Abstract
Although it is well established that hepatic macrophages play a crucial role in the development of liver fibrosis, the underlying mechanisms remain largely elusive. Moreover, it is not known whether other mononuclear phagocytes such as dendritic cells contribute to hepatic stellate cell (HSC) activation and liver fibrosis. Here we show for the first time that hepatic macrophages enhance myofibroblast survival in an NF-κB-dependent manner, and thereby promote liver fibrosis. Microarray and pathway analysis revealed no induction of HSC activation pathways by hepatic macrophages but a profound activation of the nuclear factor-kappa B (NF-κB) pathway in HSCs. Conversely, depletion of mononuclear phagocytes during fibrogenesis in vivo resulted in suppressed NF-κB activation in HSCs. Macrophage-induced activation of NF-κB in HSC in vitro and in vivo was mediated by IL-1 and TNF. Notably, IL-1 and TNF did not promote HSC activation but promoted survival of activated HSC in vitro and in vivo and thereby increased liver fibrosis, as demonstrated by neutralization in co-culture experiments, and genetic ablation of IL-1 and TNF receptor in vivo. Co-culture and in vivo ablation experiments revealed only a minor contribution to NF-κB activation in HSCs by dendritic cells, and no contribution of dendritic cells to liver fibrosis development, respectively.
Conclusion
Promotion of NF-κB-dependent myofibroblast survival by macrophages but not dendritic cells provides a novel link between inflammation and fibrosis.
Keywords: Kupffer cell, NFkB, Interleukin 1, Tumor necrosis factor, M1
The development of liver fibrosis constitutes one of the major complications of chronic liver disease with many clinical consequences such as the development of esophageal varices and ascites being directly related to the presence of liver fibrosis (1). The hepatic wound healing response is a concerted action of multiple resident and non-resident cell types that not only provides a scaffold for structural stability but also involves the removal of cellular debris by infiltrating hepatic macrophages (HM) and the regeneration of functional parenchyma (2,3). Hepatic stellate cells (HSCs) are considered the main fibrogenic cell type in the liver, and are responsible for the production of various types of extracellular matrix (ECM) (2,3). HSCs undergo a well-characterized activation process during which they lose their characteristic vitamin A and lipid stores, and obtain a myofibroblastic phenotype (2,3). The activation of HSCs is controlled by multiple soluble mediators including TGFβ and PDGF, and is part of a complex cellular network that controls the hepatic wound healing response. Previous studies have demonstrated that multiple cell populations including HM, myeloid-derived suppressor cell, B cells, T cells and natural killer cells influence the development of liver fibrosis (4-12). Among those, HM exert a profound effect on HSCs and hepatic fibrosis as shown by genetic or pharmacologic models of macrophage depletion (6,7,13). At the same time, HM also contribute to fibrosis resolution through MMP13 and matrix remodeling (6,14,15). However, the mechanisms by which HM promote liver fibrosis remain largely elusive. Dendritic cells (DC) are developmentally closely related to macrophages, and exert a profound effect on liver fibrosis regression (16) and the cytokine microenvironment during fibrogenesis (12), but their contribution to liver fibrosis development remains unknown.
In the present study, we uncovered the promotion of HSC/myofibroblast survival as novel and to-date unknown mechanism through which macrophages promote fibrosis. Moreover, we demonstrate for the first time that neither classical DC (cDC) and plasmacytoid DC (pDC) contribute to fibrogenesis.
Experimental Procedures
Mice and liver fibrosis induction
C57BL/6 mice, Balb/c mice, CD11c-DTR-eGFP mice (in C57Bl/6 background), Tnfrsf1a/Il1r1-deleted (“dko”) mice (in B6.129S background) and B6.129S mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in a specific pathogen-free facility. Collagen-GFP reporter mice have been described (17). Hepatic fibrosis was induced in 8-12 week-old male mice by ligating the common bile duct (BDL) for 5-15 days as described (18,19), by 4-20 intraperitoneal injections of carbon tetrachloride (0.125 μl/g to 0.5 μl/g body weight, all dissolved in corn oil at a ratio of 1:3), or by 18 weeks treatment with 300 mg/l thioacetamide in drinking water as described (13). Some mice received single or repeated intraperitoneal injections of 200 μl liposomal clodronate (5 mg/ml) or liposomal vehicle as described (13). All animal procedures were approved by the Columbia University or Mount Sinai School of Medicine Institutional Animal Care and Use Committee, and are in accordance with the “Guide for the Care and Use of Laboratory Animals” by the National Institutes of Health.
Dendritic cell depletion
All cDC depletion studies were performed in CD11c-DTR chimeric mice that express CD11c-DTR only in bone marrow and its progeny. In the BDL fibrosis model, cDC depletion was achieved by 2 intraperitoneal injections of diphtheria toxin (25 ng/g body weight) or PBS at days 4 and 6. In the CCl4 fibrosis model, depletion of cDC was achieved by intraperitoneal diphtheria toxin injection every 72 hrs, 25 ng/g for the first two weeks followed by 10 ng/g for the last two weeks. For the depletion of pDC, C57B6 mice were injected with pDC-depleting antibody 120G8 or isotype control (500 μg/mouse IP dissolved in 200 μL saline) every 48 hrs during the last two weeks of CCl4-induced fibrosis.
Statistical analysis
All data are expressed as means ± standard deviation. For comparison of two groups, two-sided unpaired t-test or Mann-Whitney test were used. For multiple group comparisons, ANOVA with Tukey posthoc analysis was performed. For correlation, Pearson correlation co-efficient was calculated. A p-value < 0.05 was considered statistically significant.
Additional procedures are described in the Supplementary Method Section.
Results
Macrophages activate NF-κB in HSCs in vitro and in vivo
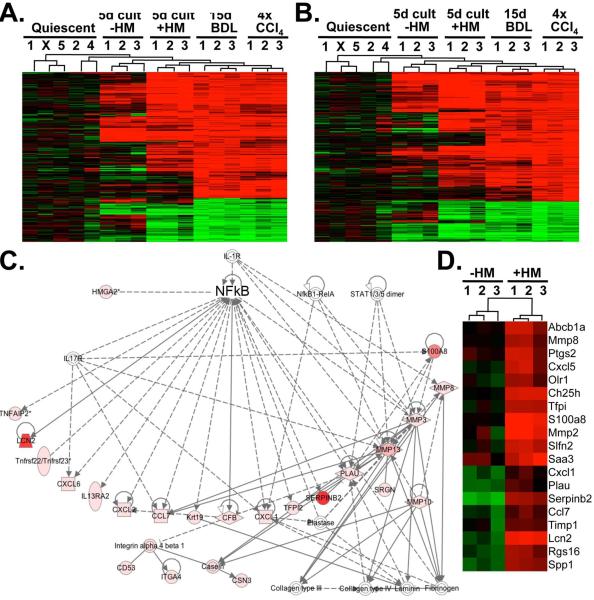
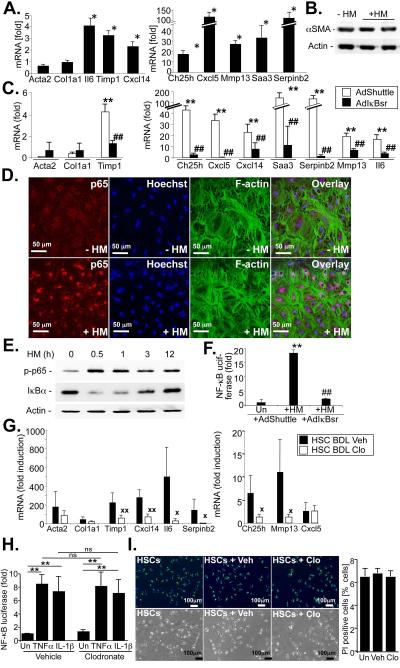
HSCs activate in a complex in vivo environment, characterized by the presence of multiple resident and recruited cell populations including macrophages. To identify signaling pathways through which hepatic macrophages (HM) exert profibrogenic effects, we determined, by microarray analysis, which genes and signaling pathways are activated in HSCs co-cultured with F4/80-positive HM from fibrotic livers (Suppl.Fig.1). Microarray analysis revealed that co-culture of HSCs with HM in a contact-independent manner resulted in a profound influence on gene expression shifting the pattern towards those observed in in vivo-activated HSCs isolated either from bile duct-ligated or CCl4-treated mice (Fig.1A-B), as previously postulated by us (18). IPA pathway analysis of the more than 1400 genes with significant and >2-fold change (Suppl.Table 1) revealed liver fibrosis and inflammatory responses as the most significant toxicological and biological functions (Suppl.Fig.2A-B), and the NF-κB pathway as center component of the highest ranked network (Fig.1C). Accordingly, NF-κB–regulated genes were significantly overrepresented among genes with more than 10-fold induction (χ2 test, p<0.00001). Out of the 82 genes with more than 10-fold upregulation, 18 have been described as NF-κB–dependent (Fig.1D, Suppl.Table 2). We confirmed these results by real-time qPCR, and found that co-culture with HM for 24h or for five days increased the expression of known NF-κB–regulated genes including Il6, Saa3, Cxcl5, Cxcl14, Serpinb2, Ch25h and Mmp13 (Fig.2A,C). Surprisingly, HM did not induce classical HSC activation markers such as Col1a1, Col1a2 or Acta2 mRNA and did not change αSMA protein levels in HSCs (Fig.2C). We confirmed that all NF-κB-dependent genes, including Timp1, were suppressed in the presence of adenoviral IκB superrepressor (Fig.2A) or by short-term treatment with IKK inhibitor Bay 11-7085 at very low non-toxic concentrations (Suppl.Fig.3). NF-κB activation was further confirmed by p65 immunohistochemistry (Fig.2D) and immunoblot (Fig.2E) demonstrating p65 translocation, p65-S536 phosphorylation and IκBα degradation in HSCs treated with conditioned media from HM but not after treatment with control media. Similar observations were made in an NF-κB reporter assay, in which co-culture with HM induced a greater than 15-fold increase in NF-κB-driven luciferase activity (Fig.2F). Based on these results, we focused on the NF-κB pathway in subsequent analyses of mechanisms by which HM affect HSCs and fibrogenesis. Next we determined whether HM alter NF-κB-dependent gene expression in HSCs in the fibrotic liver employing a depletion approach. For this purpose, we analyzed gene expression in FACS-sorted ultrapure HSCs isolates that were immediately lysed after isolation and thus provide a “snapshot” of HSC gene expression in the fibrotic liver. NF-κB dependent gene expression was highly upregulated in HSCs activated in vivo in comparison to quiescent HSCs (Fig.2G). Macrophage depletion by repeated liposomal clodronate injection efficiently reduced F4/80-positive and CD11b- and F4/80-double positive macrophages, and ameliorated liver fibrosis following BDL and CCl4 treatment (Suppl.Fig.4). Notably, macrophage depletion strongly suppressed the expression of the NF-κB dependent genes that were upregulated by HM in our co-culture system (Fig.2G). We further excluded that liposomal clodronate directly affects NF-κB activation by NF-κB reporter assay and or cell death in cultured HSCs (Fig.2H,I).
Figure 1. Microarray and pathway analysis reveal NF-κB and not fibrogenic activation as the predominant effect of hepatic macrophages on HSCs.
A-B. Heatmap from microarrays showing quiescent HSCs, 5 day in vitro-activated HSCs, 5 day in vitro-activated HSCs co-cultured with HM, HSC from mice bile duct-ligated for 15 days and HSCs from mice after 4 CCL4 injections. The heatmap (left panel) includes all genes that were significantly (adjusted p-value <0.05) and at least 4-fold up-or downregulated in HSCs from BDL livers (A). The heatmap (right panel) includes all genes that were significantly (adjusted p-value <0.05) and at least 4-fold up-or downregulated in HSCs from CCl4 livers (B). C. Shown is the highest-ranking network from IPA analysis. D. Heatmap of NF-κB-responsive genes with significant (adjusted p-value <0.05) and at least 10-fold induction in response to co-culture with HM.
Figure 2. Hepatic macrophages induce NF-κB but not myofibroblastic activation in HSCs in vitro and in vivo.
A-B. Hepatic stellate cells were co-cultured with HM for 5 days (A), followed by determination of NF-κB responsive genes by qPCR, or western blot for αSMA (B). C. HSCs were infected with AdIκBsr or an empty control adenovirus followed by co-culture with HSCs for 24h. mRNA expression of HSC activation markers and NF-κB responsive genes was determined by qPCR. D. HSCs were stimulated with conditioned media from HM for 1h. NF-κB activation was determined by immunofluorescent p65 staining (red fluorescence) in HSCs co-stained for F-actin (green fluorescence). Nuclei were marked by Hoechst (blue fluorescence). E. HSCs were stimulated with conditioned media from HM for various times. NF-κB activation was determined by immunoblot for S536-phosphorylated p65 and IκBα. F. HSCs were transduced with an adenoviral NF-κB reporter. After co-culture with HM for 24h, luciferase activity was determined and is expressed as fold induction compared to HSCs cultured with empty inserts. G. Gene expression patterns were determined in ultrapure unplated HSCs isolated from 15 day bile duct-ligated vehicle-treated mice (n=6 isolations) and clodronate-treated mice (n=4 isolations) mice by a combination of gradient centrifugation and FACS sorting without additional plating. mRNA expression of fibrogenic genes and NF-κB dependent genes was determined by qPCR and expressed in comparison to quiescent HSCs isolated from control wt mice (n=3 isolations). H-I. Hepatic stellate cells were treated with 50 μl of 5mg/ml solution per ml of media or liposomal vehicle for 12h. TNFα- and IL-1β-induced NF-κB reporter activity (H.) and cell death, showing Hoechst stained nuclei (blue), retinoids (green) and propidium iodide positive nuclei (red) were determined (I). **p<0.01 in comparison to HSCs cultured without HM. p<0.05 in comparison to HSCs cultured in the absence of HM ## p<0.01 in comparison to AdShuttle-infected HSCs co-cultured with HM.  p<0.05,
p<0.05,  p<0.01 vs HSC BDL Veh.
p<0.01 vs HSC BDL Veh.
IL-1 and TNF mediate macrophage-induced NF-κB activation in HSCs
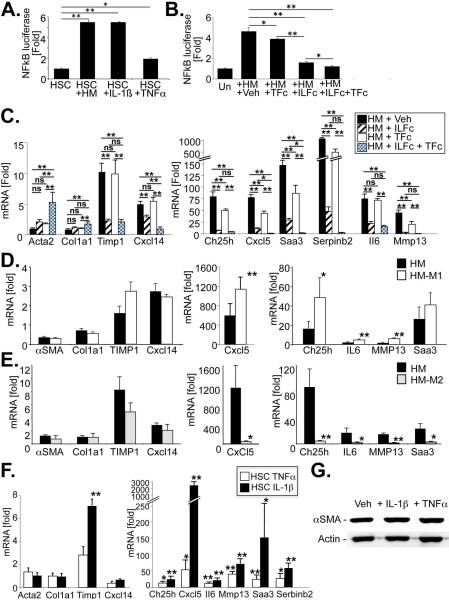
Next, we investigated mechanisms through which HM induce NF-κB activation in HSCs. First, we tested the contribution of IL-1 and TNF to HM-induced NF-κB in HSCs, based on (i) their known potent activation of NF-κB, (ii) the presence of the IL-1 receptor in the NF-κB network identified by IPA analysis (Fig.1C), and upregulated M1 markers iNOS and Cox2 in HM from BDL mice (Suppl.Fig.1C). HM induced NF-κB to the same degree as rmIL-1β, and to a higher degree than rmTNFα (Fig.3A). TNF and IL-1 neutralization in co-cultures by antagonistic TNF-receptor I-Fc and IL-1-receptor I-Fc chimera demonstrated potent inhibition of NF-κB-driven luciferase activity by IL-1 neutralization, and moderate inhibition by TNF neutralization (Fig.3B). Combined TNF and IL-1 neutralization almost completely blocked the HM-induced NF-κB-driven luciferase activity. Similarly, IL-1 and TNF neutralization also blocked HM-induced upregulation of NF-κB dependent genes Ch25h, Cxcl5, Saa3, Serpinb2, Il6 and Mmp13 with IL-1 neutralization exerting pronounced and TNF neutralization exerting moderate effects (Fig.3C). Again, combined TNF and IL-1 neutralization resulted in almost complete suppression of NF-κB-dependent gene expression. Conversely, IL-1β and TNFα upregulated all NF-κB target genes in HSCs that were induced by HM with the exception of Cxcl14 (Fig.3F). Moreover, converting the HM population that consisted of a mixed M1/M2 phenotype (Suppl.Fig.1C) as in previous studies (20), to an inflammatory M1 phenotype by treatment with LPS and IFNγ further increased the expression of NF-κB dependent genes in HSCs (Fig.3D). Conversely, converting the HM population to an M2 phenotype by combined treatment with IL-10 and IL-4 suppressed the expression of NF-κB-dependent genes in HSCs (Fig.3E).
Figure 3. Macrophage-derived IL-1 and TNF promote NF-κB activation but not myfibroblastic activation in HSCs.
A. NF-κB-driven luciferase activity was determined in HSCs that were co-cultured with HM, or treated with IL-1β (5 ng/ml) or TNFα (30 ng/mL) for 24h. B. NF-κB-driven luciferase activity was determined in HSC co-cultured with HM in the presence of vehicle (0.1%BSA in PBS), antagonistic TNFRI-Fc chimera (TFc, 0.5 μg/ml), antagonistic IL-1RI-Fc chimera (IL-Fc, 0.5 μg/ml) or both for 24h. C. mRNA expression of HSC activation markers or NF-κB responsive genes was determined by qPCR in HSC co-cultured with HM in the presence or absence of TNFRI-Fc chimera, IL-1RI-Fc chimera or both for 24h. D-E. Macrophages isolated from BDL livers were treated with treated 16 hours with LPS (10 ng/mL) and interferon gamma (10 ng/mL) to convert them into M1 phenotype (D), or with IL-4 (10 ng/ml) and IL-10 (10 ng/ml) to convert them into M2 phenotype (E). Following 5 washes, M1 or M2 macrophages were co-cultured with HSCs. Co-culture induced expression of NF-κB-dependent genes was determined by qPCR. F. HSCs were treated with rmIL-1β (5 ng/ml) or rmTNFα (30 ng/ml) for 6h followed by analysis of HSC activation markers and NF-κB dependent genes by PCR. G. αSMA levels were analyzed by immunoblotting after treating HSCs with rmIL-1β or rmTNF for 5 days. *p<0.05, ** p<0.01
IL-1 and TNF do not induce HSC activation but enhance HSC survival in vitro and in vivo
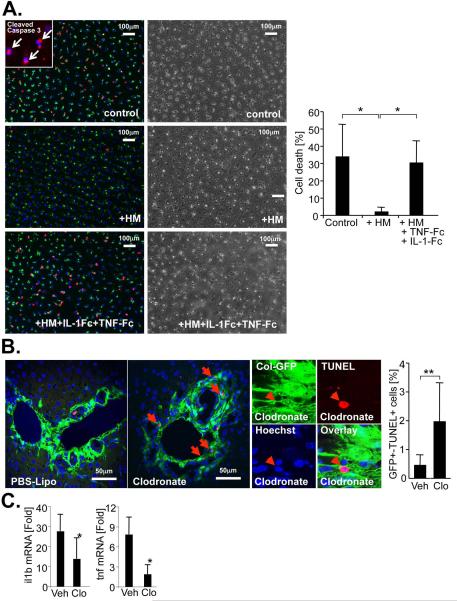
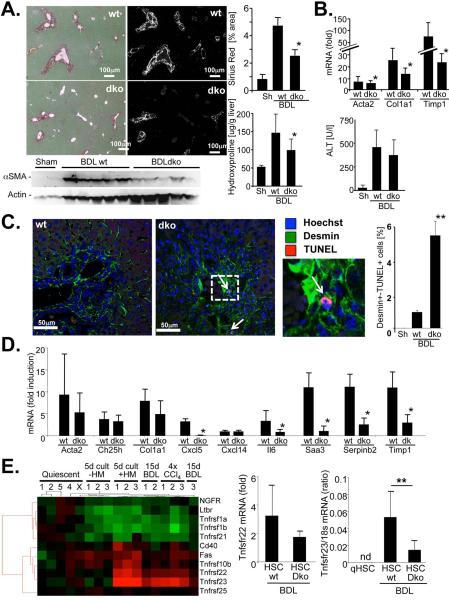
As one main function of the NF-κB pathway is the protection from cell death, we next determined whether HM-induced NF-κB prevented HSC death, or whether it directly promoted HSC activation. In contrast to previous studies (21), IL-1β and TNFα failed to directly induce HSC activation (Fig.3F-G). Co-culture of HSCs with HM strongly suppressed cell death induced by prolonged cell culture in low-serum media (Fig.4A), a well-established method of inducing HSC death (22,23) that showed signs of apoptosis such as caspase 3 cleavage (Fig.4A). The protective effects of HM were almost completely abolished by the combined neutralization of IL-1 and TNF (Fig.4A). Conversely, rmIL-1β was as efficient as HM in rescuing HSC from cell death (Suppl.Fig.5). Furthermore, neutralization of IL-1 and TNF did not reduce viability of HM, and HM supernatant could also suppress HSC death (data not shown), again emphasizing that HSCs and not HM are the relevant targets of IL-1 and TNF. To determine whether this survival pathway was also responsible for the decreased fibrosis observed in macrophage-depleted mice, we performed TUNEL assays in clodronate- or vehicle-treated collagen-GFP reporter mice following BDL to detect apoptosis in GFP-positive collagen-producing myofibroblasts. In mice receiving liposomal clodronate during fibrogenesis, we detected a five-fold increase in GFP- and TUNEL-double positive cells using confocal microscopy (Fig.4B). Notably, the rate of TUNEL-positive HSCs in vehicle-treated mice were very similar to peak rates in previous studies that investigated the contribution of HSC apoptosis to fibrosis resolution (22,24), thus underscoring the relevance of this dramatic increase in HSC apoptosis for liver fibrosis. We found no effect of liposomal clodronate on HSC viability thereby excluding that liposomal clodronate directly induces HSC apoptosis in fibrotic livers (Fig.2I). We also observed reduced IL-1β and TNFα mRNA in fibrotic livers from clodronate-treated mice (Fig.4C). To test the in vivo relevance of this pathway, we first investigated how deficiency of IL-1β, the predominant activator of NF-κB in our co-culture experiments, affects liver fibrosis. In contrast to previously published studies, we found no statistically significant difference in BDL-induced liver fibrosis between IL1R1 knockout and wild-type mice, and further confirmed this data in the CCl4 and thioacetamide models of liver fibrosis (Suppl.Fig.6). If IL-1 signaling promoted liver fibrosis by increasing NF-κB-dependent HSC survival rather than direct HSC activation, it would be likely that TNFα, the other major NF-κB activating cytokine produced by macrophages, could still achieve NF-κB activation in HSCs and thus compensate for the loss of IL-1 signaling in this model. Based on the hypothesis that absence of both IL-1 and TNF signaling would be required to reduce HSC survival and liver fibrosis, we performed BDL in TNFR1/IL1R1 double knockout mice (dko) and wild-type control mice. Compared to wild-type mice, dko mice showed significantly reduced hepatic fibrosis after 5 or 15 days of BDL (Fig.5A-B) and a five-fold increase in apoptotic TUNEL and desmin double-positive HSCs without significant differences in hepatic injury (Fig.5B) supporting our hypothesis that suppression of both IL-1 and TNF signaling are required to affect HSC survival and liver fibrosis. Moreover, we found a significant reduction of NF-κB-dependent genes including Il6, Cxcl5, Saa3, Serpinb2 and Timp1 in ultrapure unplated HSCs from dko mice thus confirming that NF-κB activation in HSC was mediated by TNF and IL-1 (Fig.5C). Our microarray analysis revealed an upregulation of two Trail decoy receptors, murine Trail decoy receptor 1 (Tntrsf23) and murine Trail decoy receptor 2 (Tnfrs22) in HSCs co-cultured with HM and in HSCs from BDL and CCl4 livers (Fig.5D, Suppl.Table 2). Notably, Trail-mediated apoptosis is major contributor to HSC cell death induced by hepatic natural killer cells in vitro and in vivo (11,25). Neutralization of TNF or IL-1 prevented the upregulation of Tnfrsf22 and Tnfrsf23 mRNA by HM in co-culture experiments (Suppl.Fig.7A). Moreover, depletion of HM by liposomal clodronate or dko of TNFR1 and IL1R1 reduced Tnfrsf22 and Tnfrsf23 expression in vivo (Suppl.Fig.7B).
Figure 4. Hepatic macrophages protect HSCs from cell death.
A. HSCs were starved in 0.1% FBS in the presence or absence of HM, and the absence or presence of antagonistic TNFRI-Fc and IL-1RI-Fc chimera. Cell death was determined by propidium iodide staining (red). Nuclei were visualized by Hoechst (blue). Vitamin A-containing HSC lipid droplets were visualized by autofluorescence (pseudocolored in green). Shown are representative images and a quantification of the average of three independent HSC and HM isolations. Insert shows cleaved caspase 3 immunofluorescent staining (red) and Hoechst (blue). B. To determine apoptosis in collagen-expressing myofibroblasts, collagen-GFP reporter mice were treated with vehicle (n=5) or clodronate (n=4) followed by BDL for 9 days and TUNEL staining of liver sections. Cell death of collagen-expressing myofibroblasts was visualized by confocal microscopy and quantified by counting cells double-positive cells for GFP (green) and TUNEL (red). Nuclei were visualized by Hoechst staining (blue). C. Hepatic levels of IL-1β and TNFα mRNA were determined by qPCR in bile duct-ligated vehicle (n=17) and clodronate-treated (n=10) mice. *p<0.05; **p<0.01.
Figure 5. TNF and IL-1 mediate NF-κB activation and protection from cell death in HSCs during liver fibrosis.
A. TNFRI and IL1-RI dko (n=5) and wild-type mice (n=10) underwent BDL for 14 days. Hepatic fibrosis was assessed by Sirius Red staining and polarized microscopy, αSMA immunoblotting and hydroxyproline assay. B. TNFRI and IL1-RI dko (n=7) and wild-type mice (n=9) underwent BDL for 5 days. Hepatic fibrogenesis was assessed qPCR for profibrogenic genes, hepatic injury by ALT measurement. C. Following 2 weeks of BDL, HSCs were isolated from mice TNFRI and IL1-RI dko (n=5 isolations) or wild-type mice (n=5 isolations) by gradient centrifugation and Vitamin A fluorescence-based FACS without plating. mRNA expression of fibrogenic and NF-κB dependent genes was determined by qPCR and expressed in comparison to quiescent HSCs isolated from wild-type mice (n=3 isolations). D. To determine apoptosis in HSCs, liver sections from 14 day bile-duct ligated TNFRI and IL1-RI dko (n=5) and wild-type mice (n=5) were stained for desmin (green fluorescence) and by TUNEL (red fluorescence). Nuclei were marked by Hoechst staining (blue fluorescence). Cells positive for both desmin and TUNEL were quantified. *p<0.05; **p<0.01. E. Heatmap of TNF receptor family members including Trail and Trail decoy receptors under different conditions, determined by microarray analysis (left panel). Expression of Trail decoy receptors Tnfrsf22 and Tnfrsf23 as determined by qPCR in ultrapure unplated HSCs isolated from 14 day bile duct-ligated wild-type mice (n=5 isolations) and double-deficient for TNFRI and IL1-RI mice (“dko”, n=5 isolations) by a combination of gradient centrifugation and FACS analysis without additional plating (center and right panel). Data is expressed as fold induction in comparison to quiescent HSCs isolated from control wt mice (n=3 isolations) or as ratio between the investigated mRNA and 18s mRNA if mRNA expression was not detectable in qHSC. *p<0.05; **p<0.01. nd: non-detectable.
Dendritic cells do not contribute to HSC activation and fibrosis in vivo
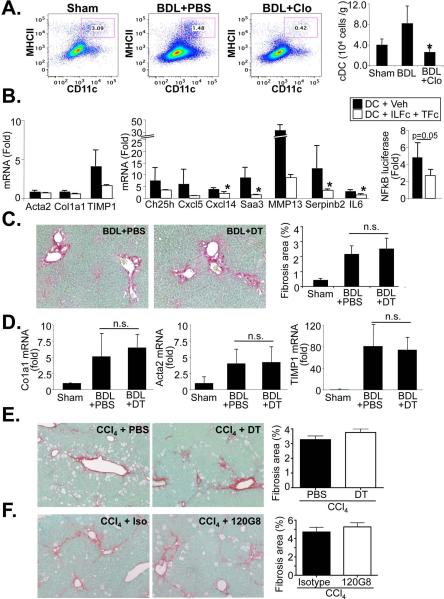
Liposomal clodronate does not affect HSC number (13) or biology (Fig.2 H,I), but may deplete dendritic cells (DC), a highly endocytotic cell population as demonstrated by our FACS analysis (Fig.6A). DC contribute to liver inflammation (12) and to the regression of liver fibrosis (16), but their role in the development of liver fibrosis is not known. To test whether DC may contribute to HSC activation and liver fibrogenesis, we first performed a co-culture of DC and HSCs. Similar to HM, DC did not activate HSCs but upregulated the expression of NF-κB dependent genes, and NF-κB-driven luciferase reporter activity through an IL-1 and TNF-dependent manner (Fig.6B). However, activation of NF-κB was considerably lower than the induction we observed in HM co-culture. Based on these results, we next determined whether ablation of DC may have contributed to the reduced fibrogenesis in clodronate-treated mice. In our first approach, we performed BDL in diphtheria toxin-treated or PBS-treated bone marrow-chimeric CD11c-DTR-eGFP mice. Bone marrow chimerism avoids the known side effects of diphtheria toxin treatment observed after long-term treatment in global CD11c-DTR-eGFP mice (26). We did not observe a significant difference in BDL-induced fibrosis as determined by Sirius Red staining and qPCR for fibrogenic genes αSMA, Col1a1 and TIMP1 (Fig.6C-D). We confirmed this data employing CCl4 injection for induction of liver fibrosis, again using bone marrow-chimeric CD11c-DTR-eGFP mice. Similar to the BDL model, we did not observe significant differences in liver fibrosis between PBS and diphtheria toxin-treated mice (Fig.6E). As a third approach, we used antibody-mediated ablation of pDC. Again, we did not observe a reduction of CCl4-induced liver fibrosis (Fig.6F). Importantly, we achieved considerable depletion of cDC and pDC by above methods (Suppl.Fig.8). Similar to previous studies (27), we observed neutrophilia in CD11c-DTR mice (Suppl.Fig.9) but consider this unlikely to exert a profound effect on fibrosis based on previous studies (28). Thus, our data suggest that neither class of DC significantly contributes to liver fibrogenesis in vivo.
Figure 6. Dendritic cells moderately induce NF-κB dependent gene transcription in HSCs but do not contribute to BDL- and CCl4-induced liver fibrosis.
A. Bile duct ligated mice were injected every 5 days for a total of 3 injections with PBS (n=4) or clodronate (n=3) followed by quantification of cDC using CD11c and MHCII flow cytometry FACS plots show percentage of double-positive cells, the bar graph shows cell numbers per gram liver. B. mRNA expression of HSC activation markers (left panel), NF-κB responsive genes (middle panel) or NF-κB-driven luciferase activity (right panel) were determined by qPCR and NF-κB reporter assay, respectively, in HSC co-cultured with DC in the presence of antagonistic TNFRI-Fc and IL-1RI-Fc chimera (both 0.5 μg/ml) or vehicle (0.1%BSA in PBS) for 24h. C-D. Liver fibrosis was induced by BDL in CD11c-DTR bone marrow-chimeric mice. Chimeric male CD11c-DTR were treated with two injections of PBS (n=5) or diphtheria toxin (25 ng/g body weight) (n=6). Mice were sacrificed after 7 days. Deposition of fibrillar collagen was determined by Sirius Red staining (left upper panel) and morphometric quantification (right upper panel) (C). Expression of profibrogenic genes Acta2, Col1a1 and TIMP1 was determined by qPCR. Fold induction was calculated to sham-operated control (D). E. Chimeric CD11c-DTR mice (n=4 each group) were used for DC depletion (25 ng/g first two weeks, 10 ng/g last two weeks) and simultaneous liver fibrosis induction using CCl4 (0.5 μl/g, three time per week). Fibrosis was determined by Sirius Red staining. F. For pDC depletion C57Bl/6 mice (n=4) were injected every 48 hours with 120G8 antibody (500 μg/mouse) or isotype control during last two weeks of CCl4 induced liver fibrosis (0.5 μl/g, three time per week for four weeks). Fibrosis was determined by Sirius Red staining. * p<0.05
Discussion
Hepatic fibrogenesis is involves multiple resident and recruited cell populations. HSCs represent the center component of this wound healing response but other population including macrophages are known positive modulators of fibrogenesis. Here, we uncover a novel function of macrophages, the promotion of HSC/myofibroblast survival. A second novel finding of our study lies in the discovery that DC do not contribute to liver fibrosis.
Employing microarray and pathway analysis, we discovered that NF-κB, the best characterized anti-apoptotic signaling pathway (29,30) and an important regulator of liver injury and fibrosis (31), was a key pathway activated in HSCs by HM. The relevance and physiologic nature of the employed in vitro co-culture system is validated by the finding that this system achieves HSC gene expression patterns highly similar to those found in vivo-activated HSCs, and that all gene expression changes and functional consequences of NF-κB activation were confirmed in vivo. Activation of the NF-κB pathway was further established by reporter assays, western blots, immunofluorescence and qPCR. Most importantly, NF-κB was activated in HSCs from fibrotic livers, and macrophage depletion reduced NF-κB activation in HSCs. The activation of NF-κB in HSCs in liver fibrosis is consistent with a previous study, but points towards macrophages instead of angiotensin II as the main trigger of NF-κB activation in HSCs (32). Surprisingly, co-culture with macrophages and macrophage-secreted cytokines such as IL-1β and TNFα did not promote HSC activation, and is consistent with the reported minor or insignificant inductions of αSMA and Col1a1 mRNA (33) and absence of increased αSMA protein expression in most studies that co-cultured human , and murine HSCs with macrophages (33,34). Only one previous study found a profound and significant activation of rat HSCs by HM (35). In our study, macrophage-induced NF-κB activation rendered activated HSCs more resistant to cell death in vitro and in vivo, thereby promoting the persistence of activated HSCs and fibrosis. Although the rate of 1% HSC apoptosis in fibrotic livers appeared low, it reflects the rapid removal of apoptotic cells in vivo (as opposed to their accumulation in vitro), and is virtually identical to peak apoptosis rates reported by Iredale et al. (22). Thus, the observed increase to 5% HSC apoptosis is biologically highly significant, reducing the number activated myofibroblasts and limiting fibrogenic responses as reported (11,22,32,36). It is likely that increased NF-κB activation protects activated HSCs from both intrinsic and extrinsic inducers of cell death. Accordingly, our study also found that HM induce the expression of Trail decoy receptors in HSCs in an NF-κB-dependent manner. This finding is of interest as natural killer cells, which are particularly enriched in the liver and activated during liver injury, significantly contribute to killing of activated HSCs during liver fibrosis in a Trail-dependent manner (11,37,38).
Our study identified IL-1 and TNF as main factors of HM-mediated NF-κB activation and cytoprotection in HSC. Notably, we observed no effect of IL-1β or TNFα on HSC activation. The key role of HM-derived IL-1 and TNF in NF-κB activation and protection from HSC death was not only found in vitro, but also in vivo as demonstrated by the profound decrease in NF-κB responsive genes in unplated, ultrapure HSCs isolates from TNFR1/IL1R1 double-knockout mice, and increased apoptosis of desmin-positive cells in TNFR1/IL1R1 dko livers after BDL. Previous studies have demonstrated reduced fibrogenesis in mice deficient in TNFR1 or IL1-R (39,40). In contrast to these studies, we could not observe reduced liver fibrosis in IL-1R knockout mice in three different models of liver fibrosis. This is consistent with the notion that both TNFα and IL-1β are powerful NF-κB activators, that they can likely functionally substitute each other.
Our study employed F4/80-positive HM from bile duct-ligated livers for co-culture experiments and therefore exposed HSCs to a mixture of resident and recruited macrophages typically found during fibrogenesis. Accordingly, our data show that these HM have a mixed M1/M2 phenotype as previously reported (20) Based on our observations that converting HM into M1 phenotype increased, and into . M2 phenotype reduced their ability to induce NF-κB-dependent gene expression in HSCs, we conclude that the inflammatory/M1 HM subpopulation contributes to NF-κB activation and HSC survival. It should be emphasized that the M1/M2 classification does not fully account for diverse and often overlapping biological functions of macrophage populations, in particular in the liver (20) . It is conceivable that different HM populations collaborate for the induction fibrosis in vivo, with inflammatory M1-type HM promoting HSC survival and M2-type HM affecting HSCs through other pathways We did not find a significant impact of Gr1 status in HM on NF-κB activation in HSCs . (data not shown) suggesting that both recruited and resident macrophages are capable of promoting NF-κB activation in HSCs. Clodronate did not affect HSC activation directly nor did it alter NF-κB activation in HSCs. Moreover, our results employing DC depletion additionally excluded DC as potential contributors to clodronate effects as we did not see a contribution of this cell type to liver fibrosis.
Dendritic cells are key regulators of inflammation and the cytokine milieu in the fibrotic liver (12). Moreover, DC contribute to the regression of liver fibrosis through an MMP9-dependent mechanism (16). However, the contribution of DC to fibrogenesis is unknown. Although we found that CD11c-positive DC induce a moderate degree of NF-κB activation in HSCs via TNF and IL-1 production, we did not observe a role for pDC or cDC in promoting liver fibrosis in BDL- and CCl4-induced liver fibrosis. Most likely, the much lower number of DC in the liver in comparison to HM and the lower potency of NF-κB activation by DC renders the contribution of DC-derived TNF and IL-1 to the overall pool and NF-κB-mediated HSC survival insignificant. In this regard, the ratio of DC to HSCs in our co-culture experiments is at least one or two magnitudes higher than the ratio that can be achieved in a fibrotic liver. Another possible explanation may be the critical role of DC in NK cell activation, cells with well established anti-fibrogenic potential (11,41). None of the available CD11c-DTR based ablation strategies can achieve a completely selective depletion of cDC without affecting the composition of other immune cells (26,27). Even recent transgenic mouse models that avoid early neutrophilia after DC depletion still lead to neutrophilia after two days (27). Although neutrophilia represents a confounding factor, we consider it unlikely that neutrophilia affects fibrogenesis based on previous studies that did not show effects on liver fibrosis (28). These data suggest that DC interact with and regulate other immune cells, or that increased granulopoiesis after ablation causes these secondary effects. Nonetheless, the ablation strategies employed in our study (i) avoid common side effects described for DC ablation (26) due to our use of CD11c-DTR chimeric mice and 120G8 antibody, (ii) address the role of both cDC and pDC, and (iii) investigate the role of cDC in two common models of liver fibrosis.
Our study contains several limitations: First, as we observed more than 1400 HM-regulated genes, it is likely that genes besides NF-κB-regulated genes affect HSC responses. Further studies are required to unravel the relevance of NF-κB-independent genes and pathways regulated by HM. These may include additional mediators secreted from HM such as IL-6 and TGFβ (35,42). Accordingly, our IPA analysis revealed Stat1/3/5 as HM-activated pathways. Second, our studies were performed in mouse models, and further studies are required to determine whether HM-induced NF-κB activation plays a role in human fibrogenesis. As patients develop fibrosis slowly over decades, pathways that promote long-term myofibroblast survival may be particularly relevant. IL-1 and TNF inhibitors may be considered for antifibrotic therapies but may cause severe side effects. In conjunction with previous studies (32,43), our data support the concept that targeting NF-κB pathway in HSCs and subsequent induction of HSC apoptosis may be a more suitable anti-fibrogenic strategy. In summary, our study shows that HM provide a novel link between inflammation, HSC survival and liver fibrosis, and suggest that inflammatory signaling pathways may provide additional targets for antifibrotic therapies in the liver. Future studies need to determine whether macrophage-mediated promotion of myofibroblast survival also promotes fibrosis in other organs.
Supplementary Material
Acknowledgments
Financial support: This study was supported NIH grants R01DK076920 and U54CA163111 (both to Robert F. Schwabe). Jean-Philippe Pradere was supported by a postdoctoral fellowship from the American Liver Foundation. Johannes Kluwe was supported by the German Research Foundation (KL2140/2-1) and a Sheila Sherlock fellowship from the European Association for the Study of the Liver. Ingmar Mederacke was supported by the German Research Foundation (ME 3723/1-1). Dianne Dapito was supported by NIH grant 1F31DK091980. Costica Aloman was supported by NIH grant 1K08DK088954
List of Abbreviations
- α-SMA
α smooth muscle actin
- BDL
bile duct ligation
- CCl4
Carbon tetrachloride
- DC
dendritic cells
- ECM
extracellular matrix
- HM
hepatic macrophages
- HSC
hepatic stellate cell
- qPCR
quantitative real-time PCR
- cDC
classical dendritic cell
- dko
double knockout
- FACS
fluorescence-activated cell sorting
- GFP
green fluorescent protein
- IL
interleukin
- IPA
Ingenuity Pathway Analysis
- mRNA
messenger RNA
- NF-κB
nuclear factor kappa B
- PBS
phosphate-buffered saline
- pDC
plasmacytoid DC
- TNF
tumor necrosis factor
- TUNEL
terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
References
- 1.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maher JJ. Interactions between hepatic stellate cells and the immune system. Semin Liver Dis. 2001;21:417–426. doi: 10.1055/s-2001-17555. [DOI] [PubMed] [Google Scholar]
- 5.Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A. 1997;94:10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, Burchardt ER, et al. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200–207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- 8.Suh YG, Kim JK, Byun JS, Yi HS, Lee YS, Eun HS, Kim SY, et al. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology. 2012 doi: 10.1002/hep.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, Shlomchik MJ, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2010;53:230–242. doi: 10.1002/hep.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 12.Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 14.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, Iredale JP. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 15.Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, Ramachandran P, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 16.Jiao J, Sastre D, Fiel MI, Lee UE, Ghiassi-Nejad Z, Ginhoux F, Vivier E, et al. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55:244–255. doi: 10.1002/hep.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, et al. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 18.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Kluwe J, Pradere JP, Gwak GY, Mencin A, De Minicis S, Osterreicher CH, Colmenero J, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–334. e327. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teixeira-Clerc F, Julien B, Grenard P, Tran Van, Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 24.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Tittel AP, Heuser C, Ohliger C, Llanto C, Yona S, Hammerling GJ, Engel DR, et al. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods. 2012;9:385–390. doi: 10.1038/nmeth.1905. [DOI] [PubMed] [Google Scholar]
- 28.Saito JM, Bostick MK, Campe CB, Xu J, Maher JJ. Infiltrating neutrophils in bile duct-ligated livers do not promote hepatic fibrosis. Hepatol Res. 2003;25:180–191. doi: 10.1016/s1386-6346(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 29.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 30.Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 31.Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oakley F, Teoh V, Ching ASG, Bataller R, Colmenero J, Jonsson JR, Eliopoulos AG, et al. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology. 2009;136:2334–2344. e2331. doi: 10.1053/j.gastro.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 33.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology. 2006;44:1487–1501. doi: 10.1002/hep.21427. [DOI] [PubMed] [Google Scholar]
- 36.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927–939. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- 37.Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–1451. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- 38.Gur C, Doron S, Kfir-Erenfeld S, Horwitz E, Abu-Tair L, Safadi R, Mandelboim O. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut. 2011 doi: 10.1136/gutjnl-2011-301400. [DOI] [PubMed] [Google Scholar]
- 39.Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1324–1331. doi: 10.1152/ajpgi.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarrats N, Moles A, Morales A, Garcia-Ruiz C, Fernandez-Checa JC, Mari M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54:319–327. doi: 10.1002/hep.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, et al. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713–723. doi: 10.1053/j.gastro.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oakley F, Meso M, Iredale JP, Green K, Marek CJ, Zhou X, May MJ, et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology. 2005;128:108–120. doi: 10.1053/j.gastro.2004.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.