Abstract
Narcolepsy is a neurological disorder characterized by excessive daytime sleepiness, cataplexy, hypnagonic hallucinations, sleep paralysis, and disturbed nocturnal sleep patterns. Narcolepsy is caused by the loss of hypocretin (orexin)-producing neurons in the lateral hypothalamus. Evidence, such as a strong association with HLA DQB1*06:02, strongly suggests an autoimmune basis targeting hypocretin neurons. Genome-wide association studies have strengthened the association between narcolepsy and immune system gene polymorphisms, including the identification of polymorphisms in the T cell receptor alpha locus, TNFSF4 (also called OX40L), Cathepsin H (CTSH) the purinergic receptor P2RY11, and the DNA methyltransferase DNMT1. Recently, attention has been raised regarding a spike in cases of childhood narcolepsy in 2010 following the 2009 H1N1 pandemic (pH1N1) in China and vaccination with Pandemrix, an adjuvanted H1N1 vaccine that was used in Europe. How the immune system may be involved in disease initiation and/or progression remains a challenge to researchers. Potential immunological pathways that could lead to the specific elimination of hypocretin producing neurons include molecular mimicry or bystander activation, and are likely a combination of genetic and environmental factors, such as upper airway infections.
Narcolepsy and the loss of hypocretin
Narcolepsy affects approximately 0.02% of the population worldwide. Narcoleptic patients frequently have severe sleepiness, which makes remaining awake during activities that demand an alert state particularly difficult. Additionally, these individuals commonly have fragments of Rapid Eye Movement (REM) sleep that intrude into wakefulness, such as hypnagogic (dream-like) hallucinations as they drift off to sleep, as well as brief episodes of cataplexy (muscle paralysis) triggered by strong emotions [1–4]. One of the most important breakthroughs in elucidating the cause of narcolepsy came with the discovery of hypocretins (orexins) [5–7]*. Hypocretins are exclusively synthesized in the lateral hypothalamus (LH) and are derived from a single protein precursor named prepro-hypocretin. Prepro-hypocretin is enzymatically cleaved into two peptides, hypocretin 1 and 2 (also called orexin A and B), which are comprised of 33 and 28 amino acids respectively. There are two cloned hypocretin receptors, HCRTR1 and HCRTR2, both of which are serpentine G-protein-coupled receptors [6]*. Hypocretin-secreting neurons project from the LH throughout the central nervous system (CNS) to neurons involved in the regulation of feeding, sleep-wakefulness, neuroendocrine homeostasis, and autonomic regulation [8, 9]. Hypocretin knockout mice and dogs with null mutations in the HCRTR2 gene develop narcoleptic symptoms, indicating that the loss of this peptide is causal for development of narcolepsy.
The loss of hypocretin has also been reported by numerous clinical studies of narcoleptic individuals [10–15]*.
Narcoleptic patients typically have low hypocretin cerebrospinal fluid (CSF) levels which can be explained by the loss of over 90% of their hypocretin-producing neurons, a process that has been associated with narcoleptic onset [16]. This loss of hypocretin-producing cells is seemingly selective, rather than general or regional destruction, as intermingling melanin-concentrating neurons appear unaffected in the same narcoleptic patients [14, 15]*. This specific depletion of hypocretin-secreting neurons led to the hypothesis that narcolepsy is an autoimmune driven process within the hypothalamus [17–19].
Increased onset of narcolepsy following infections
It has become increasingly clear that environmental factors play an important role in the development and progression of a variety of autoimmune conditions [20]. Particular attention has focused on the connection between infections and disease pathophysiology [21]. Susceptibility to narcolepsy has been associated with common upper airway infections, including those caused by streptococcus pyogenes and the influenza A virus [22–24]*. In China, the onset of narcolepsy in children appears to follow seasonal patterns, with significant increases in cases reported in the months following winter-related infections [24]**. Streptococcal infections have also been associated with various neurological autoimmune disorders, including Sydenham Chorea, suggesting that certain immunological responses to infection may result in specific neurological damage [25]. Epidemiological studies have revealed that individuals infected with streptococcus are more than five times as likely to develop narcolepsy, with streptococcus-specific antibodies found in 65% of narcoleptic patients as compared to 26% of age-matched controls [22, 23]*. The development of narcolepsy in association with upper airway infections could result from various processes, including molecular mimicry and bystander activation. Molecular mimicry would involve processing and presenting bacterial and viral peptides in the context of DQB1*06:02, which would activate a population of cross-reactive T cells present in predisposed individuals. Bystander activation from the generalized proinflammatory environment associated with infections could facilitate the destruction of hypocretin-secreting neurons in a variety of ways. Streptococcal infections can induce the production of IL-17 by T cells, and IL-17 receptor signaling has been shown to facilitate permeability of the blood brain barrier, potentially exposing hypocretin-secreting neurons to the immune system. While the relative contribution of these immunological processes to disease pathophysiology remains unclear, the connection to upper-airway infections provides additional support for an autoimmune basis of narcolepsy.
Increased onset of narcolepsy in China following the H1N1 pandemic of 2009
The correlation between influenza A infection and the onset of narcolepsy was further strengthened by reports from China showing that onset in children was highly correlated with seasonal patterns of upper airway infections, including a spike in diagnosis following the H1N1 influenza pandemic of 2009 [24]**, a phenomenon that was followed by a decrease in cases in the years that followed [26]*. Intriguingly, the vast majority (>95%) of patients diagnosed with narcolepsy following the 2009 pandemic had not received H1N1 vaccination, indicating that naturally occurring influenza A infections may increase the susceptibility of developing this disorder. As most of the newly reported cases were young children following upper-airway infections, important questions have arisen regarding the specific immunological responses generated in these young individuals that may have increased their risk of developing narcolepsy.
While these findings are of extreme interest to our understanding of narcolepsy, the connection between infection and onset is not a novel observation. Kinnier Wilson also described a cohort of similar new cases of narcolepsy due to the worldwide epidemic of encephalitis from about 1918 to 1925 secondary to the Spanish flu pandemic (Wilson SAK, The Narcolepsies. Brain 1928; 51: 63–109).
Pandemrix vaccination connected to narcolepsy in Scandinavia
Reports from Scandinavia first suggested the possibility that the development of narcolepsy could have been triggered by H1N1 vaccination [27]. Recent studies have indicated that Pandemrix, an AS03 (squalene, alpha-tocopherol) H1N1 vaccine developed in response to the 2009 H1N1 influenza pandemic, likely contributed to the spike of narcoleptic onset seen among children and young adults in Finland [28]. Finnish researchers reported a 12.7-fold increased risk of narcoleptic diagnosis in children and young adults approximately eight months following Pandemrix vaccination when compared to age-matched individuals that did not receive the vaccination [28, 29]**. Further reports revealed an association in Sweden, Finland, Ireland and in the UK following the AS03-adjuvanted vaccine and an increased risk of developing narcolepsy mostly increased in individuals between 5–19 years of age [28]. Conversely, there have been no reported increases in the incidence of narcolepsy in Italy, or the Netherlands, though the reason behind this phenomenon remains unclear [27]. Much attention has now been focused on systematically collecting data to further understand the association between H1N1 infections/vaccinations and the onset of narcolepsy [30]. What remains unclear, however is why other H1N1 vaccines, such as those with squalene alone as an adjuvant (available in Europe), or non-aduvanted vaccine (all vaccines used in the US) did not seem to be as strongly associated, if associated at all with increased narcolepsy incidence.
Immunological associations of narcolepsy
The importance of the immune system and its potential role in the onset of narcolepsy has been the focus of research and debate for many years [31–33]. Much of the initial supporting evidence was based on strong genetic linkage to 6p21.3, an incredibly gene-rich region of the genome that includes a multitude of immunologically associated genes including the human leukocyte antigen (HLA) [34]. With more than 98% of narcoleptic patients with low CSF hypocretin-1 carrying HLA DQB1*06:02, frequently in combination with HLA DRB1*15:01, narcolepsy has one of the strongest known associations with HLA [35, 36]. While nearly all narcoleptic patients express DQB1*06:02, expression of DQB1*06:02 is not limited to narcoleptic individuals; Between 12–38% of the general population are carriers of this allele [37]. The association between various HLA class II encoded HLA-DRB1-DQA1-DQB1 haplotype and several autoimmune diseases has been previously described, including Graves’s disease, rheumatoid arthritis, and type 1 diabetes [38–42]. The significant association with DQB1*06:02 strongly suggests an interaction between a specific T cell receptor subtype leading to the destruction of hypocretin producing neurons.
Other reported immunological associations with narcolepsy include polymorphisms in the T cell receptor alpha (TCRα) locus [43–45]**. Following somatic DNA recombination of V, D and J segments in TCR loci, alpha and beta chains are generated by developing T cells to form a unique, antigen-specific heterodimeric protein called the TCR. Expressed on the surface of T cells, the TCR plays an integral role in the recognition of antigens bound to HLA molecules on the surface of antigen presenting cells (APCs) (Figure 1A, [II]) [46]. Specific variants in the TCR alpha locus J region segment were shown to confer an increased risk of developing narcolepsy [43]**. These findings in addition to the strong association with DQB1*06:02 strongly suggest a T cell-mediated autoimmune basis in the development of narcolepsy.
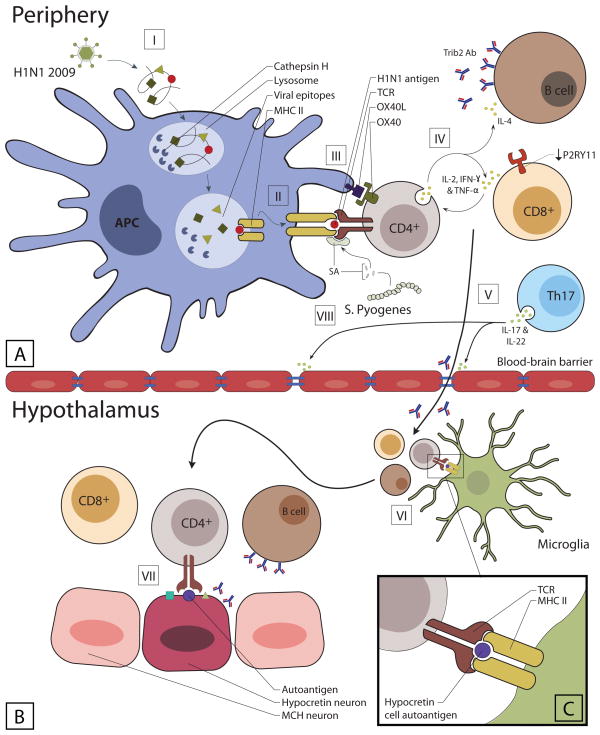
Figure 1. Autoimmunity and hypocretin cell loss.
[I] Following phagocytosis, APCs degrade H1N1 influenza virus in the lysosome, a process facilitated by cathepsin H.
[II] Peptides from the virus are presented on the surface of APCs in the context of MHC class II (DQA1*01:02-DQB1*06:02) for cognate TCR recognition.
[III] Hypocretin specific CD4+ T cells recognizes the presented antigen and is activated. After being activated, the CD4+ T cells upregulate the expression of OX40 which is an important costimulatory molecule recognized by APCs that express OX40L.
[IV] Activated CD4+ T cells secrete IFN-γ, TNF-α and IL-2, which stimulate CD8+ T cells and drive a Th1 mediated immune response. IL-4 from activated CD4+ T cells promotes a humoral response leading to the production of Tribbles2 antibody by B cells.
[V] Generation of super antigens as a result of streptococcus infection activates Th17 cells, facilitating the breakdown of the blood-brain barrier through the secretion of IL-17 and IL-22. Increased permeability allows for the migration of activated T cells, B cells, and TRIBBLES2 antibodies into the CNS.
[VI] Migration of activated T cells and B cells through the BBB is followed by interaction with CNS APCs (microglia) that present autoantigens (hypocretin cell autoantigens) in the context of DQA1*01:02-DQB1*06:02
[VII and inset C] Reactivated T cells reach the hypothalamus and recognize hypocretin neurons, inducing effector functions such as the secretion of cytokines or cytotoxic compounds.
Moreover, super-antigens from streptococcus cross-link the MHC and TCR molecules independent of antigen specificity activating the autoreactive T cell. This may lead to sensitization to hypocretin-specific antigens and form the basis for subsequent booster reactions of cross-reactive CD4+ T cells, a process that finally will end in an autoimmune reaction against hypocretin neurons.
APC, antigen presenting cell; MCH, melanin concentrating hormone; MHC, major histocompatibility complex; TCR, T cell receptor.
Recent Immunochip studies found that polymorphisms in Cathepsin H (CTSH), a member of the Cathepsin family, were also associated with narcolepsy [47]. Cathepsins are endosomal/lysosomal enzymes that play an important role in numerous cellular processes, including protein recycling, antigen processing, and peptide-MHC II interactions. CTSH is highly expressed in APCs such as monocytes, dendritic cells, and B cells. It is reasonable to hypothesize that changes in CTSH activity could alter the MHC II-peptide repertoire presented to T cells leading to an increased risk of developing narcolepsy (Figure 1A, [II]).
Associations between narcolepsy and polymorphisms within the Tumor Necrosis Factor (ligand) Superfamily member 4 (TNFSF4, also called OX40L) were also found in the same Immunochip study [47]. OX40L is an important costimulatory molecule that is upregulated in APCs in proinflammatory environments and functions to promote T cell survival, expansion, and effector functions. In addition, OX40-OX40L interactions have been implicated in the regulation of regulatory T cells, and are important in maintaining tolerance to prevent autoimmunity. It is possible that altered co-stimulation of T cells could skew the immune response to infection (Figure 1A, [III]), thus increasing the risk of developing narcolepsy.
Reports of narcoleptic patients with additional clinical symptoms including deafness, cerebellar ataxia, and neuropathy have recently been associated with mutations in the exon 21 of the DNMT1 gene [48]. DNMT1 is a DNA methytransferase that is highly expressed in the immune system and has been shown to be important for T regulatory cell differentiation following TCR stimulation [49]. The consequences of this mutation on the immune system for narcoleptic patients is unknown, though an alteration in T regulatory cell development and or function is known to result in various autoimmune conditions. It is also possible that DNMT1 has effect on neurodegeneration.
Further strengthening the case for an autoimmune basis, a SNP in the 3′ untranslated region of P2RY11, a purinergic receptor gene, has also shown strong association with narcolepsy [45, 50]**. P2RY11 is an ATP receptor that modulates leukocyte maturation, chemotaxis, and apoptosis. Further analysis revealed a correlation between this P2RY11 allele and reduced expression in cytotoxic lymphocytes as well as natural killer (NK) cells. Reduced expression of P2RY11 could lead to reduced resistance to ATP-mediated cell death, potentially altering the immune response to infection (Figure 1A, [IV]), or possibly directly effecting hypocretin-secreting neurons. Interestingly, P2RY11 is located extremely close to DNMT1, and this proximity (synteny) is conserved in phylogeny up to zebrafish, suggesting that these two genes may have common or intertwined regulatory elements.
Researchers have long searched for evidence of hypocretin-specific antibodies as a possible mechanism of the specific destruction of hypocretin-producing neurons in the brain. Many of the findings were either negative or inconclusive, though a humoral contribution cannot be completely ruled out as an important component [33]. Recent studies have shown altered levels of total IgG and hypocretin-specific IgM in narcoleptic patients, though these findings were not specific to patients with low levels of hypocretin [51, 52]. Using a transgenic mouse model to identify proteins co-localized with hypocretin, researchers were able to detect autoantibodies against tribbles homologue 2 (TRIB2) in the serum of 14% of narcoleptic individuals compared to 5% in controls [53]*. This observation was replicated in multiple ethnic groups and anti-TRIB2 autoantibodies were associated with the onset of narcolepsy [54, 55], although not in post H1N1 cases. While TRIB2 is highly expressed by hypocretin producing neurons, TRIB2 can be found in many other cell types, indicating this is unlikely the causative mechanism that results in the specific destruction of hypocretin producing neurons (Figure 1A, [IV]) [56].
Autoimmunity and narcolepsy
An autoimmune basis for the hypocretin cell loss in narcolepsy has long been suspected due to its strong genetic association with selected HLA alleles. HLA encodes multiple subtypes of MHC class I and II proteins that present foreign peptides to T cells during infections thereby triggering immune responses via TCR activation. In autoimmunity, self-peptides are believed to be mistakenly perceived as foreign, thus leading to tissue destruction, which often occurs in context of specific HLA alleles.
Among autoimmune diseases, narcolepsy is uniquely positioned to demonstrate molecular mimicry in humans. First, narcolepsy occurs nearly exclusively in individuals with DQB1*06:02. Second, a specific association with the TCRα locus also confers an increased risk, indicating a crucial role for specific J segments of TCRα in the “immunological synapse’ of narcolepsy (Figure 1A, [II]). Finally, studies have shown increased rates of narcoleptic onset in children following exposure to influenza A H1N1 infections and selected H1N1 vaccine preparations. These findings strongly suggest that some T cells that can be activated by H1N1 epitopes that leads to the destruction of hypocretin-producing neurons (Figure 1B, [VII]). A parsimonious explanation could involve mimicry between H1N1 and a peptide contained in hypocretin cells (Figure 2G, 2B). Many cofactors have recently been identified as co-localizing with hypocretin and could be involved. The fact the AS03-containing vaccine was particularly associated with increased onset could be the result of the strength and nature of the AS03 adjuvant that would catalyze molecular mimicry between H1N1 proteins and hypocretin-cell containing proteins.
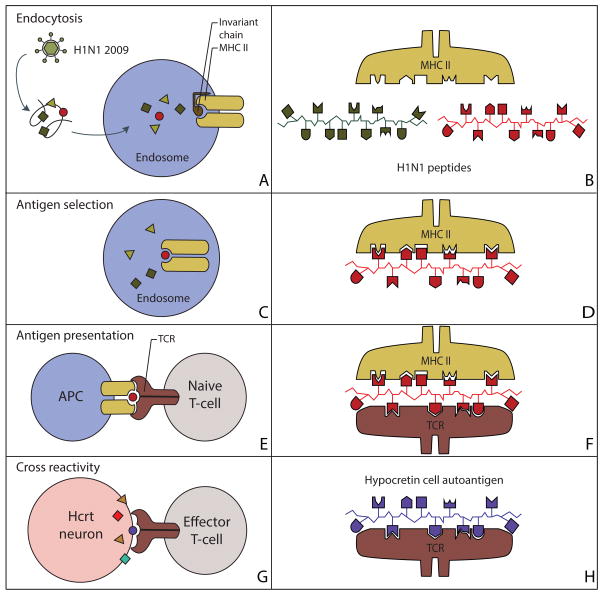
Figure 2. Molecular mimicry between H1N1 peptides and hypocretin neuron autoantigens.
Sequence and structural homology between foreign and self-peptides are required for molecular mimicry to occur.
(A) The H1N1 virus is engulfed by an APC and the foreign antigenic material is digested into peptide fragments in the endosome/lysosome.
(B) The invariant chain is digested and the MHC groove is ready for occupancy by the antigenic fragment.
(C, D) The MHC binding groove selects the H1N1 fragment with a specific amino acid sequence in the context of DQA1*01:02-DQB1*06:02.
(E) The vesicles move to the plasma membrane and the complex is displayed at the cell surface for TCR recognition.
(F) The TCR recognizes a presented peptide with a specific amino acid sequence.
(G, H) Activated CD4+ T cells cross-react and recognize hypocretin neuron autoantigens as foreign molecules, prompting an autoimmune response against hypocretin neurons.
MHC, major histocompatibility complex; TCR, T-cell receptor.
Controversy still remains regarding the likely contribution of the immune system to the development of narcolepsy. Neither key targets of immune cells nor a molecular mechanism to explain how the immune system is responsible for the specific depletion of hypocretin-producing neurons have yet been identified. While the body of evidence clearly implicates the immune system, future studies will be required to confirm the autoimmune basis of narcolepsy. We believe that one of the reasons why the mechanism may be so difficult to detect is an absence of epitope spreading and autoantibodies in the disease. This may however turn out to be an advantage eventually, as if the autoimmune process is finally discovered, it is more likely to be closer to causality.
Supplementary Material
Table 1.
| Protein | Expression | Function | Reference Study | Relationship to narcolepsy |
|---|---|---|---|---|
|
| ||||
| HLA DQB1*06:02 | Antigen presenting cells | Encode MHC Class II proteins | Mignot et al., 1997. | 99% of narcoleptic patients carry this allele |
| TCR alpha | T cells | Recognize antigens presented by APCs | Hallmayer et al., 2009. | An amino acid variant in the TCR alpha locus J24 segment confers an increased risk of developing narcolepsy |
| CTSH | Antigen presenting cells | Degradation of lysosomal proteins | ||
| OX40L | Antigen presenting cells | Amplifies Th2 cell differentiation | ||
| DNMT1 | Lymphoblasts, CD4+ and CD8+ cells | Regulates the addition of methyl groups to DNA to effect gene expression | Winkelmann et al., 2012. | Mutations in exon 21 of this gene cause autosomal dominant cerebellar ataxia, deafness and narcolepsy |
| P2RY11 | Lymphoblasts, CD4+ and CD8+ cells | Modulates leukocyte maturation, chemotaxis, and apoptosis | Kornum et al., 2011. | There is a reduced expression in cytotoxic lymphocytes NK cells of narcoleptic patients |
| TRIB2 | Wide expression | Induces apoptosis of cells mainly of the hematopoietic origin | Toyoda et al., 2010; Kawashima et al., 2010. | Anti-TRIB2 autoantibodies were associated with the onset of narcolepsy |
Highlights.
Narcolepsy is caused by the loss of hypocretin-producing neurons in the brain.
HLA DQB1*06:02 association suggests an autoimmune basis for narcolepsy.
Association with H1N1 infections indicates molecular mimicry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sonka K, Susta M. Diagnosis and management of central hypersomnias. Ther Adv Neurol Disord. 2012;5(5):297–305. doi: 10.1177/1756285612454692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgess CR, Scammell TE. Narcolepsy: neural mechanisms of sleepiness and cataplexy. J Neurosci. 2012;32(36):12305–11. doi: 10.1523/JNEUROSCI.2630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. 2012;9(4):739–52. doi: 10.1007/s13311-012-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viorritto EN, Kureshi SA, Owens JA. Narcolepsy in the pediatric population. Curr Neurol Neurosci Rep. 2012;12(2):175–81. doi: 10.1007/s11910-011-0246-3. [DOI] [PubMed] [Google Scholar]
- 5.Nevsimalova S, et al. Narcolepsy: clinical differences and association with other sleep disorders in different age groups. J Neurol. 2012 doi: 10.1007/s00415-012-6702-4. [DOI] [PubMed] [Google Scholar]
- 6*.Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–85. doi: 10.1016/s0092-8674(00)80949-6. This research identified two novel neuropeptides named orexin-A and -B (Hcrt 1 and -2) that bind and activate two closely related orphan GPCRs that appeared to have a role in the control of feeding and energy homeostasis. [DOI] [PubMed] [Google Scholar]
- 7*.de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–7. doi: 10.1073/pnas.95.1.322. Analysis of the expression patterns of subtracted hypothalamus-enriched sequences revealed a 130-residue putative secretory protein (preprohypocretin), which gave rise to two products structurally related both to each other (hypocretin-1 and 2) and to secretin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnavion P, de Lecea L. Hypocretins in the control of sleep and wakefulness. Curr Neurol Neurosci Rep. 2010;10(3):174–9. doi: 10.1007/s11910-010-0101-y. [DOI] [PubMed] [Google Scholar]
- 9.Dalal J, et al. Translational profiling of hypocretin neurons identifies candidate molecules for sleep regulation. Genes Dev. 2013;27(5):565–78. doi: 10.1101/gad.207654.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–76. doi: 10.1016/s0092-8674(00)81965-0. This work determined that canine narcolepsy is caused by disruption of the hypocretin (orexin) receptor 2 gene (Hcrtr2), and it is the first to establish that disruptions in the hypocretin system result in narcolepsy. [DOI] [PubMed] [Google Scholar]
- 11*.Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–51. doi: 10.1016/s0092-8674(00)81973-x. This paper established that genetic ablation of hypocretin (orexin) in mice results in narcolepsy. [DOI] [PubMed] [Google Scholar]
- 12*.Nishino S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355(9197):39–40. doi: 10.1016/S0140-6736(99)05582-8. This is the first description of hypocretin deficiency in human narcolepsy. [DOI] [PubMed] [Google Scholar]
- 13.Mignot E, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59(10):1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 14.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 15*.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–74. doi: 10.1016/s0896-6273(00)00058-1. This work corroborated that hypocretin cell loss befalls in human narcolepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savvidou A, et al. Hypocretin deficiency develops during onset of human narcolepsy with cataplexy. Sleep. 2013;36(1):147–8. doi: 10.5665/sleep.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andlauer O, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35(9):1247–55F. doi: 10.5665/sleep.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornum BR, Faraco J, Mignot E. Narcolepsy with hypocretin/orexin deficiency, infections and autoimmunity of the brain. Curr Opin Neurobiol. 2011;21(6):897–903. doi: 10.1016/j.conb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Fontana A, et al. Narcolepsy: autoimmunity, effector T cell activation due to infection, or T cell independent, major histocompatibility complex class II induced neuronal loss? Brain. 2010;133(Pt 5):1300–11. doi: 10.1093/brain/awq086. [DOI] [PubMed] [Google Scholar]
- 20.Molina V, Shoenfeld Y. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity. 2005;38(3):235–45. doi: 10.1080/08916930500050277. [DOI] [PubMed] [Google Scholar]
- 21.Sfriso P, et al. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010;87(3):385–95. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 22.Koepsell TD, Longstreth WT, Ton TG. Medical exposures in youth and the frequency of narcolepsy with cataplexy: a population-based case-control study in genetically predisposed people. J Sleep Res. 2010;19(1 Pt 1):80–6. doi: 10.1111/j.1365-2869.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Aran A, et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32(8):979–83. doi: 10.1093/sleep/32.8.979. A case–control study evaluating whether Streptococcus Pyogenes and Helicobacter pylori infections are triggers for narcolepsy using blood markers of infection. Antibodies against streptococcal proteins were significantly elevated in narcoleptic patients close to onset. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Han F, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70(3):410–7. doi: 10.1002/ana.22587. This study shows using clinical reports from China a strong seasonality in the onset of narcolepsy, and further demonstrates a sharp increase in the number of new cases following the H1N1 pandemic. [DOI] [PubMed] [Google Scholar]
- 25.Snider LA, Swedo SE. Post-streptococcal autoimmune disorders of the central nervous system. Curr Opin Neurol. 2003;16(3):359–65. doi: 10.1097/01.wco.0000073938.19076.31. [DOI] [PubMed] [Google Scholar]
- 26*.Han F, et al. Decreased incidence of childhood narcolepsy 2 years after the 2009 H1N1 winter flu pandemic. Ann Neurol. 2012 doi: 10.1002/ana.23799. A follow-up study which revealed that narcolepsy onset decreased after the the 2009 H1N1 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijnans L, et al. The incidence of narcolepsy in Europe: Before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Nohynek H, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7(3):e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Partinen M, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7(3):e33723. doi: 10.1371/journal.pone.0033723. Report on the occurrence of narcolepsy after Pandemrix H1N1 vaccinations in Finland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poli F, et al. Narcolepsy as an adverse event following immunization: Case definition and guidelines for data collection, analysis and presentation. Vaccine. 2013;31(6):994–1007. doi: 10.1016/j.vaccine.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Mignot E, et al. Narcolepsy and immunity. Adv Neuroimmunol. 1995;5(1):23–37. doi: 10.1016/0960-5428(94)00043-n. [DOI] [PubMed] [Google Scholar]
- 32.Black JL., 3rd Narcolepsy: a review of evidence for autoimmune diathesis. Int Rev Psychiatry. 2005;17(6):461–9. doi: 10.1080/02646830500381492. [DOI] [PubMed] [Google Scholar]
- 33.Overeem S, Black JL, 3rd, Lammers GJ. Narcolepsy: immunological aspects. Sleep Med Rev. 2008;12(2):95–107. doi: 10.1016/j.smrv.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton R, et al. Gene map of the extended human MHC. Nature reviews Genetics. 2004;5(12):889–99. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 35.Rogers AE, et al. HLA DR15 (DR2) and DQB1*0602 typing studies in 188 narcoleptic patients with cataplexy. Neurology. 1997;48(6):1550–6. doi: 10.1212/wnl.48.6.1550. [DOI] [PubMed] [Google Scholar]
- 36.Mignot E, et al. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20(11):1012–20. [PubMed] [Google Scholar]
- 37.Han F, et al. HLA-DQ association and allele competition in Chinese narcolepsy. Tissue Antigens. 2012;80(4):328–35. doi: 10.1111/j.1399-0039.2012.01948.x. [DOI] [PubMed] [Google Scholar]
- 38.Singal DP, Blajchman MA. Histocompatibility (HL-A) antigens, lymphocytotoxic antibodies and tissue antibodies in patients with diabetes mellitus. Diabetes. 1973;22(6):429–32. doi: 10.2337/diab.22.6.429. [DOI] [PubMed] [Google Scholar]
- 39.Dorman JS, Bunker CH. HLA-DQ locus of the human leukocyte antigen complex and type 1 diabetes mellitus: a HuGE review. Epidemiologic reviews. 2000;22(2):218–27. doi: 10.1093/oxfordjournals.epirev.a018034. [DOI] [PubMed] [Google Scholar]
- 40.van der Horst-Bruinsma IE, et al. HLA-DQ-associated predisposition to and dominant HLA-DR-associated protection against rheumatoid arthritis. Human immunology. 1999;60(2):152–8. doi: 10.1016/s0198-8859(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 41.Newton JL, et al. A review of the MHC genetics of rheumatoid arthritis. Genes and immunity. 2004;5(3):151–7. doi: 10.1038/sj.gene.6364045. [DOI] [PubMed] [Google Scholar]
- 42.Simmonds MJ, et al. A novel and major association of HLA-C in Graves’ disease that eclipses the classical HLA-DRB1 effect. Human molecular genetics. 2007;16(18):2149–53. doi: 10.1093/hmg/ddm165. [DOI] [PubMed] [Google Scholar]
- 43**.Hallmayer J, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nature genetics. 2009;41(6):708–11. doi: 10.1038/ng.372. In a genome wide association study of narcolepsy with cataplexy the tree top hits are all SNPs in the T cell receptor alpha locus. This is the first disease association of a common polymorphism in the TCR locus, and the finding strongly supports the autoimmune hypothesis of narcolepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernardini C, et al. Genome-wide gene expression profiling of human narcolepsy. Gene Expr. 2012;15(4):171–81. doi: 10.3727/105221612x13372578119652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han F, et al. TCRA, P2RY11, and CPT1B/CHKB associations in Chinese narcolepsy. Sleep Med. 2012;13(3):269–72. doi: 10.1016/j.sleep.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrie HT, et al. T cell receptor gene recombination patterns and mechanisms: cell death, rescue, and T cell production. The Journal of experimental medicine. 1995;182(1):121–7. doi: 10.1084/jem.182.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faraco J, et al. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS Genet. 2013;9(2):e1003270. doi: 10.1371/journal.pgen.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkelmann J, et al. Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Human molecular genetics. 2012;21(10):2205–10. doi: 10.1093/hmg/dds035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Josefowicz SZ, Wilson CB, Rudensky AY. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. Journal of immunology. 2009;182(11):6648–52. doi: 10.4049/jimmunol.0803320. [DOI] [PubMed] [Google Scholar]
- 50**.Kornum BR, et al. Common variants in P2RY11 are associated with narcolepsy. Nature genetics. 2011;43(1):66–71. doi: 10.1038/ng.734. In a genome wide association study of narcolepsy with cataplexy an association is found with SNPs in the purinergic receptor subtype 2Y11 gene. An effect of the polymorphism on regulation of lymphocytes is also shown. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka S, Honda M. IgG abnormality in narcolepsy and idiopathic hypersomnia. PLoS One. 2010;5(3):e9555. doi: 10.1371/journal.pone.0009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deloumeau A, et al. Increased immune complexes of hypocretin autoantibodies in narcolepsy. PLoS One. 2010;5(10):e13320. doi: 10.1371/journal.pone.0013320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Cvetkovic-Lopes V, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120(3):713–9. doi: 10.1172/JCI41366. Searched for potential autoantigens in Hcrt neurons by transgene expression of a flag-tagged poly(A)-binding protein driven by the Hcrt promoter for capturing mRNAs, which are expressed exclusively by hcrt neurons. A systematic screen for autoantibodies against the corresponding proteins revealed autoantibodies against tribbles homologue 2 (Trib2) in the blood of narcoleptic patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toyoda H, et al. Anti-Tribbles homolog 2 autoantibodies in Japanese patients with narcolepsy. Sleep. 2010;33(7):875–8. doi: 10.1093/sleep/33.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawashima M, et al. Anti-Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. Sleep. 2010;33(7):869–74. doi: 10.1093/sleep/33.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim AS, Scammell TE. The trouble with Tribbles: do antibodies against TRIB2 cause narcolepsy? Sleep. 2010;33(7):857–8. doi: 10.1093/sleep/33.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.