Abstract
Despite its success in almost all farm and laboratory animals, somatic cell nuclear transfer (SCNT) is still a low-efficiency technique. In this investigation, we determined the impact of each enucleation step on oocyte viability (assessed by parthenogenetic activation): Hoechst (HO) staining, cytochalasin B, ultraviolet (UV) exposure, and demecolcine. Our data showed that of all the factors analyzed, UV exposure impaired oocyte development (cleavage, 59% for untreated oocytes vs. 8% UV exposed; blastocyst stage, 32% untreated vs. 0% UV exposed). A minor toxicity was detected following demecolcine treatment (cleavage, 62%; blastocyst stage, 13%). Next, we compared HO/UV (canonical) and demecolcine-assisted enucleation (DAE), with a straight removal of metaphase chromosomes without any chemical or physical aid (straight enucleation). DAE improved the preimplantation development of sheep cloned embryos compared to HO/UV enucleation (cleavage, 38% vs. 19%; blastocysts, 17% vs. 4%), yet straight enucleation resulted in the highest cleavage and blastocysts rates (61% and 30%, respectively). We concluded that: (1) UV exposure harms sheep oocyte and embryo development; (2) DAE may represent an alternative approach, especially for unskilled operators; and (3) straight enucleation remains, in our estimation, the most reliable and least harmful protocol for SCNT.
Introduction
Somatic cell nuclear transfer (SCNT) remains the most effective method for nuclear reprogramming of differentiated nuclei (Gurdon and Wilmut, 2011). Despite the broad spectrum of its potential applications, SCNT efficiency remains low (Thuan et al., 2010). The primary factor that hinders SCNT efficiency is biological in nature—the epigenetic resistance of the differentiated donor nucleus to nuclear reprogramming (Pasque et al., 2011).
However, technical factors also play a role (Wakayama et al., 2010). SCNT is a multistep technique, and the efficiency of each step accounts for the final outcome. First, the oocyte is enucleated. In large animal oocytes, this is typically facilitated by Hoechst 33342–cytochalasin B treatment and ultraviolet (UV) exposure. Then a nucleus from a differentiated cell is injected into that enucleated oocyte or electrofused to it. The removal of metaphase II (MII) chromosomes from the oocyte is a crucial moment in SCNT. In laboratory animals such as mouse and rat, the MII chromosomes are identifiable and hence easy to remove. However, in large animals, the MII plate is hardly detectable due to the high lipid content in the cytoplasm. For this reason, oocyte enucleation in large animals is normally assisted by Hoechst dye (HO) staining and subsequent short UV exposure to locate the DNA (Wilmut et al., 1997). Although UV exposure is known to have harmful effects (Takaneda et al., 2007), it has been routinely used in the majority of cloning laboratories, although it is not an ideal method for oocyte enucleation. Recently, the use of UV in SCNT procedures is being reconsidered in the light of reports suggesting a higher toxic effect than previously thought (Gil et al., 2012; Maalouf et al., 2008; Maside et al., 2011). Given that our recent SCNT data are in line with these observations, we decided to simplify the established enucleation procedures.
To this extent, we critically dissected each step required in oocyte enucleation in both canonical HO/UV and demecolcine-assisted enucleation (DAE)—HO staining, cytochalasin B, UV, demecolcine—and determined the impact of each on oocyte development, as assessed by parthenogenetic development. Untreated/unmanipulated oocytes and oocytes enucleated without any chemical or UV exposure served as controls in the experiment. We demonstrate that of all factors tested, UV exposure had the most detrimental effect on oocyte development, whereas demecolcine exerted a milder toxic effect. We also show that enucleation can be successfully accomplished without any chemical or physical agents, and that this “straight” enucleation results in the highest developmental potential of oocytes reconstructed with somatic nuclei.
Materials and Methods
All chemicals, unless otherwise indicated, were obtained from Sigma Chemicals Co. (St. Louis, MO, USA).
In vitro maturation of sheep oocytes
Methods of in vitro embryo production were adapted from those previously described (Ptak et al., 2002). The ovaries were transferred at 37°C to the laboratory within 1–2 h. Oocytes were aspirated in the presence of tissue culture medium-199 (TCM-199) medium (Gibco, Life Technologies, Milan, Italy) containing HEPES and heparin. Then oocytes with at least two to three layers of compact cumulus cells and uniform cytoplasm were selected for in vitro maturation (IVM). All selected oocytes were washed and then matured in vitro in bicarbonate-buffered TCM-199 containing 2 mM glutamine, 0.3 mM sodium pyruvate, 100 μM cysteamine, 10% fetal bovine serum (FBS; Gibco Life Technologies, Milan, Italy), 5 μg/mL follicle-stimulating hormone (FSH; Ovagen, ICP, Auckland, New Zealand), 5 μg/mL lutenizing hormone (LH), and 1 μg/mL estradiol. Maturation was conducted in a humidified atmosphere of 5% CO2 in air at 39°C for 24 h.
Exp. 1—Oocyte treatment and in vitro activation
To evaluate the effect of each single step required for oocyte enucleation, MII oocytes were divided into six groups: (1) Untreated oocytes (control); (2) oocytes treated with Hoechst 33342 (5 μg/mL) for 10 min; (3) oocytes treated with Hoechst 33342 for 10 min and then exposed under UV light for 1–3 sec (mercury short arc HBO 103 W/2 lamp, OSRAM); (4) oocytes treated with HO for 10 min and partially exposed under UV, shielding the MII chromosomes (see Fig. 1); (5) oocytes incubated with cytochalasin B (7.5 μg/mL) for 1 h; and (6) oocytes incubated with demecolcine (0.04 μg/mL) for 2 h. At the end of each treatment, all groups were in vitro activated (IVA) as previously described (Loi et al., 1998; Ptak et al., 2002). In vitro development was recorded every 24 h.
FIG. 1.
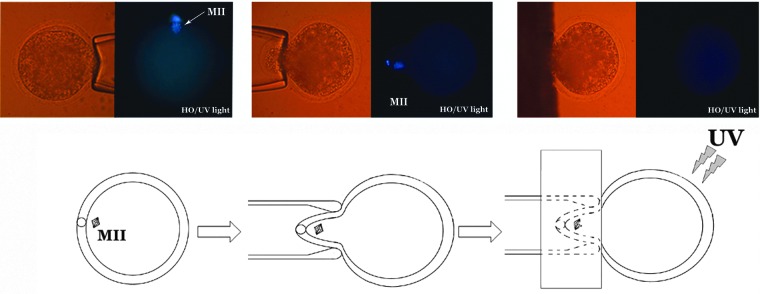
Oocytes partially exposed under UV shielding the MII plate. The MII plate is aspirated into a holding pipette and the cytoplasm is shielded from UV light.
Exp. 2— Comparing “canonical” (HO/UV), DAE, and “straight” oocyte enucleation in nuclear transfer
Canonical enucleation (HO/UV)
At 22 h of maturation, oocytes were denuded of granulosa cells in the presence of hyaluronidase (300 U/mL). Oocytes with extruded first polar bodies (1PB) were incubated in Hoechst 33342 (5 μg/mL) in TCM-199 medium for 10 min. They were then enucleated by aspiration of the metaphase II plate in 5-μL drops of TCM-199 medium with 4 mg/mL bovine serum albumin (BSA), gentamicin (5 mg/mL), and 7.5 μg/mL cytochalasin B, under UV light with a piezo-driven unit (PiezoXpert Eppendorf, Milan, Italy), assembled on a Narishige Micromanipulator and fitted to an inverted Nikon microscope.
Demecolcine-assisted enucleation
At 22 h of maturation, oocytes were transferred in IVM medium enriched with demecolcine (0.04 μg/mL) and incubated for 2 h. Then, oocytes were denuded of granulosa cells in the presence of hyaluronidase and gentle pipetting. Oocytes with extruded 1PB and demecolcine-induced cytoplasmatic protrusions (see Fig. 2) were incubated in Hoechst 33342 (5 μg/mL) in TCM-199 medium for 10 min. Enucleation was performed using a piezo-driven pipette partially filled with mercury. Single oocytes were enucleated in 5-μL drops of TCM-199 enriched with cytochalasin B (7.5 μg/mL) under mineral oil. Only the aspirated cytoplasts were observed under UV light to ensure the MII chromosomes' removal without oocyte UV exposure.
FIG. 2.
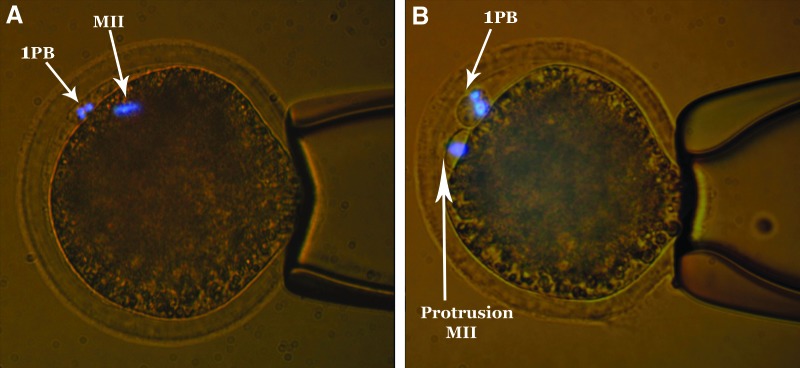
Karyoplast protrusion induced by demecolcine. (A) MII oocytes without treatment. (B) MII chromosomes accumulation in cytoplasm protrusion after treatment. 1PB, first polar body.
Straight enucleation
At 22 h post-IVM, matured oocytes with extruded 1PB were incubated in Hoechst 33342 (5 μg/mL) in TCM-199 medium for 10 min. Single oocytes were enucleated in 5-μL drops of TCM-199 without cytochalasin B under mineral oil. The small portion of cytoplasm under 1PB was aspirated by enucleation pipette. The enucleation was confirmed by exposure under UV light to the aspirated cytoplasts (no oocyte UV exposure).
Somatic cell nuclear transfer
Enucleated oocytes were transferred to TCM-199 medium for 15 min and injected with adult sheep fibroblasts by PiezoXpert in TCM-199 under mineral oil. Donor cells were kept in 11% polyvinylpyrrolidone (PVP) solution. Reconstructed oocytes were activated in vitro with ionomycin (5 μM, 5 min) and 6-dimethylaminopurine (10 mM) with 7.5 μg/mL cytochalasin B for 3–5 h. The in vitro development was recorded every 24 h.
In vitro culture of preimplantation embryos
In vitro culture of preimplantation embryos was done as previously described (Ptak et al., 2002). Presumptive zygotes were transferred into 20-μL drops consisting of synthetic oviductal fluid (SOF) enriched with 1% (vol/vol) basal medium Eagle (BME) essential amino acids (Gibco, Life Technologies, Milan, Italy), 1% (vol/vol) minimum essential medium (MEM) nonessential amino acids (Gibco, Life Technologies, Milan, Italy), and 8 mg/mL BSA. Cultures were carried out in a humidified atmosphere of 5% CO2, 7% O2, and 88% N2 at 39–C. On days 3 and 5 of culture (where day 0=day of in vitro activation), the medium was changed. On day 5, 10% of FBS charcoal stripped was added to the medium.
Statistical analyses
The Fisher exact test was used to compare data on in vitro development. Probability values less than 0.05 were considered to be statistically significant. Statistical analyses were performed using GraphPad Prism 5.0 software.
Results
UV exposure of oocytes compromise parthenogenetic embryo development
No effects were observed on in vitro development of oocytes treated with Hoechst 33342 (group 2), which reached the blastocyst stage at rates comparable to the controls (Table 1). Reduced cleavage and blastocyst development rates were instead observed in oocytes treated with cytochalasin B (group 5) and demecolcine (group 6), whereas total and partial UV exposure (groups 3 and 4, respectively) compromised blastocyst development (see Table 1).
Table 1.
Developmental Potential of Oocytes
| Groups | Cleavage/oocytes (%) | Blastocyst/cleavage (%) |
|---|---|---|
| 1. CTR, untreated | 68/116 (59%) | 22/68 (32%) |
| 2. Hoechst | 28/45 (62%) | 10/28 (36%) |
| 3. UV (both cytoplasm and MII plate) | 9/119 (8%)a | 0/9 (0%)a |
| 4. UV (only cytoplasm) | 20/95 (21%)b | 0/20 (0%)b |
| 5. Cytochalasin B | 48/90 (53%) | 6/48 (13%)c |
| 6. Demecolcine | 37/60 (62%) | 5/37 (13%)d |
Treated with:
UV (cytoplasm+MII) vs. CTR: cleavage p<0.0001.
UV (only cytoplasm) vs. CTR: cleavage p<00001; blastocysts p=0.0394; blastocysts p=0.0023.
Cytochalasin B vs. CTR: blastocysts p=0.0158.
Demecolcine vs. CTR: blastocysts p=0.0382. Fisher exact test.
CTR, control; UV, ultraviolet; MII, metaphase II.
DAE and straight enucleation improve cloned blastocyst development
DAE significantly improved the production of cloned embryos compared to canonical enucleation (HO staining and UV light exposure). Table 2 shows that cleavage and blastocyst rates of demecolcine-assisted enucleated clones were higher than UV-enucleated ones (cleavage, 38% in DAE clones vs. 19% in UV-enucleated clones; blastocyst, 17% vs. 4%, respectively). However, the “straight” enucleation far exceeded DAE in both cleavage (61% vs. 38%) and blastocyst rates (30% vs. 17%, respectively).
Table 2.
Influence of Different Enucleation Protocols on Developmental Potential of Somatic Cell Nuclear Transfer Embryos
| Oocyte enucleation | Cleavage/oocytes (%) | Blastocyst/cleavage (%) |
|---|---|---|
| Canonical (HO/UV) | 52/270 (19%) | 2/52 (4%) |
| Demecolcine assisted | 96/250 (38%)a | 16/96 (17%)a |
| Straight | 79/130 (61%)b,c | 24/79 (30%)b,c |
Demecolcine assisted vs. canonical: cleavage p<0.0001, blastocysts p=0.0326.
Straight vs. canonical: cleavage p<0.0001; blastocyst p=0.0001.
Straight vs. demecolcine assisted: cleavage p<0.0001; blastocysts p=0.0458.
HO, Hoechst; UV, ultraviolet.
Discussion
The best-known biological effect of UV exposure is DNA damage (Yang W, 2011); for this reason, UV exposure has been adopted in SCNT, given that the oocyte chromosomes are completely removed and substituted with somatic cell nuclei. Here we decided to totally reset the established procedures for oocyte enucleation, demonstrating that the MII chromosomes can be successfully removed without the need for UV-induced DNA dyes or disabling the oocyte's cytoskeleton, as already suggested by Maalouf et al. (2008).
As expected, the simple exposure of Hoechst 33342 to the oocytes was harmless (blastocysts, 36% HO vs. 32% control (CTR)). A milder reduction of the oocyte competence was observed following cytochalasin B and demecolcine treatment (13% and 13% blastocysts, respectively, comparing with 32% CTR). It is likely that the long interference of cytoplasmic trafficking resulting from actin–tubulin depolymerization negatively affects development (Miyamoto and Gurdon, 2011).
The stronger factor we found to compromise oocyte viability was UV exposure, with a major impairment of the first cleavage (8%) and no development to the blastocyst stage following parthenogenetic activation. These results are in line with recent studies (Terashita et al., 2011).
To dissect the effects of UV on DNA and cytoplasm, we shielded the metaphase plate of a group of oocytes (group 4), causing only the cytoplasm to be to irradiated, as actually occurs in SCNT protocols. The resulting damage was less intense than total exposure (total UV 8% vs. UV cytoplasm 21%), yet no blastocysts were observed following 7 days in vitro culture (Table 1). Hence, we can infer that UV exposure effectively impairs most of oocyte's functions (i.e., mitochondrial activity), as previously shown (Leal et al, 1999; Smith, 1993).
The alternatives to canonical (OH/UV) enucleation proposed so far are: (1) Polarized light microscopy, that locates the metaphase spindle (Liu et al., 2000); (2) the use of softer filter systems with traditional halogen lamps, associated with fluorescent antibody directed against the phosphorylated serine 10 of histone H3 (H3S10ph) binding to M-phase chromosomes (Yamagata et al., 2012); and (3) DAE (Yin et al., 2002).
Polarized light microscopy works very well in oocytes with a clear cytoplasm, such as mouse, rat, or human. Particularly for human oocytes, this microscopy is now widely used in assisted reproduction techniques (ART) (Keefe et al., 2003). The use of polarized light in large animal oocytes is less advisable due to the high lipid droplet content. The softer halogen lamp associated with fluorescent antibody anti-histone to locate the chromosomes reduces the phototoxic effects but adds the further step of antibody injection prior to enucleation (Yamagata et al., 2012), hence it is unpractical.
As for the large animals, we are interested in the use of demecolcine, which was first suggested for the enucleation of pig oocytes (Yin et al., 2002) and next extended to other large animal such as bovines (Tani et al., 2006). Although we recorded here a mild toxic effect of demecolcine, we believe that DAE is a worthy alternative to canonical HO/UV enucleation because less damaging than UV exposure.
Many technical improvements have been validated since the first SCNT report (Maside et al., 2011); one of the most relevant is the use of piezo-driven enucleation pipettes, originally devised for intracytoplasmic sperm injection (ICSI) in mice (Kimura et al., 1995).
Piezo-driven pipettes are used to carry out the enucleation without cytochalasin B, DNA dyes, or UV exposure in a procedure we have termed straight enucleation. We then compared the cloned blastocyst outcome derived from straight approach with those produced by the canonical HO/UV-based and DAE methods.
The enucleation rate of DAE, assessed a posteriori by HO staining of the aspirated fragment, was 100%, which is similar to the rate of the HO/UV group. Straight enucleation was slightly less efficient, with 85% of oocytes effectively enucleated (data not shown). As expected, HO/UV gave the worst results in terms of embryo development (4%), compared to demecolcine-assisted and straight enucleation (17% vs. 30% blastocysts, respectively). These results confirm previous studies in the sheep model, where it has been observed that canonical enucleation of in vivo–matured oocytes led to 11% blastocysts (Wilmut et al., 1997), whereas the removal of UV exposure increases blastocyst rate up to 35% (Lee and Campbell, 2006), even if oocytes were in vitro matured, as in our work.
To conclude, we have confirmed that UV radiation exerts harmful effects on embryonic development through direct cytoplasmic damage. Although moderate cytotoxicity was also found for demecolcine, DAE is user friendly compared to others methods; however, straight enucleation resulted in the highest frequencies of development. Straight enucleation requires trained operators, but completely eliminates other minor factors that partially erode oocyte competence, providing a defined platform on which to untangle the major issue in SCNT, the epigenetic resistance of somatic cells to nuclear reprogramming.
Acknowledgments
The research leading to these results received funding from the European Research Council (ERC) (FP7/2007-2013)/ERC grant agreement no. 210103 to G.P. and PRIN 2007, no. 2007MY2M92 to G.P. P.L. acknowledges the support of the EU FP7-KBBE−2009-13 Programme, project no. 244356, NextGene, PRIN MIUR founding (protocol no. 2009JE3CHM), and Project “GenHome.” Funds from the Bank Foundation Tercas (Teramo, Italy) are also acknowledged.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Gil M.A. Maside C. Cuello C. Parrilla I. Vazquez J.M. Roca J. Martinez E.A. Effects of Hoechst 33342 staining and ultraviolet irradiation exposure on mitochondrial distribution and DNA copy number in porcine oocytes and preimplantation embryos. Mol. Reprod. Dev. 2012;79:651–663. doi: 10.1002/mrd.22071. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B. Wilmut I. Nuclear transfer to eggs and oocytes. Cold Spring Harb. Perspect. Biol. 2011;3(6):1. doi: 10.1101/cshperspect.a002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe D. Liu L. Wang W. Silva C. Imaging meiotic spindles by polarization light microscopy: Principles and applications to IVF. Reprod. Biomed. Online. 2003;7:24–29. doi: 10.1016/s1472-6483(10)61724-5. [DOI] [PubMed] [Google Scholar]
- Kimura Y. Yanagimashi R. Intracytoplasmatic sperm injection in the mouse. Biol. Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- Leal C.L. Lee C. Moor R.M. UV irradiation of pig metaphase chromosomes: Maturation-promoting factor degradation, nuclear cytology and cell cycle progression. J. Reprod. Fertil. 1999;116:363–371. doi: 10.1530/jrf.0.1160363. [DOI] [PubMed] [Google Scholar]
- Lee J.H. Campbell K.H. Effects of enucleation and caffeine on maturation-promoting factor (MPF) and mitogen-activated protein kinase (MAPK) activities in ovine oocytes used as recipient cytoplasts for nucleartransfer. Biol. Reprod. 2006;74:691–698. doi: 10.1095/biolreprod.105.043885. [DOI] [PubMed] [Google Scholar]
- Liu L. Oldenbourg R. Trimarchi J.R. Keefe D.L. A reliable, noninvasive technique for spindle imaging and enucleation of mammalian oocytes. Nat. Biotechnol. 2000;18:223–225. doi: 10.1038/72692. [DOI] [PubMed] [Google Scholar]
- Loi P. Ledda S. Fulka J., Jr. Cappai P. Moor R.M. Development of parthenogenetic and cloned ovine embryos: Effect of activation protocols. Biol. Reprod. 1998;58:1177–1187. doi: 10.1095/biolreprod58.5.1177. [DOI] [PubMed] [Google Scholar]
- Maalouf W.E. Alberio R. Campbell K.H. Differential acetylation of histone H4 lysine during development of in vitro fertilized, cloned and parthenogenetically activated bovine embryos. Epigenetics. 2008;3:199–209. doi: 10.4161/epi.3.4.6497. [DOI] [PubMed] [Google Scholar]
- Maside C. Gil M.A. Cuello C. Sanchez-Osorio J. Parrilla I. Lucas X. Caamaño J.N. Vazquez J.M. Roca J. Martinez E.A. Effects of Hoechst 33342 staining and ultraviolet irradiation exposure on the developmental competence of in vitro-matured porcine oocytes. Theriogenology. 2011;76:1667–1675. doi: 10.1016/j.theriogenology.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Miyamoto K. Gurdon J.B. Nuclear actin and transcriptional activation. Commun. Integr. Biol. 2011;4:582–583. doi: 10.4161/cib.4.5.16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasque V. Jullien J. Miyamoto K. Halley-Stott R.P. Gurdon J.B. Epigenetic factors influencing resistance to nuclear reprogramming. Trends Genet. 2011;27:516–525. doi: 10.1016/j.tig.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak G. Clinton M. Tischner M. Barboni B. Mattioli M. Loi P. Improving delivery and offspring viability of in vitro-produced and cloned sheep embryos. Biol. Reprod. 2002;67:1719–1725. doi: 10.1095/biolreprod.102.006171. [DOI] [PubMed] [Google Scholar]
- Smith L.C. Membrane and intracellular effects of ultraviolet irradiation with Hoechst 33342 on bovine secondary oocytes matured in vitro. J. Reprod. Fertil. 1993;99:39–44. doi: 10.1530/jrf.0.0990039. [DOI] [PubMed] [Google Scholar]
- Takenaka M. Horiuchi T. Yanagimachi R. Effect of light on development of mammalian zygote. Proc. Natl. Acad. Sci. USA. 2007;104:14289–14293. doi: 10.1073/pnas.0706687104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani T. Shimada H. Kato Y. Tsunoda Y. Demecolcine-assisted enucleation for bovine cloning. Cloning Stem Cells. 2006;8:61–66. doi: 10.1089/clo.2006.8.61. [DOI] [PubMed] [Google Scholar]
- Terashita Y. Li C. Yamagata K. Sato E. Wakayama T. Effect of fluorescent mercury light irradiation on in vitro and in vivo development of mouse oocytes after parthenogenetic activation or sperm microinjection. J. Reprod. Dev. 2011;57:564–571. doi: 10.1262/jrd.11-015h. [DOI] [PubMed] [Google Scholar]
- Thuan N.V. Kishigami S. Wakayama T. How to improve the success rate of mouse cloning technology. J. Reprod. Dev. 2010;56:20–30. doi: 10.1262/jrd.09-221a. [DOI] [PubMed] [Google Scholar]
- Wakayama S. Mizutani E. Wakayama T. Production of cloned mice from somatic cells, ES cells, and frozen bodies. Methods Enzymol. 2010;476:151–169. doi: 10.1016/S0076-6879(10)76009-2. [DOI] [PubMed] [Google Scholar]
- Wilmut I. Schnieke A.E. McWhir J. Kind A.J. Campbell K.H. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Yamagata K. Iwamoto D. Terashita Y. Li C. Wakayama S. Hayashi-Takanaka Y. Kimura H. Saeki K. Wakayama T. Fluorescence cell imaging and manipulation using conventional halogen lamp microscopy. PLoS One. 2012;7:e31638. doi: 10.1371/journal.pone.0031638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. Surviving the sun: Repair and bypass of DNA UV lesions. Protein Sci. 2011;20:1781–1789. doi: 10.1002/pro.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X.J. Tani T. Yonemura I. Kawakami M. Miyamoto K. Hasegawa R. Kato Y. Tsunoda Y. Production of cloned pigs from adult somatic cells by chemically assisted removal of maternal chromosomes. Biol. Reprod. 2002;67:442–446. doi: 10.1095/biolreprod67.2.442. [DOI] [PubMed] [Google Scholar]


