorectal cancer. Dis Colon Rectum. 2003;46:A55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


