Abstract
Antibody titers to vaccine-preventable diseases such as tetanus, polio, measles, mumps, and rubella decline within 1-10 years after allogeneic or autologous hematopoietic stem cell transplantation (SCT) if the recipient is not vaccinated. Vaccine-preventable diseases such as pneumococcal diseases, Haemophilus influenzae type b infections, influenza, measles, and varicella can pose an increased risk for SCT recipients. Therefore, after SCT, the recipients should be routinely revaccinated. Vaccination recommendations have previously been developed and published by the European Group of Blood and Marrow Transplantation and the Centers for Disease Control, by the Infectious Diseases Society of America, and by the American Society for Blood and Marrow Transplantation in 2009. Different epidemiologies and strategies have existed in Korea. In 2012, the Korean Society of Infectious Diseases published "Vaccination for Adult" describing the guidelines for vaccination, one of the chapters assigned for vaccination of SCT recipients. The present article reviews the current available vaccination strategies for SCT recipients, their family members, and healthcare workers, with the focus on recent Korean perspectives.
Keywords: Stem cell transplantation, Vaccination, Graft versus host disease, Immune response; Infection
Introduction
Hematopoietic stem cell transplantation (SCT) is used to treat two categories of diseases [1]. The first category consists of non-malignant diseases that result in bone marrow failure syndrome (for example, aplastic anemia, myelodysplastic syndrome, immune deficiency syndrome such as severe combined immunodeficiency or chronic granulomatous disease, genetic disorders such as glycogen storage diseases or mucopolysaccharidoses, and hemoglinopathies such as thalassemia or sickle cell anemia), in which SCT serves to replace a failed tissue and organ. The second category includes neoplastic diseases, particularly hematologic malignancies such as acute and chronic leukemia, lymphoma, multiple myeloma, and myeloproliferative diseases. Transplantation serves two functions: to facilitate the safe use of cytotoxic therapies (intensive chemotherapy with or without total body irradiation) by reversing the myelosuppressive or myeloablative effects of cytotoxic therapy, and to provide immune cells to attack remnant malignancies that express tumor-specific antigens.
SCT can also be classified as autologous and allogeneic according to the donor origin. Techniques for allogeneic SCT have been developed and diversified according to the source of stem cells (bone marrow, peripheral blood, cord blood, or mesenchymal stem cells), the type of donor (sibling, unrelated, or HLA haploidentical), and the intensity of conditioning (standard myeloablative or reduced non-myeloablative). Other supportive methods such as transfusion, colony stimulating factor treatment, and antibiotics also contribute to improved survival after SCT [2, 3].
Since the first successful allogeneic SCT was performed in Korea in 1983, noticeable progress has been achieved [4]. The numbers of SCT survivors returning to family life, school, work, and travel has gradually increased. Infectious complications frequently caused by bacteria, viruses, and fungi vary according to the time after SCT and cause significant morbidity and mortality after SCT. Therefore, it is reasonable to immunize SCT recipients to reduce the risk from vaccine-preventable diseases that may occur during the variable period after SCT [5-10].
In this article, recent advances and recommendations about vaccination after SCT are reviewed. Because differences from western countries exist with regard to the prevalent infectious diseases, sero-status of the recipients and donors, and the available vaccines and strategies, Korean perspectives are included.
Immune recovery and responses to vaccine after SCT
After allogeneic SCT, the immune system of the recipients is replaced by the immune system of the host. Immunodeficiency is caused by a combination of factors, including pre-conditioning regimen, type of SCT, source of stem cells, donor cell treatment, graft-versus-host disease (GVHD), and immunosuppressants [11].
For a vaccine-derived immune response to be clinically significant, post-SCT adaptive T and B cell immunity must have been at least partially reconstituted. B cell counts usually return to normal by 3-12 months after SCT [2]. Regardless of the time to recovery, the newly generated B cells often demonstrate impaired antigen specific responses because of the limited capability of naïve B cells to undergo somatic mutation and isotype switching during the first year after SCT. T cell counts are low in the first 1-3 months after SCT. Thereafter, T cell recovery, especially the recovery of CD4+ T cells, is influenced by recipient age, manipulation of donor grafts, and the presence of chronic GVHD. The T cell response to vaccines for pathogens encountered prior to transplantation can be observed as soon as 1-6 months post-SCT. Antibody responses to vaccines for pathogens encountered prior to transplantation can be observed at 6-12 months after SCT. T cell or antibody responses to vaccines for pathogens not encountered prior to transplantation are usually observed later (1 year after SCT) [2, 11].
Chronic GVHD is associated with functional asplenia and contributes not only to susceptibility to infectious diseases but also to poor responses to polysaccharide-based vaccines. GVHD and its treatment interrupt T cell and antibody responses to vaccines. However, recent guidelines have not delayed vaccination with non-live vaccines in patients with GVHD. In particular, vaccination against Streptococcus pneumoniae, Haemophilus influenzae type b, and influenza should be performed in a timely manner because of the high risk of developing life-threatening infections by these microorganisms [12, 13]. If patients receive prednisolone (> 0.5 mg/kg) as part of a combination immunosuppressive therapy or three immunosuppressant agents, vaccination may be postponed until the immunosuppressants dosing is reduced to a double combination or prednisolone (< 0.5 mg/kg) to achieve a better vaccine response. Live attenuated vaccines are contraindicated in patients with active chronic GVHD [14].
Because no immunosuppressants are given after autologous SCT, immune reconstitution occurs rapidly, with humoral and T-cell responses recovering in 3-9 months. Most published recommendations do not differentiate between allogeneic and autologous SCT recipients.
Guidelines for vaccination after SCT
The Centers for Disease Control and Prevention (CDC) [15] and the European Blood and Marrow Transplantation group (EBMT) [16] published and updated international guidelines for vaccination of SCT recipients. Although these guidelines differ with regard to the number of recommended doses of tetanus, polio, and H. influenzae vaccines (2 vs. 3); the number of doses of pneumococcal polysaccharide vaccines (1 vs. 2); and the time to initiate re-vaccination (6-12 months vs. 12 months) initially, Europe and North America developed updated, unified international guidelines together for autologous and allogeneic SCT recipients under the auspices of the Center for International Blood and Marrow Transplant Research (CIBMTR), and many associated committee and societies approved these guidelines [12, 13, 17], which differ from prior guidelines primarily by the following:
inclusion of seven-valent protein conjugate pneumococcal vaccine (PCV7) in all SCT recipients starting at 3-6 months post SCT, followed by the 23-valent pneumococcal polysaccharide vaccine (PPV23) in patients without chronic GVHD, and consideration of a fourth PCV7 in patients with chronic GVHD.
addition of recommendations for live varicella vaccine in selected patient groups starting at 24 months post-SCT (Varivax is optional, Zostavax is contraindicated).
optional use of vaccines licensed since 2005, such as the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine for adolescents and adults; the recombinant human papilloma virus (HPV) vaccine; and the protein conjugate meningococcal vaccine.
vaccination with inactivated vaccines starting as early as 6 months post-SCT (and earlier for PCV and influenza).
The guidelines were revised based on the 2010 approval of the 13-valent PCV (PCV13) [18]. In 2011, the German-Austrian-Swiss-Consensus Conference on Clinical Practice in Chronic GVHD summarized and updated the currently available recommendations with a particular focus on patients suffering from chronic GVHD [14]. The Korean Society of Infectious Diseases (KSID) also published guidelines for vaccination after SCT in the book of "Vaccination for Adult" in 2012 (Table 1) [19].
Table 1.
Recommended vaccinations for hematopoietic stem cell transplantation (SCT) recipients by the Korean Society of Infectious Diseases (KSID)
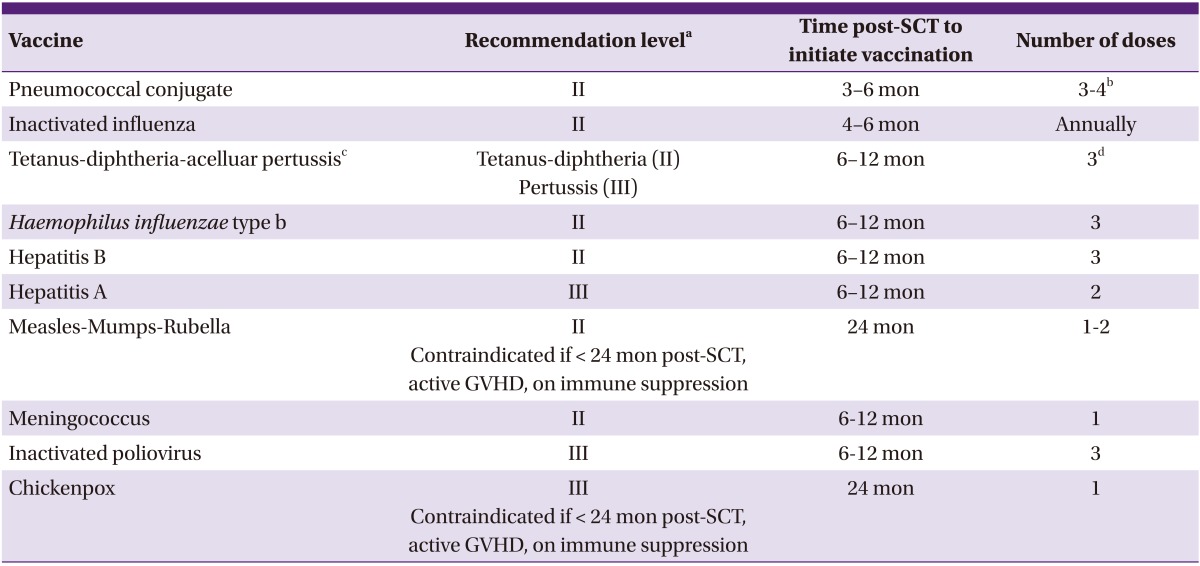
DTaP, diphtheria-tetanus-reduced acellular pertussis vaccine; GVHD, graft versus host disease; PCV, pneumococcal conjugate vaccine; SCT, hematopoietic stem cell transplantation; Td; tetanus toxoid-reduced diphtheria toxoid vaccine; Tdap, tetanus toxoid-reduced diphtheria toxoid-reduced acellular pertussis vaccine.
aStrength of recommendation: (I) Very strongly recommended: immunization may reduce mortality and be cost-effective. Most countries recommend the vaccination. (II) Strongly recommended: immunization may reduce mortality, but cost-effectiveness is unknown in Korea. Most developed countries recommend the vaccination. (III) Recommended: immunization may reduce morbidity rather than mortality. Cost-effectiveness is unknown. (U) Recommended reserved: lack of evidence for recommendation.
bFollowing the three doses of PCV, a dose of 23-valent polysaccharide pneumococcal vaccine may be given to broaden the covered spectrum (II). In SCT recipients with chronic GVHD who are likely to respond poorly to polysaccharide vaccine, a fourth PCV should be considered (III).
cDTaP is preferred over Tdap. If only Tdap is available, it can be used.
dRe-immunization with Td or Tdap at least every 10 years.
The currently available guidelines recommend the initiation of vaccination of all recipients at the same time post-SCT, irrespective of the level of immune competence (i.e., type of SCT, source of stem cells, manipulation of specific cell depletion, or intensity of conditioning). Vaccine strategies should be varied according to these critical variables in the future [12-14, 19, 20].
Recommended vaccines
1. Pneumococcus vaccine
Pneumococcal infections are among the most important causes of morbidity and mortality after allogeneic SCT because of functional hyposplenism or decreased production of Streptococcus pneumoniae-specific opsonizing antibodies as a result of pre-conditioning regimens, incomplete immune reconstitution, and/or chronic GVHD [5, 6, 21-24]. Epidemiological studies have shown that invasive pneumococcal disease occurs in 590 out of 100,000 and 199 out of 100,000 allogeneic and autologous SCT cases per year, respectively, compared with 11.5 out of 100,000 age-matched controls [25].
Currently, two types of pneumococcal vaccine are available in Korea: PCV and PPV23 [26]. PPV23 has the advantage of covering most circulating serotypes, but it has shown poor responses in SCT recipients, especially in patients with chronic GVHD. Neither repeated vaccination nor donor immunization improved this response. However, by stimulating T cell-dependent antibody responses, PCV could be immunogenic in the setting of incomplete immune reconstitution and may thus be preferred in SCT recipients [12-14, 20]. Several studies have shown a response of 60-74% with PCV7. In 2009, Cordonnier et al. [27] demonstrated a lack of inferiority of the vaccine response to all seven serotypes contained in PCV7 1 month after receipt of three monthly doses of PCV7 starting 3 or 9 months after SCT. Cordonnier et al. [28] showed that the immune response to PPV23 after PCV7 in allogeneic SCT increased the response rate to PCV7 serotypes and also broadened pneumococcal coverage, regardless of the time of the PPV23 vaccine dose, i.e., 12 or 18 months. New conjugate vaccines (PCV10, PCV13) have been approved and hold promise for broadening pneumococcal immunity after SCT [18]. Although PCV7 was recommended in the 2009 CIBMTR guidelines [12, 13], the committee also agreed that PCV13 could be used as a replacement for PCV7 [18].
Thus, according to current guidelines, all SCT recipients should be vaccinated with three doses of PCV starting 3-6 months after SCT. The three doses are given in monthly intervals. Following the three doses of PCV, a dose of PPV23 may be given to broaden the covered spectrum. In SCT recipients with chronic GVHD who are likely to respond poorly to polysaccharide vaccines, a fourth PCV should be considered [12, 13, 19, 20]. The German-Austrian-Swiss consensus conference suggested that the booster dose should be given at 18 months after allogeneic SCT (12 months after the first dose) [13].
2. Influenza vaccine
SCT recipients are recognized to be at increased risk for complicated and even fatal influenza A and B [29]. The European study of the pandemic A/H1N1 2009 influenza included 286 patients: 222 after allogeneic SCT and 64 after autologous SCT. In total, 267 patients were treated with oseltamivir and 15 with zanamivir; 125 patients (44%) were hospitalized; 93 (33%) developed lower respiratory tract infection (LRTI); 33 (16%) patients needed mechanical ventilation; and 18 (6%) patients died of the pandemic influenza or its complications 2-96 days after SCT [30].
Several risk factors for complicated influenza in SCT recipients have been reported [29-35]. Lymphopenia (note that the cutoff for absolute lymphocyte number differed in the different studies) was found to be a risk factor for LRTI [31]. Corticosteroid use is still controversial. Choi et al. [33] suggested that high-dose corticosteroid treatment (≥ 1 mg/kg) given at the time of influenza diagnosis was associated with a reduced risk for mechanical ventilation. However, some reports showed that chronic or adjuvant steroid use was significantly associated with LRTI and mortality [34, 35]. Additional risks for complications included a lack of early antiviral therapy, older age, and development of influenza soon after SCT [31].
Immunization with the inactivated influenza vaccine has been shown to decrease the risk of influenza when administered more than 6 months post-SCT [36]. The current 2009 CIBMTR guidelines highly recommend annual vaccination with the seasonal trivalent inactivated influenza vaccine in both allogeneic and autologous SCT recipients. The vaccination should be given before the influenza season starting at 6 months after SCT [12, 13, 37]. During outbreaks, SCT recipients who have not yet received the influenza vaccine should be receive it if more than 4 months have elapsed after SCT. A second dose of the vaccine after 3-4 weeks is advised in this situation, although it may only have marginal benefit. A recent update shortened the interval for the vaccine administered prior to the influenza season to 3 months after SCT [31]. No data are about live attenuated influenza vaccine (LAIV) safety and efficacy in SCT recipients; therefore, LAIV should not be used [20, 31].
3. Tetanus-diphtheria-pertussis vaccine
Most recipients will lose specific immunity to tetanus and diphtheria following SCT; therefore, it is important to have an immunization strategy against these infections in SCT recipients [38]. Currently, adults and adolescents are the main reservoir for Bordetella pertussis and an increased rate of disease and several outbreaks have been reported worldwide and in Korea [39, 40]. SCT recipients may be vulnerable to complications from pertussis because of lung damage from previous cancer chemotherapy and/or pre-conditioning regimens such as total body irradiation, even in the absence of chronic GVHD [12, 13].
In Korea, there are two types of diphtheria and tetanus vaccines, i.e., Td (tetanus-reduced diphtheria toxoid) and Tdap (tetanus-reduced diphtheria toxoid-reduced acellular pertussis) for adults, which have been available since 2004 and 2009, respectively. These vaccines contain the same full doses of tetanus toxoid (T) and reduced diphtheria toxoid (d) with or without a reduced dose of pertussis (ap). Another vaccine, DTaP (diphtheria-tetanus toxoid-acellular pertussis) for children and adolescents, has been available in Korea since 1982, and it contains full-dose diphtheria toxoid (D) and tetanus toxoid (T) in addition to full-dose (not reduced dose) acellular pertussis toxoid (aP) [26].
Full post-transplant revaccination is initiated 12 months after transplantation and consists of an average of three dose schedules of vaccine that result in long-term persistence of humoral immunity against tetanus and diphtheria in SCT recipients, for an average of 8.6 and 9.0 years, respectively [41]. Because post-SCT recipients should be regarded as "never vaccinated" and the response to the reduced dose may be poor, full toxoid vaccines should be used if possible. Current guidelines prefer three doses of full toxoid vaccine (DTaP) rather than reduced doses (Tdap or Td) starting at 6-12 months after SCT and given in monthly intervals [12-14, 19]. Recipients should be re-immunized with Td at least every 10 years. In the general population in Korea, at least one dose of Tdap is recommended [19].
4. Haemophilus influenzae type b (Hib) conjugate vaccine
H. influenzae is an important cause of infection after SCT, although fatal cases have rarely been reported in most transplantation centers. Vaccination with a conjugate Hib vaccine can elicit protective immunity after SCT [12-14]. Responses to a series of three courses of Hib vaccine ranged from 47% to 92%, with a higher response rated observed in patients whose donors were also immunized before stem cell collection [42, 43]. Because it was known that early vaccination resulted in poor responses in children, the timing of the vaccination after SCT is important for the immune response. It is recommended that three doses be given in monthly intervals starting at 6-12 months after SCT [12-14, 19]
5. Hepatitis B and A vaccine
Hepatitis B virus (HBV) infection continues to be a common problem after SCT as a result of both immunosuppression and frequent transfusions of blood product. A substantial portion of allo-SCT recipients lose their immunity to HBV over time [44, 45]. Therefore, all recipients who are sero-negative for HBV markers and recipients who are positive for anti-HBc only or show resolved hepatitis B (anti-HBc plus anti-HBs) should be vaccinated [14]. Jaffe et al. [46] evaluated immunity to the three-dose regimen. To be eligible for vaccination, recipients had to have completed immune reconstitution (CD4 cell count >200 cells/µl, IgG > 5 g/L, and adequate in vitro T cell response) and to have stopped taking immunosuppressants. Early post-SCT vaccination is likely to yield unsuccessful immunity; given the nature of antigen, it is recommended to start the vaccination for hepatitis B no earlier than 6-12 months post-SCT. Because chronic GVHD was associated with reduced sero-conversion after vaccination, the antibody response to the Hbs antigen should be determined within 1-3 months of the last vaccine. If the response is not adequate, repeat of a three-dose vaccine should be considered [46].
In the 2009 CIBMTR guidelines, hepatitis A is not routinely recommended (travelers to an endemic area only) [12, 13]. However, there still have been outbreak reports in Korea; therefore, KSID recommends vaccinations for sero-negative SCT recipients starting at 6-12 months after SCT [19]. The dosing schedule is the same as that for patients in the general population.
6. Measles-mumps-rubella (MMR) vaccine
Outbreaks of measles and mumps have been reported in many parts of the world. In 2000-2001, a measles epidemic occurred in Korea, with more than 55,000 reported cases [47]. During the outbreak, 16 SCT recipients in our center were presumptively diagnosed with measles, and one of them died of fatal measles pneumonia [48-50]. Although the risk in SCT patients has not been well studied, MMR vaccine titers are known to decline gradually after SCT [51-53].
The MMR is a live, attenuated virus vaccine and is recommended to recipients who are without chronic GVHD and not taking immunosuppressants; it is performed 24 months after SCT [12-14], and it has been shown to be safe and successful. Limited data are available on the immunogenicity and durability of the response to MMR following alternative donor SCT or in response to mumps following any type of SCT. During an outbreak of measles in Brazil, earlier MMR vaccination between 9 and 19 months after SCT was reported to be safe and immunogenic [54]. However, this strategy is reserved for during epidemics.
The risk for severe rubella infection after allogeneic SCT is likely low. However, with the current improvement in survival together with reduced-intensity conditioning, the potential for pregnancy is also likely to increase. Therefore, the rubella vaccine could be indicated in female recipients who have retained the potential for pregnancy [12-14].
7. Neisseria meningitidis vaccine
As in the cases of vaccines against pneumococcus and Hib, it is expected that the protein conjugate meningococcal vaccine will provide significantly better and stable immune responses than does the polysaccharide vaccines [12-14]. Although a tetravalent conjugate vaccine is available in Korea now, no data have been collected regarding the immunogenicity and safety of this vaccine in adult SCT recipients [19].
8. Inactivated poliovirus vaccine
Although polio has been eradicated in many countries, including Korea, areas of activity still exist [14]. Recipients lose early immunity to poliovirus after SCT and they can be vulnerable. Although both a live attenuated polio and an inactivated polio vaccine exist, the live vaccine is contraindicated in SCT recipients or their close contacts. The inactivated vaccine has been shown to be safe and immunogenic, and protective levels were obtained. Guidelines recommend three doses of the inactivated vaccine with an interval of at least 1 month starting at 6 months after SCT [12-14, 19].
9. Chickenpox, Herpes zoster vaccine
Varicella zoster virus (VZV) causes extensive morbidity late after SCT, with associated risks for both dermatomal reactivation and disseminated diseases [5, 55]. Severe, potentially fatal diseases, including hepatitis and meningoencephalitis, can occur late after HCT even in the absence of skin lesions. Recent studies have suggested that prolonged acyclovir prophylaxis effectively decreases VZV-related morbidity. The results of these studies have propelled many centers to administer acyclovir for the first year after allogeneic SCT [2]. In our center, where we do not recommend application of long-term use of acyclovir, herpes zoster developed in 34.3% of patients (79/230) at a median of 235 days post-SCT. The cumulative HZ incidence was 22% at 1 year, 30.8% at 2 years, 38.6% at 3 years, and 41.2% at 4 years [5].
There are 2 main vaccines: one for chickenpox and the other for shingles. The difference between these vaccines is the number of plaque-forming units of attenuated virus. The chickenpox vaccine has lower viral titers, and limited data are available regarding the use of the varicella vaccine after allogeneic SCT. It could be used for all SCT recipients who have met the criteria for liver virus vaccination [56, 57]. However, a vaccine for herpes zoster has not been tested in an immunocompromised population and is not recommended in the 2009 CIBMTR because of its high viral titer [12,13]. Clinical trials are underway for an inactivated herpes zoster vaccine in recipients of autologous SCT.
10. Human papilloma virus vaccine
HPV infection is known to promote cervical cancer. Among immunosuppressed patients, the risk of developing squamous cell carcinoma is 64-250 times higher than that among the general population. Data on the immunogenicity and clinical effectiveness of the quadrivalent HPV vaccine in allo-SCT recipients are lacking. Optimal HPV screening strategies and the effect of HPV vaccination on transplant recipients remain largely unknown. Because of the increased risk of secondary malignancy in SCT recipients, regular follow-up by a gynecologist is advised with HPV screening [14, 58]. The 2009 CIBMTR guidelines recommend that clinicians follow the policy used for the general population in each country [12, 13].
11. Japanese B encephalitis virus vaccine
The 2009 CIBMTR guidelines recommend that the Japanese B encephalitis virus vaccine be used according to local policy when residing in or traveling to endemic areas. No data are available regarding the time at which vaccination can be expected to induce an immune response after SCT [12, 13].
12. Other vaccines
Vaccination for SCT recipients travelling to endemic areas is either contraindicated (live-attenuated vaccines) or not rated because of limited experience. The BCG vaccine can cause severe infection in patients with depressed T cell function and is not recommended in SCT recipients [12-14].
Donor vaccination
Donor vaccination has been demonstrated to improve the post-transplantation immunity of SCT recipients, especially in the case of pneumococcus, Hib, tetanus, and HBV [12-14]. For example, vaccination against HBV is recommended in all donors without acquired or natural immunization because of the possibility of adoptive cell-mediated immunotherapy. Although insufficient time is usually available to complete vaccination in sero-negative donors, even if a single dose can be administered before donation, it should be given [43, 45, 59]. Re-vaccination after SCT is frequently necessary to ensure long-term immunity. However, because of ethical and practical problems, there is no recommendation regarding donor vaccination [12, 13, 19].
Vaccination of family members, close contacts, and healthcare workers
Vaccination of family members, close contacts, and healthcare workers is recommended to minimize exposure to vaccine-preventable diseases among SCT recipients (Table 2) [19].
Table 2.
Recommended vaccinations for family members and healthcare workers in contact with SCT recipients by the Korean Society of Infectious Diseases (KSID)

aStrength of recommendation; (I) Very strongly recommended: immunization may reduce mortality and be cost-effective. Most countries recommend the vaccination. (II) Strongly recommended: immunization may reduce mortality but cost effectiveness is unknown in Korea. Most developed countries recommend the vaccination. (III) Recommended: immunization may reduce morbidity rather than mortality. Cost-effectiveness is unknown. (U) Recommended reserved: lack of evidence for recommendation.
Annual influenza vaccination of family members, close contacts, and staff in the transplant unit is highly recommended. These individuals should not be immunized by LAIV because of the transmission risk to the patients [12, 13, 31].
MMR vaccination can be used safely in family members and individuals in close contact with patients. It could also be considered in other individuals with frequent social contact (e.g., teachers and kindergarten workers). Vaccination is indicated for all individuals who are ≥12 months old and not pregnant or immunocompromised. No evidence exists for person-to-person transmission when MMR is administered [52].
Vaccination of family members and close contacts for VZV is recommended, as healthy vaccinated individuals have a minimal risk of transmitting the vaccine virus to their contacts. However, if rashes develop after VZV vaccination, the vaccinated healthy individual should avoid contact with SCT recipients until all rashes have crusted or the rash has resolved [14].
Conclusion and perspectives
There have been remarkable advances in understanding post-SCT immune recovery that may make it possible to develop new vaccine strategies. The current vaccination guidelines do not differentiate among donor type. Because SCT using alternative donors is increasing, prospective, multi-center, international clinical trials for recommending different vaccine strategies should be undertaken. It is also anticipated that new vaccines (such as inactivated vaccines for herpes zoster and a cytomegalovirus vaccine) will be available soon.
References
- 1.Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am. 2010;24:257–272. doi: 10.1016/j.idc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Rezvani AR, Deeg HJ. Introduction to hematopoietic cell transplantation. In: Bowden RA, Ljungman P, Snydman DR, editors. Transplant infections. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 1–12. [Google Scholar]
- 3.Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–1475. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- 4.Lee JW, Kim CC. The activity of hematopoietic stem cell transplantation in Korea. Bone Marrow Transplant. 2008;42(Suppl 1):S92–S95. doi: 10.1038/bmt.2008.127. [DOI] [PubMed] [Google Scholar]
- 5.Lee DG. Common infectious diseases in hematopoietic stem cell transplant recipients. Korean J Med. 2013;84:158–167. [Google Scholar]
- 6.Dropulic LK, Lederman HM. Overview of infections in the immunocompromised host. In: Hayden RT, Carroll KC, Tang YW, Wolk DM, editors. Diagnostic microbiology of the immunocompromised host. 1st ed. Washinton, DC: ASM Press; 2009. pp. 3–43. [Google Scholar]
- 7.Safdar A, Armstrong D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin Infect Dis. 2011;53:798–806. doi: 10.1093/cid/cir492. [DOI] [PubMed] [Google Scholar]
- 8.Socié G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, Korthof E, Weis J, Levy V, Tichelli A Late Effects Working Party of the European Study Group for Blood and Marrow Transplantation. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 9.Chen CS, Boeckh M, Seidel K, Clark JG, Kansu E, Madtes DK, Wagner JL, Witherspoon RP, Anasetti C, Appelbaum FR, Bensinger WI, Deeg HJ, Martin PJ, Sanders JE, Storb R, Storek J, Wade J, Siadak M, Flowers ME, Sullivan KM. Incidence, risk factors and mortality from pneumonia developing late after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:515–522. doi: 10.1038/sj.bmt.1704162. [DOI] [PubMed] [Google Scholar]
- 10.Bjorklund A, Aschan J, Labopin M, Remberger M, Ringden O, Winiarski J, Ljungman P. Risk factors for fatal infectious complications developing late after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;40:1055–1062. doi: 10.1038/sj.bmt.1705856. [DOI] [PubMed] [Google Scholar]
- 11.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115:3861–3868. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 12.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungman P, Cordonnier C, Einsele H, Englund J, Machado CM, Storek J, Small T Center for International Blood and Marrow Transplant Research; National Marrow Donor Program; European Blood and Marrow Transplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Disease Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Diseases Canada; Centers for Disease Control and Prevention. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44:521–526. doi: 10.1038/bmt.2009.263. [DOI] [PubMed] [Google Scholar]
- 14.Hilgendorf I, Freund M, Jilg W, Einsele H, Gea-Banacloche J, Greinix H, Halter J, Lawitschka A, Wolff D, Meisel R. Vaccination of allogeneic haematopoietic stem cell transplant recipients: report from the international consensus conference on clinical practice in chronic GVHD. Vaccine. 2011;29:2825–2833. doi: 10.1016/j.vaccine.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan KM, Dykewicz CA, Longworth DL, Boeckh M, Baden LR, Rubin RH, Sepkowitz KA Centers for Disease Control and Prevention; Infectious Diseases Society of America; American Society for Blood and Marrow Transplantation Practice Guidelines and beyond. Preventing opportunistic infections after hematopoietic stem cell transplantation: the Centers for Disease Control and Prevention, Infectious Diseases Society of America, and American Society for Blood and Marrow Transplantation Practice Guidelines and beyond. Hematology Am Soc Hematol Educ Program. 2001:392–421. doi: 10.1182/asheducation-2001.1.392. [DOI] [PubMed] [Google Scholar]
- 16.Ljungman P, Engelhard D, de la Cámara R, Einsele H, Locasciulli A, Martino R, Ribaud P, Ward K, Cordonnier C Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 2005;35:737–746. doi: 10.1038/sj.bmt.1704870. [DOI] [PubMed] [Google Scholar]
- 17.Styczynski J, Gil L Paediatric Diseases Working Party. Prevention of infectious complications in pediatric HSCT. Bone Marrow Transplant. 2008;42(Suppl 2):S77–S81. doi: 10.1038/bmt.2008.289. [DOI] [PubMed] [Google Scholar]
- 18.Ljungman P, Small TN vaccination recommendations writing group. Update to vaccination guidelines. Biol Blood Marrow Transplant. 2010;16:1608–1609. doi: 10.1016/j.bbmt.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Lee DG, Yoo JH. Hematopoietic stem cell transplantation. In: Cheong HJ, editor. Vaccination for adult. 2nd ed. Seoul: MIP Press; 2012. pp. 380–388. [Google Scholar]
- 20.Small TN, Cowan MJ. Immunization of hematopoietic stem cell transplant recipients against vaccine-preventable diseases. Expert Rev Clin Immunol. 2011;7:193–203. doi: 10.1586/eci.10.103. [DOI] [PubMed] [Google Scholar]
- 21.Engelhard D, Cordonnier C, Shaw PJ, Parkalli T, Guenther C, Martino R, Dekker AW, Prentice HG, Gustavsson A, Nurnberger W, Ljungman P Infectious Disease Working Party of the European Bone Marrow Transplantation (IDWP-EBMT) Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol. 2002;117:444–450. doi: 10.1046/j.1365-2141.2002.03457.x. [DOI] [PubMed] [Google Scholar]
- 22.Youssef S, Rodriguez G, Rolston KV, Champlin RE, Raad II, Safdar A. Streptococcus pneumoniae infection in 47 hematopoietic stem cell transplantation recipients: clinical characteristics of infections and vaccine-breakthrough infections, 1989-2005. Medicine (Baltimore) 2007;86:69–77. doi: 10.1097/md.0b013e31803eb176. [DOI] [PubMed] [Google Scholar]
- 23.Debbache K, Varon E, Hicheri Y, Legrand P, Donay JL, Ribaud P, Cordonnier C. The epidemiology of invasive Streptococcus pneumoniae infections in onco-haematology and haematopoietic stem cell transplant patients in France. Are the serotypes covered by the available anti-pneumococcal vaccines? Clin Microbiol Infect. 2009;15:865–868. doi: 10.1111/j.1469-0691.2009.02810.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumar D, Chen MH, Welsh B, Siegal D, Cobos I, Messner HA, Lipton J, Humar A. A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. Clin Infect Dis. 2007;45:1576–1582. doi: 10.1086/523583. [DOI] [PubMed] [Google Scholar]
- 25.Kumar D, Humar A, Plevneshi A, Siegal D, Franke N, Green K, McGeer A Toronto Invasive Bacterial Diseases Network. Invasive pneumococcal disease in adult hematopoietic stem cell transplant recipients: a decade of prospective population-based surveillance. Bone Marrow Transplant. 2008;41:743–747. doi: 10.1038/sj.bmt.1705964. [DOI] [PubMed] [Google Scholar]
- 26.Cheong HJ. Vaccination necessary for Korean adults. J Korean Med Assoc. 2011;54:1289–1296. [Google Scholar]
- 27.Cordonnier C, Labopin M, Chesnel V, Ribaud P, De La Camara R, Martino R, Ullmann AJ, Parkkali T, Locasciulli A, Yakouben K, Pauksens K, Einsele H, Niederwieser D, Apperley J, Ljungman P Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis. 2009;48:1392–1401. doi: 10.1086/598324. [DOI] [PubMed] [Google Scholar]
- 28.Cordonnier C, Labopin M, Chesnel V, Ribaud P, Camara Rde L, Martino R, Ullmann AJ, Parkkali T, Locasciulli A, Yakouben K, Pauksens K, Bonnet E, Einsele H, Niederwieser D, Apperley J, Ljungman P. Immune response to the 23-valent polysaccharide pneumococcal vaccine after the 7-valent conjugate vaccine in allogeneic stem cell transplant recipients: results from the EBMT IDWP01 trial. Vaccine. 2010;28:2730–2734. doi: 10.1016/j.vaccine.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 30.Ljungman P, de la Camara R, Perez-Bercoff L, Abecasis M, Nieto Campuzano JB, Cannata-Ortiz MJ, Cordonnier C, Einsele H, Gonzalez-Vicent M, Espigado I, Halter J, Martino R, Mohty B, Sucak G, Ullmann AJ, Vázquez L, Ward KN, Engelhard D Infectious Diseases Working Party, European Group for Blood and Marrow Transplantation; Infectious Complications Subcommittee, Spanish Group of Haematopoietic Stem-cell Transplantation. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1231–1235. doi: 10.3324/haematol.2011.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelhard D, Mohty B, de la Camara R, Cordonnier C, Ljungman P. European guidelines for prevention and management of influenza in hematopoietic stem cell transplantation and leukemia patients: summary of ECIL-4 (2011), on behalf of ECIL, a joint venture of EBMT, EORTC, ICHS, and ELN. Transpl Infect Dis. 2013;15:219–232. doi: 10.1111/tid.12054. [DOI] [PubMed] [Google Scholar]
- 32.Ditschkowski M, Elmaagacli AH, Beelen DW. H1N1 in allogeneic stem cell recipients: courses of infection and influence of vaccination on graft-versus-host disease (GVHD) Ann Hematol. 2011;90:117–118. doi: 10.1007/s00277-010-0971-8. [DOI] [PubMed] [Google Scholar]
- 33.Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117:5050–5056. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SH, Hong SB, Yun SC, Choi WI, Ahn JJ, Lee YJ, Lee HB, Lim CM, Koh Y Korean Society of Critical Care Medicine H1N1 Collaborative. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med. 2011;183:1207–1214. doi: 10.1164/rccm.201101-0110OC. [DOI] [PubMed] [Google Scholar]
- 35.Espinosa-Aguilar L, Green JS, Forrest GN, Ball ED, Mariaz RT, Strasfeld L, Taplitz RA. Novel H1N1 influenza in hematopoietic stem cell transplantation recipients: two centers' experiences. Biol Blood Marrow Transplant. 2011;17:566–573. doi: 10.1016/j.bbmt.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Machado CM, Cardoso MR, da Rocha IF, Boas LS, Dulley FL, Pannuti CS. The benefits of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant. 2005;36:897–900. doi: 10.1038/sj.bmt.1705159. [DOI] [PubMed] [Google Scholar]
- 37.Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant. 2008;42:637–641. doi: 10.1038/bmt.2008.264. [DOI] [PubMed] [Google Scholar]
- 38.Li Volti S, Mauro L, Di Gregorio F, Romeo MA, Lupo L, Pizzarelli G, Mangiagli A, Giammanco G, Russo G. Immune status and immune response todiphtheria-tetanus and polio vaccines in allogeneic bone marrow-transplanted thalassemic patients. Bone Marrow Transplant. 1994;14:225–227. [PubMed] [Google Scholar]
- 39.Hewlett EL, Edwards KM. Pertussis-not just for kids. N Engl J Med. 2005;352:1215–1222. doi: 10.1056/NEJMcp041025. [DOI] [PubMed] [Google Scholar]
- 40.Choi JH, Choo EJ, Huh A, Choi SM, Eom JS, Lee JS, Park SH, Kang JH. Immunogenicity and safety of diphtheria-tetanus vaccine in adult. J Korean Med Sci. 2010;25:1727–1732. doi: 10.3346/jkms.2010.25.12.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Škovránková J, Petráš M. Persistence of humoral immunity to tetanus and diphtheria in hematopoietic stem cell transplant recipients after post-transplant immunization. Pediatr Blood Cancer. 2012;59:908–913. doi: 10.1002/pbc.24186. [DOI] [PubMed] [Google Scholar]
- 42.Parkkali T, Käyhty H, Hovi T, Olander RM, Roivainen M, Volin L, Ruutu T, Lahdenkari M, Ruutu P. A randomized study on donor immunization with tetanus-diphtheria, Haemophilus influenzae type b and inactivated poliovirus vaccines to improve the recipient responses to the same vaccines after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2007;39:179–188. doi: 10.1038/sj.bmt.1705562. [DOI] [PubMed] [Google Scholar]
- 43.Storek J, Dawson MA, Lim LC, Burman BE, Stevens-Ayers T, Viganego F, Herremans MM, Flowers ME, Witherspoon RP, Maloney DG, Boeckh M. Efficacy of donor vaccination before hematopoietic cell transplantation and recipient vaccination both before and early after transplantation. Bone Marrow Transplant. 2004;33:337–346. doi: 10.1038/sj.bmt.1704336. [DOI] [PubMed] [Google Scholar]
- 44.Idilman R, Ustün C, Karayalçin S, Aktemel A, Turkyilmaz AR, Ozcan M, Arslan O, Bozdayi AM, Van Thiel DH, Akan H. Hepatitis B virus vaccination of recipients and donors of allogeneic peripheral blood stem cell transplantation. Clin Transplant. 2003;17:438–443. doi: 10.1034/j.1399-0012.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 45.Idilman R, Arat M. Evaluation and management of hepatitis B virus infectionin hematopoietic stem cell transplantation: before and after transplantation. Expert Rev Anti Infect Ther. 2011;9:641–652. doi: 10.1586/eri.11.79. [DOI] [PubMed] [Google Scholar]
- 46.Jaffe D, Papadopoulos EB, Young JW, Orelly RJ, Prockop S, Kerman NA, Jakubowski A, Boulad F, Perales MA, Castro-Malaspina H, Small TN. Immunogenicity of recombinant hepatitis B vaccine (rHBV) in recipients of unrelated or related allogeneic hematopoietic cell (HC) transplants. Blood. 2006;108:2470–2475. doi: 10.1182/blood-2006-04-006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Na BK, Shin JM, Lee JY, Shin GC, Kim YY, Lee JS, Lee JK, Cho HW, Lee HJ, Rota PA, Bellini WJ, Kim WJ, Kang C. Genetic and antigenic characterization of measles viruses that circulated in Korea during the 2000-2001 epidemic. J Med Virol. 2003;70:649–654. doi: 10.1002/jmv.10444. [DOI] [PubMed] [Google Scholar]
- 48.Lee DG, Yoo JH, Choi JH, Choi SM, Park SH, Kim YJ, Kim DW, Shin WS, Kim CC. A fatal case of measles pneumonia complicating an adult recipient of hemopoietic stem cell transplantation during the nationwide epidemic in Korea. Int J Infect Dis. 2006;10:410–411. doi: 10.1016/j.ijid.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Yoo JH, Lee DG, Choi SM, Choi JH, Park YH, Kim YJ, Kim HJ, Lee S, Kim DW, Lee JW, Min WS, Shin WS, Kim CC. Infectious complications and outcomes after allogeneic hematopoietic stem cell transplantation in Korea. Bone Marrow Transplant. 2004;34:497–504. doi: 10.1038/sj.bmt.1704636. [DOI] [PubMed] [Google Scholar]
- 50.Lee DG, Park ST, Na BK, Choi JH, Shin WS, Paik SY, Shin JM, Kang C, Kim WJ, Lee HJ, Kim CC. Characteristics of respiratory tract infections in the hematopoietic stem cell transplantation population. Korean J Infect Dis. 2001;33:419–429. [Google Scholar]
- 51.Ljungman P, Fridell E, Lönnqvist B, Bolme P, Böttiger M, Gahrton G, Linde A, Ringdén O, Wahren B. Efficacy and safety of vaccination of marrow transplant recipients with a live attenuated measles, mumps, and rubella vaccine. J Infect Dis. 1989;159:610–615. doi: 10.1093/infdis/159.4.610. [DOI] [PubMed] [Google Scholar]
- 52.Ljungman P, Aschan J, Barkholt L, Broliden PA, Gustafsson B, Lewensohn-Fuchs I, Löfgren C, Winiarski J, Ringdén O. Measles immunity after allogeneic stem cell transplantation influence of donor type, graft type, intensity of conditioning, and graft-versus host disease. Bone Marrow Transplant. 2004;34:589–593. doi: 10.1038/sj.bmt.1704634. [DOI] [PubMed] [Google Scholar]
- 53.Lee SH, Choi SM, Park YH, Park SG, Kim YJ, Chung MS, Choi JH, Yoo JH, Shin WS, Min WS, Kim CC, Kim DJ. Changes in antibody titers of measles, mumps, rubella, and hepatitis B virus after bone marrow transplantation in Korea: a preliminary report. Korean J Infect Dis. 1998;30:558–563. [Google Scholar]
- 54.Machado CM, de Souza VA, Sumita LM, da Rocha IF, Dulley FL, Pannuti CS. Early measles vaccination in bone marrow transplant recipients. Bone Marrow Transplant. 2005;35:787–791. doi: 10.1038/sj.bmt.1704878. [DOI] [PubMed] [Google Scholar]
- 55.Onozawa M, Hashino S, Haseyama Y, Hirayama Y, Iizuka S, Ishida T, Kaneda M, Kobayashi H, Kobayashi R, Koda K, Kurosawa M, Masauji N, Matsunaga T, Mori A, Mukai M, Nishio M, Noto S, Ota S, Sakai H, Suzuki N, Takahashi T, Tanaka J, Torimoto Y, Yoshida M, Fukuhara T. Incidence and risk of postherpetic neuralgia after varicella zoster virus infection in hematopoietic cell transplantation recipients: Hokkaido Hematology Study Group. Biol Blood Marrow Transplant. 2009;15:724–729. doi: 10.1016/j.bbmt.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Chou JF, Kernan NA, Prockop S, Heller G, Scaradavou A, Kobos R, Knowles MA, Papadopoulos EB, Casson A, Copeland C, Torok-Castanza J, Zakak N, Ruggiero J, Small TN. Safety and immunogenicity of the live attenuated varicella vaccine following T replete or T cell-depleted related and unrelated allogeneic hematopoietic cell transplantation (alloHCT) Biol Blood Marrow Transplant. 2011;17:1708–1713. doi: 10.1016/j.bbmt.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kussmaul SC, Horn BN, Dvorak CC, Abramovitz L, Cowan MJ, Weintrub PS. Safety of the live, attenuated varicella vaccine in pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant. 2010;45:1602–1606. doi: 10.1038/bmt.2010.31. [DOI] [PubMed] [Google Scholar]
- 58.Tedeschi SK, Savani BN, Jagasia M, Engelhardt B, Anasetti C, Barrett AJ, Lee S. Time to consider HPV vaccination after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1033–1036. doi: 10.1016/j.bbmt.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carreras E. Risk assessment in haematopoietic stem cell transplantation: the liver as a risk factor. Best Pract Res Clin Haematol. 2007;20:231–246. doi: 10.1016/j.beha.2006.10.010. [DOI] [PubMed] [Google Scholar]


