Abstract
Ectopic expression of a set of transcription factors in somatic cells could reprogram the differentiated cell fate into the pluripotent state, and the resultant so-called induced pluripotent stem cells (iPSCs) have been proposed as seed cells for cell therapy–based regenerative medicine. However, their tumorigenicity limited the further application of iPSCs clinically. More recently, collected evidence has shown that differentiated somatic cells could be directly converted into other types of somatic cells through overexpression of transcription factors enriched in the targeted cell types. Induced neurons have been recently converted from fibroblasts; however, it remains unknown if other cell types could be used for neuron induction. One easily accessible cell type, adipocyte progenitor cells (APCs), has the advantage of steady proliferation in vitro and lower mortality rate. In the present study, we demonstrated that APCs could also be converted into functional neurons using the three transcriptional factors (Ascl1, Brn2, Myt1l) that could convert fibroblasts into neurons. Moreover, we also demonstrated that vitamin C could elevate the efficiency of conversion of the APCs and fibroblasts into neurons. The converted cells represent another appropriate cell resource for clinical application and disease modeling.
Introduction
The differentiated cell state can be reprogrammed to the pluripotent state by forced overexpression of a set of transcriptional factors (Takahashi and Yamanaka, 2006; Takahashi et al., 2007), somatic nuclear transfer (Campbell et al., 1996; Gurdon et al., 1958), or fusion with embryonic stem cells (ESCs) (Cowan et al., 2005; Tada et al., 2001; Tada et al., 2003). Subsequently, the pluripotent stem cells can be further differentiated into many kinds of functional cells, such as hematopoietic cells (Grigoriadis et al., 2010; Inoue-Yokoo et al., 2012), hepatocytes (Saito et al., 2006; Sasaki et al., 2009), cardiomyocytes (Klug et al., 1996; Zhang et al., 2009), and neurons (Bain et al., 1995; Hu and Zhang, 2009; Karumbayaram et al., 2009), that can be used for in vitro disease modeling or future application in regenerative medicine. However, the inefficient and time-consuming induction of induced pluripotent stem cells (iPSCs) and tumorigenesis of the resulting iPSCs greatly limited the further application of iPSCs (Pera, 2011).
Recently, the direct conversion of the differentiated fibroblast cells to neuronal cells by overexpression of a cluster of forebrain transcriptional factors (Ambasudhan et al., 2011; Caiazzo et al., 2011; Pang et al., 2011; Son et al., 2011) or microRNAs was reported (Ambasudhan et al., 2011). Direct conversion of mature cells into neurons that will overcome the drawbacks of iPSCs is of potential clinical utility. Although collected evidence has shown that the conversion of fibroblasts into neurons can be achieved (Caiazzo et al., 2011; Sasaki et al., 2009), the requirement of skin biopsies and slow growth rate in vitro make it a cumbersome source for the direct conversion. Adipocyte progenitor cells (APCs) can be easily accessible and have the advantage of slight trauma and steady proliferation, which may be used as an alternative resource for neuronal induction. In this study, we successfully converted mouse APCs into neurons using three transcriptional factors—Ascl1, Brn2, and Myt1l.
Vitamin C, as a neural compound, could elevate the efficiency of generation of iPSCs (Esteban et al., 2010). However, it remains unknown whether vitamin C can elevate the efficiency of neuronal induction. In this study, we found that vitamin C can significantly elevate the efficiency of conversion mouse embryonic fibroblasts and APCs into neurons.
Materials and Methods
Isolation and culture of mouse APCs
Brown adipose tissue of newborn mice was isolated and digested at 37°C in phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA, USA) containing 2 mg/mL collagenase (collagenase A; Roche, Indianapolis, IN, USA), with constant shaking in a centrifugal tube at about 100 rpm for 30 min. After filtration through a 40-μm nylon filter mesh (BD Falcon, San Jose, CA, USA) and centrifugation, the pellet was suspended in medium, counted with a hemocytometer, plated at 5×104 cells/mL in 6-cm tissue culture plates, and cultured in Dulbecco's modified Eagle medium (DMEM; Gibco, Invitrogen, Carlsbad, CA) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Hyclone, Logan, UT, USA). The cells were observed daily under an inverted phase-contrast microscope and passaged after 80–90% confluence. The culture medium was refreshed every 2 days.
Viral infection
Tet-O-Fuw-Ascl1, Brn2, and Myt1l (obtained from Addgene) were used separately along with lentivirus packaging plasmids psPAX-2 and pMD2G to transfect 293T cells. After 48 h, viral particles in the supernatant were collected to infect the APCs. After infection for 6–8 h, the infected cells were cultured with the fresh APC medium. The next day, the medium was changed to APC medium containing doxycycline (2 mg/mL, Sigma, Ronkonkoma, NY, USA). After 48 h, the medium was switched to neuronal induction media consisting of Neurobasal medium (Invitrogen, Carlsbad, CA, USA), 2% B27 (Invitrogen, Carlsbad, CA, USA), 1 mM glutamine (Invitrogen, Carlsbad, CA, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma, Ronkonkoma, NY, USA), and brain-derived neurotrophic factor (BDNF;10 ng/mL, Peprotech, Rocky Hill, NJ, USA) containing doxycycline (2 mg/mL, Sigma, Ronkonkoma, NY, USA). The medium was replaced with the fresh medium every 2–3 days for 14–20 days.
Flow cytometry analysis
To analyze the phenotype of the APCs, cells were collected and washed with FACS buffer (phosphate-buffered salt solution with 2% FBS). Cells were stained with anti-mouse CD90.2-PE/Cy7 monoclonal antibody (BD, San Diego, CA, USA), anti-mouse Sca-1-PE/Cy7 monoclonal antibody (BD, San Diego, CA, USA), anti-mouse CD34-PE monoclonal antibody (BD, San Diego, CA, USA), and anti-mouse CD45-PE monoclonal antibody (BD, San Diego, CA, USA). Cells were then washed and resuspended in FACS buffer. All analyses were performed on a LSRII cell sorter (Beckman Coulter Inc).
Immunofluorescence
For immunofluorescence analysis, mouse APCs and induced neurons were plated onto poly-D-lysine (PDL)-coated glass coverslips. The cells were fixed for overnight at 4°C in 4% paraformaldehyde in PBS, permeabilized for 15 min in PBS containing 0.5% (vol/vol) Triton X-100, blocked with 5% (wt/vol) goat BSA for 1 h at room temperature, and incubated overnight at 4°C in PBS containing 1.25% (wt/vol) bovine serum albumin (BSA) and primary antibodies. Then cells were washed three times with PBS and incubated for 1 h at room temperature using anti-rabbit or anti-mouse secondary antibodies: Alexa Fluor-488, Alexa Fluro-543, or Alexa Fluor-633 (1:500, Invitrogen, Carlsbad, CA, USA). Primary antibodies were as follows: rabbit anti-βIII-tubulin (Tuj1) (1:500, Covance, San Diego, CA, USA), mouse anti-MAP2a (1:500, Millipore Corporation, Billerica, MA, USA), mouse anti-NeuN (1:300, Millipore Corporation, Billerica, MA, USA), rabbit anti-vGLUT1 (1:1000, Synaptic Systems, Goettingen, Germany), mouse anti-GABA (1:500, Millipore Corporation, Billerica, MA, USA), and mouse anti-Nestin (1:300, Developmental Studies Hybridoma Bank, IA, USA).
Reverse transcription-polymerase chain reaction
Total RNAs were extracted from mouse APCs and the neurons derived from APCs using the TRIzol isolation system (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions. The yield and integrity of the RNA were determined by the Nanodrop and agarose gel electrophoresis, respectively. A 2-μg amount of total RNA was transcribed into cDNA using oligo(dT), primers, and reverse transcriptase (Promega, Madison, WI, USA). The primers used to amplify cDNA samples are listed in Table S1 (Supplementary Data are available at www.liebertpub.com/cell/.)
Electrophysiology
Electrophysiological experiments were performed on APC-derived neurons and mouse embryonic fibroblast (MEF)-derived neurons treated with vitamin C. Whole-cell patch-clamp recordings in either voltage or current clamp mode were conducted to measure voltage-activated sodium/potassium currents or action potentials. The EPC-10 (HEKA) amplifier was used for recording the electrophysiological signals, and the data were acquired by the Patchmaster software (HEKA) and analyzed using IGOR Pro (Wavemetrics). The glass micropipette contained a solution that consisted of 130 mM K+-gluconate, 20 mM KCl, 10 mM HEPES, 0.2 mM EGTA, 4 mM Mg2ATP, 0.3 mM Na2GTP, and 10 mM Na+-phosphocreatine (at pH 7.3, 290–310 MOsm). The pipette resistance was in the range of 2.0–4.0 MΩ. The bath solution contained 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, and 10 mM glucose (at pH 7.4). All electrophysiological experiments were performed at room temperature.
Statistical analysis
After infection for 14–20 days, the total number of neurons that were treated with vitamin C and were mock-treated were quantified. The cells were counted on 10 fields from three replicates and normalized with the number of cells before infection. Data were analyzed by analysis of variance (ANOVA) using software. The statistical significance was at p<0.05.
Results and Discussion
Adipose tissue was collected from the newborn mice and the APCs were further enriched by culture. To preclude the possibility of pre-existence of neurons in the culture, we passaged the cells at least three times before induction to eliminate the neurons that could not attach to the surface of dishes upon passage. The in vitro differentiation capacity and gene expression characteristics of established APCs were characterized further. As shown in Figure S1A, the cultured cells exhibited the typical morphology of APCs. After induction, the APCs could efficiently differentiate into mature adipocytes, as revealed by Oil Red staining (Fig. S1B). Furthermore, high-level expression of PPAR-2, SMO-2, and KROX-20, which are considered to be marker genes of APCs, could also be observed in the cultured APCs (Fig. S1C). Because some of the APCs markers can also be expressed by neural crest stem cells (NCSCs), we further tested the expression of NCSC markers in our primary culture to preclude contamination by NCSCs. Using real-time PCR and an immunofluorescence staining assay, we found that the APCs we derived did not express the NCSC markers P75, Sox10, and Nestin (Fig. S1E). To detect the heterogeneity of the cell population of the derived APCs, we characterized the cultured mouse APC population according to its surface marker profile using flow cytometry. In this study, we found that the APCs were positive for Thy-1 (CD90.2, 92.8%) and Ly-6A/E (Sca-1, 87.4%), but they did not express the hematopoietic lineage markers CD34 (0.0%) or CD45 (0.2%) (Fig. S1D). This result illustrated that the APCs we derived were highly homogeneous.
To further investigate if the APCs could be directly converted into neuron-like-cells by overexpression of the transcription factors discovered previously for induction of fibroblasts into neurons, lentiviruses carrying Ascl1, Brn2, and Myt1l (ABM) were used for the subsequent experiments. The lentiviruses were first produced in 293T cells, and the infection procedure was performed as shown in Figure 1A. After infection by these viruses, the APCs underwent rapid changes in morphology (Figs. 1B and S1F). Many cells gradually formed compact cell bodies with long cytoplasmic extensions within 3 days after infection. At about 10 days after infection, the cell bodies became spherical and the extrusion of the cells extended further and developed more branches, resembling cultured neurons (Fig. 1B). To confirm that the neuronal-like cells were indeed derived from the APCs by ABM induction rather than the pre-existing neurons in the APCs, we infected the cells with lentivirus carrying green fluorescent protein (GFP) alone in parallel. None of the cells overexpressing GFP showed neuronal-like morphology (Fig. 1C).
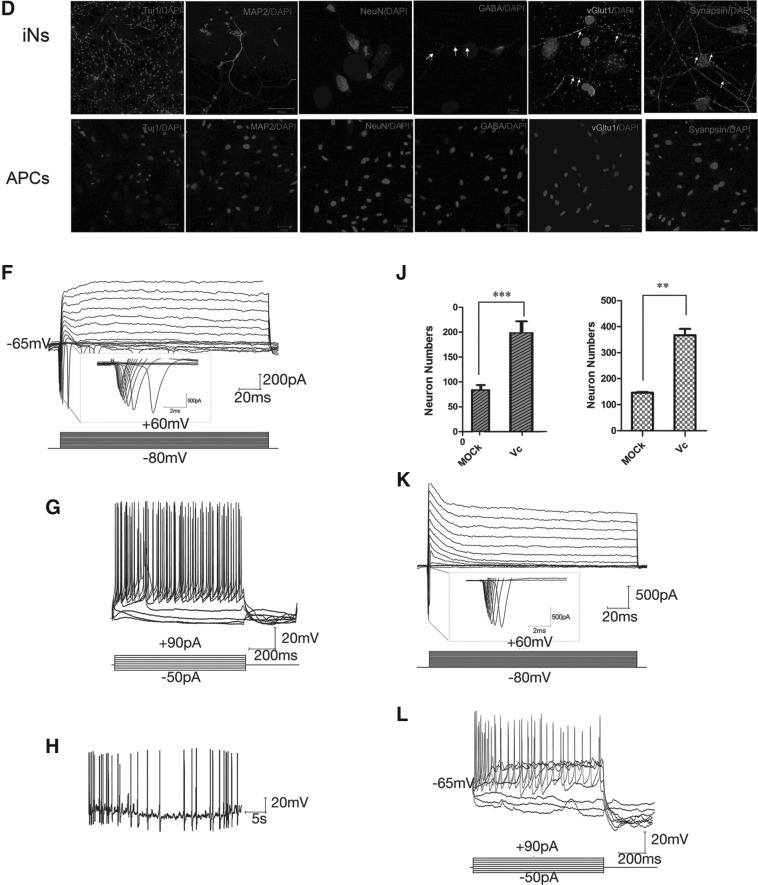
FIG. 1.
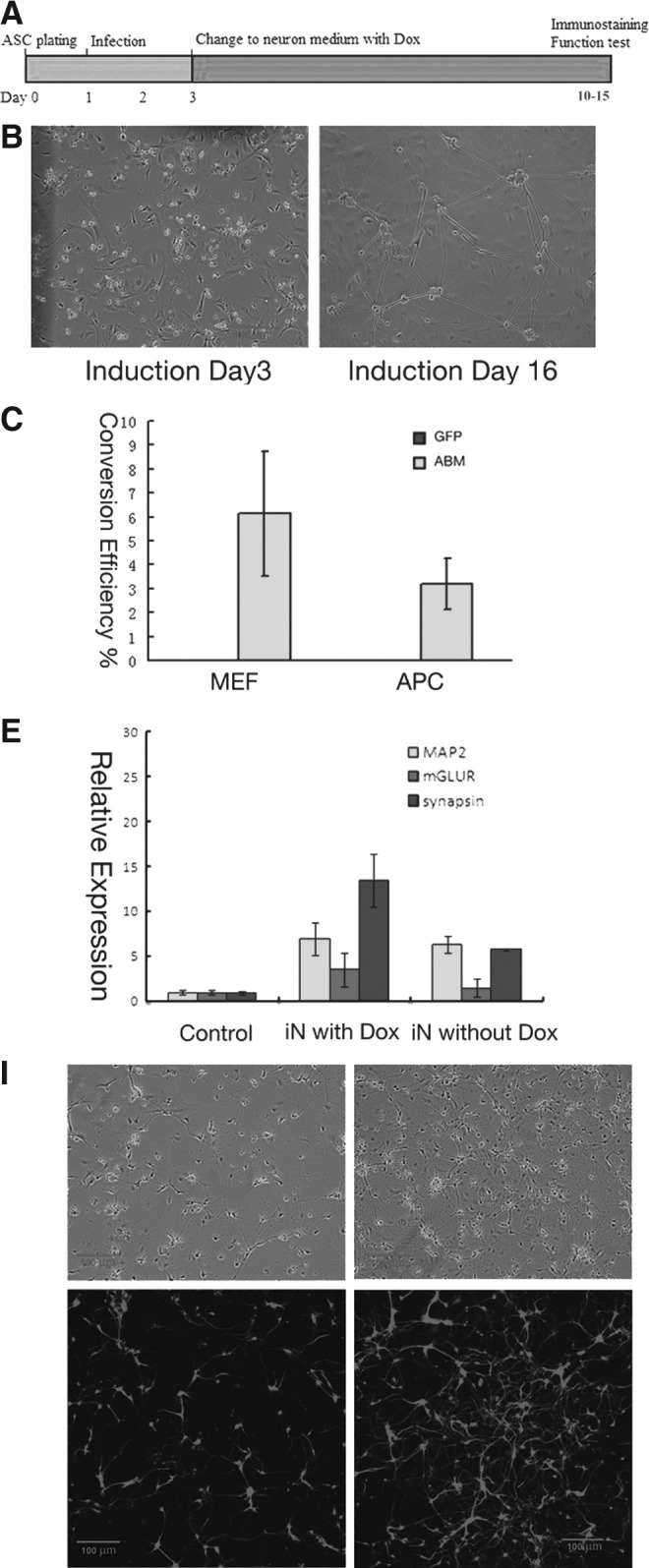
(A) Diagram depicting the transdifferentiation procedures of APCs to neurons by lentiviruses carrying ABM. (B) Morphology of the induced neurons at day 3 and day 16 after virus infection. (C) Induction efficiencies of MEFs and APCs to Tuj-1–positive neurons. Cells in the “GFP” group infected by viruses carrying GFP alone showed no neuron-like cells. (D) The induced neurons were stained positive for Tuj-1, MAP2, NeuN, Synapsin, vGLUT1, and GABA. Arrows indicate the typical punctuate pattern. Scale bars, 100 μm in Tuj-1, MAP2; 10 μm in NeuN, Synapsin, vGLut1, and GABA. The APCs as the negative control did not express neural markers Tuj1, MAP2, NeuN, Synapsin, vGlut1, and GABA. Scale bars, 50 μm. (E) Relative expression level of neuronal marker genes MAP2, Synapsin, and mGLUR. Comparison was between the cells infected with GFP alone (as control), and iN with ABM at day 25 postinfection (iN with Dox), and iN with ABM expression for 25 days and then with Dox removed to stop the exogeneous ABM expression (iN without Dox). (F) Whole cell current recording of depolarizing voltage steps (−80 mV to 60 mV) in APC-derived neurons. (G) APs recording the neurons derived from APCs. (H) Spontaneous APs presumably caused by membrane potential fluctuations recording of the iN converted from an APC 25 days after induction. (I) Morphology of MEFs infected by lentiviruses carrying ABM at day 20, vitamin C treated, and mock treated. Tuj-1 antibody was used for staining the induced cells. Scale bar, 200 μm. (J) Counting the induced neurons from MEFs (left) and APCs (right) treated with vitamin C and mock treated. (K) Whole-cell current recording of depolarizing voltage steps (−80 mV to 60 mV) in MEF-derived neurons treated with vitamin C. (L) AP recording of the vitamin C–treated MEF-derived neurons.
To further characterize the converted cells, we stained the neuronal-like cells with neuronal markers 16 days after infection. The induced neuronal-like cells expressed the pan-neuronal markers MAP2 and Tuj-1 (Fig. 1D). When the induced neurons were co-cultured with mouse glia, we found that the induced neurons were positive for NeuN and Synapsin. We further investigated the neuronal type of the converted neurons and found that among the cells with typical mature neuronal morphology, about 90% of the cells stained positive for vGLUT1, and about 10% stained positive for γ-aminobutyric acid (GABA), indicating that most of the neurons were glutamatergic neurons and a small proportion were GABAergic neurons (Fig. 1D). By contrast, the APCs we obtained did not express a series of neural markers, including Tuj1, MAP2, NeuN, Synapsin, vGltu1, and GABA (Fig. 1D). After removal of doxycycline, the exogenous expression of Ascl1, Brn2, and Myt1l was silenced, and the cells still expressed neuronal markers such as Map2, mGLUR, and Synapsin (Fig. 1E). To compare the efficiencies of induction of APCs versus MEFs, we induced the two types of donor cells with ABM (Ascl1, Brn2, Myt1l) in parallel. After about 15 days, we divided the cell culture dishes into several visual fields and counted total cell numbers and total Tuj1-positive iN (induced neurons) cell numbers. We repeated the experiment three times and found that the induction efficiency of APCs (∼3%) was slightly lower than the MEFs (∼6%) (Fig. 1C).
To ascertain the functional membrane properties of APC-derived neurons, we performed patch-clamp recordings of cells with neuronal morphology at days 15–20 after infection (Fig. 1F–H). In voltage-clamp mode, step depolarization induced inward sodium currents characterized by a typical current density–voltage relationship (Fig. 1F). Outward potassium currents were readily apparent. Consistent with such channel properties, most APC-iN cells were able to fire in response to depolarizing current injections in current clamp mode (three cells fired out of four cells tested) (Fig. 1G) and spontaneous action potentials were observed in the test (Fig. 1H). When tetrodotoxin (TTX), a specific inhibitor of Na+ ion channels, was applied in the tested cells, action potentials (APs) and inward sodium currents were abolished. Therefore, we successfully generated functional iN cells from APCs.
Apart from this, several drugs were tested for their effect on the direct conversion efficiency of somatic cells to neurons. Among these drugs, vitamin C was able to elevate the direct conversion efficiency significantly. We treated fibroblasts and APCs that were infected by viruses carrying ABM with vitamin C in the conversion process. At 20 days after induction, we detected significantly more neuronal-like cells in the vitamin C–treated cells than the mock-treated cells (Fig. 1I). Staining of the neuronal marker Tuj-1 further showed that there were more neurons in the converted neurons treated by vitamin C in the conversion process than the converted neurons that were mock treated (Fig. 1I). The cells were counted and showed distinguished elevation of conversion efficiency in the vitamin C–treated cells (Fig. 1J). We also tested the electrophysiological properties of derived neurons treated with vitamin C, and they also showed typical sodium currents and APs in these tests (Fig. 1K, L). Both of the functional membrane properties of the converted neurons from APCs and MEFs treated with vitamin C had typical responses as mature neurons. Therefore, we successfully generated functional iN cells from APCs and found that vitamin C could elevate conversion efficiency of APCs and fibroblasts to neurons.
In our study, we could successfully convert mouse APCS to functional neurons, and we also found that vitamin C can elevate the efficiency of conversion from MEFs and APCs to neurons. APCs can be obtained easily from adipose tissue and stable proliferation. Therefore, we demonstrated that direct conversion could be achieved from APCs to functional neurons, and this may provide a new possible cell resource obtained by direct conversion.
Vitamin C was found to facilitate reprogramming from fibroblasts to iPSCs (Esteban et al., 2010), and further research has shown that vitamin C functions by influencing the histone modification (Shi et al., 2010; Wang et al., 2011). Here we report that conversion from fibroblasts and APCs to neurons can be facilitated by vitamin C. This is until now the first small molecule reported to help conversion from somatic cells to neurons. However, the underlying mechanism is still not clear. Whether vitamin C functions in the same pathway or by a different mechanism of iPSC generation and in direct conversion to neurons and whether vitamin C could facilitate conversion to other cell types are issues still to be answered.
Supplementary Material
Acknowledgments
We are grateful to Dr. Li Yingji from ICE BIOSCIENCE Co., for his assistance on electrophysiological experiments. We are grateful to our colleagues in our laboratory for their assistance with experiments and in the preparation of this manuscript. This project was supported by the Ministry of Science and Technology (grants 2010CB944900 and 2011CB964800), the National Natural Science Foundation of China (31171229 to X.F. Sun), NSFC-Guangdong Joint Fund (U1132005 to X.F. Sun), and Gang Zhou City Science and Technology Administration (2011Y1-00038).
Author Disclosure Statement
The authors declare that there are no potential conflicts of interest.
References
- Ambasudhan R. Talantova M. Coleman R. Yuan X. Zhu S. Lipton S.A. Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G. Kitchens D. Yao M. Huettner J.E. Gottlieb D.I. Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Caiazzo M. Dell'Anno M.T. Dvoretskova E. Lazarevic D. Taverna S. Leo D. Sotnikova T.D. Menegon A. Roncaglia P. Colciago G. Russo G. Carninci P. Pezzoli G. Gainetdinov R.R. Gustincich D. Dityatev A. Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Campbell K.H. Loi P. Otaegui P.J. Wilmut I. Cell cycle co-ordination in embryo cloning by nuclear transfer. Rev. Reprod. 1996;1:40–46. doi: 10.1530/ror.0.0010040. [DOI] [PubMed] [Google Scholar]
- Cowan C.A. Atienza J. Melton D.A. Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- Esteban M.A. Wang T. Qin B. Yang J. Qin D. Cai J. Li W. Weng Z. Chen J. Ni S. Chen K. Li Y. Liu X. Xu J. Zhang S. Li F. He W. Labuda K. Song Y. Peterbauer A. Wolbank S. Redl H. Zhong M. Cai D. Zeng L. Pei D. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Grigoriadis A.E. Kennedy M. Bozec A. Brunton F. Stenbeck G. Park I.-H. Wagner E.F. Keller G.M. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood. 2010;115:2769–2776. doi: 10.1182/blood-2009-07-234690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J.B. Elsdale T.R. Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182:64–65. doi: 10.1038/182064a0. [DOI] [PubMed] [Google Scholar]
- Hu B.Y. Zhang S.C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Yokoo T. Tani K. Sugiyama D. Mesodermal and hematopoietic differentiation from ES and iPS cells. Stem Cell Rev. 2012;9:422–434. doi: 10.1007/s12015-012-9388-1. [DOI] [PubMed] [Google Scholar]
- Karumbayaram S. Novitch B.G. Patterson M. Umbach J.A. Richter L. Lindgren A. Conway A.E. Clark A.T. Goldman S.A. Plath K. Wiedau-pazos M. Kornblum H.I. Lowry W.E. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M.G. Soonpaa M.H. Koh G.Y. Field L.J. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J. Clin. Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z.P. Yang N. Vierbuchen T. Ostermeier A. Fuentes D.R. Yang T.Q. Citri A. Sebastiano V. Marro S. Sudhof T.C. Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera M.F. Stem cells: The dark side of induced pluripotency. Nature. 2011;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- Saito K. Yoshikawa M. Ouji Y. Moriya K. Nishiofuku M. Ueda S. Hayashi N. Ishizaka S. Fukui H. Promoted differentiation of cynomolgus monkey ES cells into hepatocyte-like cells by co-culture with mouse fetal liver-derived cells. World J. Gastroenterol. 2006;12:6818–6827. doi: 10.3748/wjg.v12.i42.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K. Ichikawa H. Takei S. No H.S. Tomotsune D. Kano Y. Yokoyama T. Sirasawa S. Mogi A. Yoshie S. Sasaki S. Yamada S. Matsumoto K. Mizuguchi M. Yue F. Tanaka Y. Hepatocyte differentiation from human ES cells using the simple embryoid body formation method and the staged-additional cocktail. ScientificWorld Journal. 2009;9:884–890. doi: 10.1100/tsw.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Zhao Y. Deng H. Powering reprogramming with vitamin C. Cell Stem Cell. 2010;6:1–2. doi: 10.1016/j.stem.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Son E.Y. Ichida J.K. Wainger B.J. Toma J.S. Rafuse V.F. Woolf C.J. Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M. Takahama Y. Abe K. Nakatsuji N. Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Tada M. Morizane A. Kimura H. Kawasaki H. Ainscough J.F. Sasai Y. Nakatsuji N. Tada T. Pluripotency of reprogrammed somatic genomes in embryonic stem hybrid cells. Dev. Dyn. 2003;227:504–510. doi: 10.1002/dvdy.10337. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Wang T. Chen K. Zeng X. Yang J. Wu Y. Shi X. Qin B. Zeng L. Esteban M.A. Pan G. Pei D. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell. 2011;9:575–587. doi: 10.1016/j.stem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Zhang J. Wilson G.F. Soerens A.G. Koonce C.H. Yu J. Palecek S.P. Thomson J.A. Kamp T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.