Abstract
The mutagenicity and carcinogenicity of the important commodity chemical 1,3-butadiene are attributed to the epoxide products. We confirmed our previous work showing that expression of rat glutathione (GSH) transferase 5-5 enhances the mutagenicity of butadiene diepoxide in Salmonella typhimurium TA1535. A GSH-butadiene diepoxide was isolated and fully characterized by mass spectrometry and NMR as S-(2-hydroxy-3,4-epoxybutyl)GSH. The conjugate had a t1/2 of 2.6 h (pH 7.4, 37 °C) and was considerably more mutagenic than butadiene diepoxide or monoepoxide in S. typhimurium. We propose that the GSH conjugate may be a major species involved in butadiene genotoxicity, not a detoxication product.
1,3-Butadiene is an important commodity chemical in industry, particularly in the production of synthetic rubber and other polymers. In 2008, 1.3 × 107 kg were produced in the United States alone (1). Butadiene is of concern in toxicology because the compound is carcinogenic in mice and rats, particularly the former species (2). In addition, some epidemiology studies suggest that butadiene is a human carcinogen (1, 3). The metabolism of butadiene is understood in the pathways shown in Scheme 1, and it is highly likely that one or more of the epoxide products of butadiene are responsible for its carcinogenicity, probably due to genotoxic mechanisms (4, 5). At least 23 adducts of DNA bases have been reported, with the number being even higher when stereochemistry is considered (6-13).
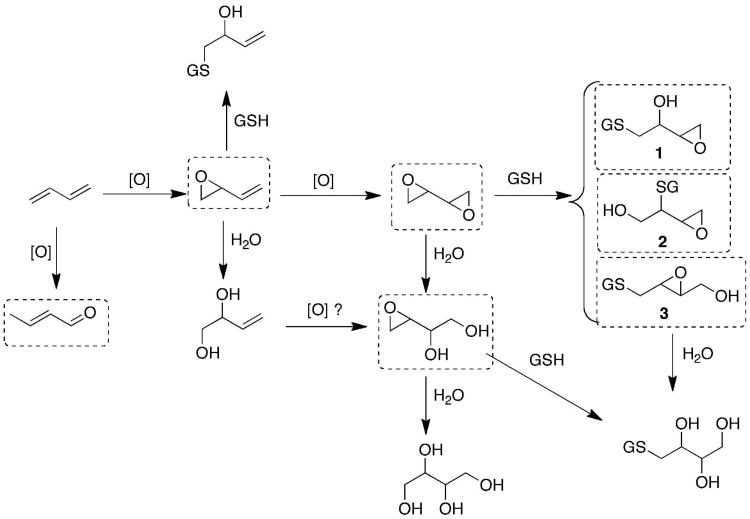
Scheme 1.
Metabolism of butadiene. Three possible GSH adducts formed from butadiene diepoxide are shown. All compounds in dotted boxes can potentially react with DNA.
We previously reported that the base pair mutagenicity of butadiene diepoxide in Salmonella typhimurium TA1535 is considerably increased by the cellular expression of rat GSH transferase 5-5 (14). That result, unexpected at the time, has been repeatable (Supporting Information Figure S1) and implies that a GSH conjugate is genotoxic. GSH conjugation has been reported but the exact structure of the conjugate was not determined (15, 16). However, GSH conjugation has usually been considered to be a detoxication process in the metabolism of butadiene diepoxide (17).
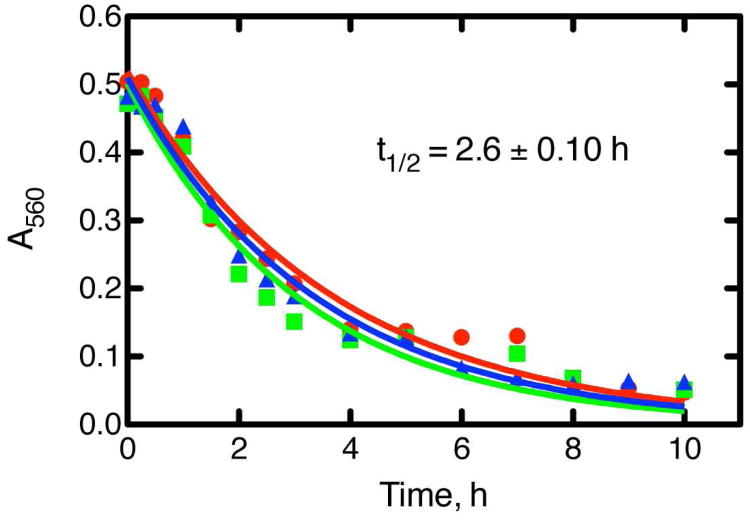
A GSH-butadiene diepoxide conjugate (1) was prepared by either (i) reacting butadiene diepoxide with GSH in CH3OH in the presence of 3 equivalents of Na° or NaOCH3 (18) or (ii) enzymatic reaction of butadiene diepoxide (2 mM) with GSH (5 mM) in the presence of a commercial mixture of rat liver GSH transferase (Sigma, St. Louis, MO; 0.1 mg mL-1) in 0.10 M Tris-HCl buffer (pH 7.7) for 1 h at 37 °C. The latter approach yielded less complex products and was used in the work presented here. LC-MS analysis showed the formation of a peak with the features expected for a GSH conjugate (Supporting Information Figure S2), and high resolution analysis yielded an MH+ ion at m/z 394.1276 (calc. for C14H24N3O8S, 394.1284, -2.0 ppm). Of the HPLC peaks (Supporting Information Figure S3), only one gave a positive colorimetric assay with p-nitrobenzylpyridine reagent (19), indicating the presence of an electrophile. The conjugate was isolated in preparative incubations, frozen, and lyophilized. The t½ was determined in 0.10 M potassium phosphate buffer (pH 7.4) at 37 °C and was 2.6 ± 0.1 h (Figure 1). The structure was established by a combination of NMR methods (1H, 13C, total correlation spectroscopy (TOCSY), heteronuclear multiple bond correlation (HBMC), heteronuclear single quantum correlation (HSQC); Supporting Information Figures S4-S8) and is shown as 1 in Scheme 1. In particular, GSH attack at C-2 is ruled out (2), as is structure 3. The product was quantified using 1H NMR and an internal standard (benzene). An ε560 value of 6.57 mM-1 cm-1 was determined for the p-nitrobenzylpyridine assay based on the 1H-NMR signals (which can be used for convenient determination of stock concentrations).
Figure 1.
Determination of the t1/2 of the purified GSH-butadiene diepoxide conjugate using a p-nitrobenzylpyridine assay (18, 19). The results shown are from three separate experiments (separate symbols), with the mean t1/2 ± SD indicated.
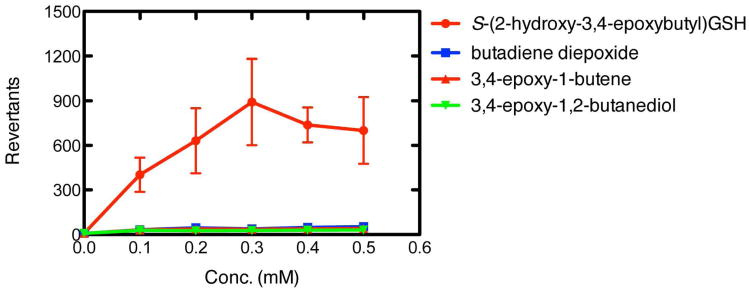
The conjugate, S-(2-hydroxy-3,4-epoxybutyl)GSH, was considerably more mutagenic than several other related epoxides in S. typhimurium TA1535, a tester strain that reports base pair mutations at a GC site (Figure 2). Other compounds tested included butadiene monoepoxide, butadiene diepoxide, and butadiene diol epoxide (prepared by reaction of 3,4-dihydroxybutene with m-chloroperbenzoic acid, Supporting Information Figures S9, S10). These results, along with the enhancement of butadiene diepoxide by GSH transferase expression, argue for a prominent role of the characterized GSH-butadiene diepoxide conjugate in butadiene genotoxicity.
Figure 2.
Mutagenicity of GSH-butadiene diepoxide conjugate in S. typhimurium TA1535. Direct comparisons were made with the indicated other three epoxides, using the methods described in (14). Results are shown as means and ranges obtained for duplicate assays.
Although many butadiene-derived adducts of DNA bases have been prepared (6-13), limited information is available about their occurrence in biological systems and/or their biological activities. We show a role for a defined GSH conjugate of butadiene diepoxide in (base pair) mutagenicity (Figure 2), congruent with previous results on the role of GSH conjugation in butadiene bioactivation (14, 20) (Supporting Information Figure S1). We have not yet evaluated the mutagenicity and other properties of S-(2-hydroxy-3,4-epoxybutyl)GSH in mammalian systems, which will be of interest. Preliminary LC-MS studies have shown the formation of Gua and Ade adducts when the GSH-butadiene diepoxide conjugate was incubated with DNA or DNA bases (results not presented). Future studies are focused on characterization of the DNA adducts formed from GSH-butadiene diepoxide, identification of which of these are miscoding, and analysis of these adducts in vivo. Three stereoisomers of butadiene diepoxide (R,R, S,S, and meso) are known and some differences have been reported for their rates of formation and reactivity (21-25) but we have not prepared the corresponding conjugates yet. The GSH transferase selectivity of the conjugation reaction also remains to be established. Some of the work involved rat and human theta-class enzymes (14, 20) (Supporting Information Figure S1) but a mixture of other rat GSH transferases could be used, as shown in the preparative work.
Supplementary Material
Acknowledgments
This work was supported in part by United States Public Health Service Grants R01 ES010546, T32 ES007028, and P30 ES00267. We thank K. D. Hardy for performing the confirming experiments presented in Supporting Information Figure S1 and D. A. Hachey, M. W. Calcutt, and D. F. Stec for assistance with mass spectrometry and NMR.
Abbreviations
- HBMC
heteronuclear multiple bond correlation
- HSQC
heteronuclear single quantum correlation
- TOCSY
total correlation spectroscopy
Footnotes
Supporting Information Available: GSH transferase-dependent mutagenicity of butadiene diepoxide in S. typhimurium TA1535, LC-MS characterization of GSH-butadiene diepoxide conjugate, NMR spectra of GSH-butadiene diepoxide conjugate, and MS and NMR spectra of butadiene diol epoxide. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Agency for Toxic Substances & Disease Registry. Toxicological Profile for 1,3-butadiene. U. S. Dept. Health Human Services, Centers for Disease Control. [accessed 2 September 2010]; http://www.atsdr.cd.gov/toxprofiles/tp28.html.
- 2.Bolt HM. Butadiene and isoprene: future studies and implications. Toxicology. 1996;113:356–360. doi: 10.1016/0300-483x(96)03473-7. [DOI] [PubMed] [Google Scholar]
- 3.Himmelstein MW, Acquavella JF, Recio L, Medinsky MA, Bond JA. Toxicology and epidemiology of 1,3-butadiene. Crit Rev Toxicol. 1997;27:1–108. doi: 10.3109/10408449709037482. [DOI] [PubMed] [Google Scholar]
- 4.de Meester C. Genotoxic properties of 1,3-butadiene. Mut Res. 1988;195:273–281. doi: 10.1016/0165-1110(88)90005-x. [DOI] [PubMed] [Google Scholar]
- 5.Cochrane JE, Skopek TR. Mutagenicity of butadiene and its epoxide metabolites: I. Mutagenic potential of 1,2-epoxybutene, 1,2,3,4-diepoxybutane and 3,4-epoxy-1,2-butanediol in cultured human lymphoblasts. Carcinogenesis. 1994;15:713–717. doi: 10.1093/carcin/15.4.713. [DOI] [PubMed] [Google Scholar]
- 6.Citti L, Gervasi PG, Turchi G, Bellucci G, Bianchini R. The reaction of 3,4-epoxy-1-butene with deoxyguanosine and DNA in vitro: synthesis and characterization of the main adducts. Carcinogenesis. 1984;5:47–52. doi: 10.1093/carcin/5.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Selzer RR, Elfarra AA. Characterization of N1- and N6-adenosine adducts and N1-inosine adducts formed by the reaction of butadiene monoxide with adenosine: evidence for the N1-adenosine adducts as major initial products. Chem Res Toxicol. 1996;9:875–881. doi: 10.1021/tx960039a. [DOI] [PubMed] [Google Scholar]
- 8.Tretyakova N, Sangaiah R, Yen TY, Gold A, Swenberg JA. Adenine adducts with diepoxybutane: isolation and analysis in exposed calf thymus DNA. Chem Res Toxicol. 1997;10:1171–1179. doi: 10.1021/tx9700681. [DOI] [PubMed] [Google Scholar]
- 9.Selzer RR, Elfarra AA. Characterization of four N-3-thymidine adducts formed in vitro by the reaction of thymidine and butadiene monoxide. Carcinogenesis. 1997;18:1993–1998. doi: 10.1093/carcin/18.10.1993. [DOI] [PubMed] [Google Scholar]
- 10.Tretyakova NY, Sangaiah R, Yen TY, Swenberg JA. Synthesis, characterization, and in vitro quantitation of N7-guanine adducts of diepoxybutane. Chem Res Toxicol. 1997;10:779–785. doi: 10.1021/tx970004q. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XY, Elfarra AA. Characterization of 1,2,3,4-diepoxybutane-2′-deoxyguanosine cross-linking products formed at physiological and nonphysiological conditions. Chem Res Toxicol. 2006;19:547–555. doi: 10.1021/tx0503395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes PH, Hackfeld LC, Kozekov ID, Hodge RP, Lloyd RS. Synthesis and mutagenesis of the butadiene-derived N3 2′-deoxyuridine adducts. Chem Res Toxicol. 2006;19:968–976. doi: 10.1021/tx060016o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seneviratne U, Antsypovich S, Goggin M, Dorr DQ, Guza R, Moser A, Thompson C, York DM, Tretyakova N. Exocyclic deoxyadenosine adducts of 1,2,3,4-diepoxybutane: synthesis, structural elucidation, and mechanistic studies. Chem Res Toxicol. 23:118–133. doi: 10.1021/tx900312e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thier R, Müller M, Taylor JB, Pemble SE, Ketterer B, Guengerich FP. Enhancement of bacterial mutagenicity of bifunctional alkylating agents by expression of mammalian glutathione S-transferase. Chem Res Toxicol. 1995;8:465–472. doi: 10.1021/tx00045a019. [DOI] [PubMed] [Google Scholar]
- 15.Boogaard PJ, Sumner SC, Turner MJ, Bond JA. Hepatic and pulmonary glutathione conjugation of 1,2:3,4-diepoxybutane in human, rat, and mouse in vitro. Toxicology. 1996;113:297–299. doi: 10.1016/0300-483x(96)03460-9. [DOI] [PubMed] [Google Scholar]
- 16.Boogaard PJ, Sumner SC, Bond JA. Glutathione conjugation of 1,2:3,4- diepoxybutane in human liver and rat and mouse liver and lung in vitro. Toxicol Appl Pharmacol. 1996;136:307–316. doi: 10.1006/taap.1996.0037. [DOI] [PubMed] [Google Scholar]
- 17.Vlachodimitropoulos D, Norppa H, Autio K, Catalán J, Hirovonen A, Tasa G, Uusküla M, Demopoulos NA, Sorsa M. GSTT1-dependent induction of centromere-negative and -positive micronuclei by 1,2:3,4-diepoxybutane in cultured human lymphocytes. Mutagenesis. 1997;12:397–403. doi: 10.1093/mutage/12.5.397. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys WG, Kim DH, Cmarik JL, Shimada T, Guengerich FP. Comparison of the DNA alkylating properties and mutagenic responses caused by a series of S-(2-haloethyl)-substituted cysteine and glutathione derivatives. Biochemistry. 1990;29:10342–10350. doi: 10.1021/bi00497a008. [DOI] [PubMed] [Google Scholar]
- 19.Epstein J, Rosenthal RW, Ess RJ. Use of p-(4-nitrobenzyl)pyridine as analytical reagent for ethylenimines and alkylating agents. Anal Chem. 1955;27:1435–1439. [Google Scholar]
- 20.Thier R, Pemble S, Kramer H, Taylor JB, Guengerich FP, Ketterer B. Human glutathione S-transferase T1-1 enhances mutagenicity of 1,2-dibromoethane, dibromomethane, and 1,2,3,4-diepoxybutane in Salmonella typhimurium. Carcinogenesis. 1996;17:163–166. doi: 10.1093/carcin/17.1.163. [DOI] [PubMed] [Google Scholar]
- 21.Krause RJ, Elfarra AA. Oxidation of butadiene monoxide to meso- and (±)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch Biochem Biophys. 1997;337:176–184. doi: 10.1006/abbi.1996.9781. [DOI] [PubMed] [Google Scholar]
- 22.Nieusma JL, Claffey DJ, Maniglier-Poulet C, Imiolczyk T, Ross D, Ruth JA. Stereochemical aspects of 1,3-butadiene metabolism and toxicity in rat and mouse liver microsomes and freshly isolated rat hepatocytes. Chem Res Toxicol. 1997;10:450–456. doi: 10.1021/tx960199m. [DOI] [PubMed] [Google Scholar]
- 23.Nieusma JL, Claffey DJ, Ruth JA, Ross D. Stereochemical aspects of the conjugation fo epoxide metabolites of butadiene with glutathione in rat liver cytosol and freshly isolated rat hepatocytes. Toxicol Sci. 1998;43:102–109. doi: 10.1006/toxs.1998.2461. [DOI] [PubMed] [Google Scholar]
- 24.Park S, Anderson C, Loeber R, Seetharaman M, Jones R, Tretyakova N. Interstrand and intrastrand DNA—DNA cross-linking by 1,2,3,4-diepoxybutane: role of sterochemistry. J Am Chem Soc. 2005;127:14355–14365. doi: 10.1021/ja051979x. [DOI] [PubMed] [Google Scholar]
- 25.Kim MY, Tretyakova N, Wogan GN. Mutagenesis of the supF gene by stereoisomers of 1,2,3,4,-diepoxybutane. Chem Res Toxicol. 2007;20:790–797. doi: 10.1021/tx700003b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.