Abstract
Background
Postoperative bacterial meningitis (PBM) is a serious potential complication after neurosurgery. Early diagnosis and introduction of antimicrobial therapy are necessary to reduce the rate of fatal outcomes from PBM. However, PBM is not easily differentiated from postoperative aseptic meningitis (PAM), which usually has favorable clinical outcomes. Serum procalcitonin (S-PCT) has been found to be a useful marker for distinguishing community-acquired bacterial from viral meningitis. We investigated the predictive performance of S-PCT for PBM in patients who underwent neurosurgery.
Materials and Methods
Between September 2009 and August 2010, we prospectively collected data from patients who underwent neurosurgery and had cerebrospinal fluid (CSF) pleocytosis within 14 days of surgery. Based on the CSF culture results, patients were categorized as either PBM or PAM cases. We compared the laboratory test results including S-PCT levels between PBM and PAM cases, and investigated the predictive performance of S-PCT for PBM.
Results
During the study period, PBM and PAM occurred in 14 and 64 patients, respectively. There was no significant difference in CSF profiles between PBM and PAM cases. S-PCT level ≥ 0.15 ng/mL (50.0% vs. 20.0%, P = 0.07) and C-reactive protein (CRP) level ≥ 2.5 mg/dL (75.0% vs. 46.5%, P = 0.16) tended to be more frequent in PBM than in PAM cases. A blood white blood cell (B-WBC) count ≥ 9,500/mm3 was more frequently found in PBM cases (85.7% vs. 50.8%, P = 0.02) than in PAM cases. For the diagnosis of PBM, an S-PCT level ≥ 0.15 ng/mL had a specificity of 80.0%. The combined criteria of a CRP level ≥ 2.5 mg/dL, B-WBC count ≥ 9,500/mm3, and an S-PCT level ≥ 0.15 ng/mL had the highest specificity (92.6%) of all the criteria. An S-PCT level ≥0.15 ng/mL had low sensitivity (50.0%), and the combined criteria of CRP level ≥ 2.5 mg/dL, B-WBC count ≥ 9,500/mm3, and S-PCT level ≥ 0.15 ng/mL had an improved sensitivity of 85.7%. However, the sensitivity did not significantly differ from that of a B-WBC count ≥ 9,500/mm3 (85.7%).
Conclusions
S-PCT showed limited performance for the diagnosis of postoperative meningitis. However, it could be a useful adjunct for the improvement of diagnostic sensitivity when used in combination with other inflammatory markers.
Keywords: Meningitis, Procalcitonin, Adenosine deaminase, Neurosurgery
Introduction
Bacterial meningitis is an important potential complication after brain surgery [1, 2]. Unlike postoperative aseptic meningitis (PAM), which has favorable outcomes [3], postoperative bacterial meningitis (PBM) may be fatal if the diagnosis and introduction of appropriate antimicrobial therapy are delayed [4, 5]. To the contrary, indiscriminate antimicrobial use for all postoperative meningitis cases may result in the development of more resistant organisms, occurrence of adverse reactions, or high medical costs. However, early and appropriate diagnosis of PBM is difficult because of the rarity of specific clinical manifestations, the postoperative changes in cerebrospinal fluid (CSF) profile, and the frequent negative results of CSF Gram stains associated with antibiotic use [6]. Although researchers have tried to identify specific laboratory markers to differentiate PBM from PAM, there have been no consistent results reported to date [6-10].
Recently, serum procalcitonin (S-PCT) has been found to be a strong predictor for distinguishing bacterial from viral meningitis [11-14]. However, the value of S-PCT in the diagnosis of PBM has not been evaluated in patients who have undergone neurosurgery. Therefore, we collected clinical data from patients who underwent neurosurgery and evaluated the predictive performance of S-PCT in the differential diagnosis between PBM and PAM.
CSF adenosine deaminase (ADA) has been recognized as a valuable diagnostic aid in patients with tuberculous meningitis; CSF ADA values are higher in tuberculous meningitis patients than in non-tuberculous meningitis patients. However, a few studies have also shown that CSF ADA values were higher in bacterial meningitis than in viral meningitis [15, 16]. Considering the paucity of helpful laboratory markers for early differentiation of PBM from PAM, we also evaluated the potential usefulness of CSF ADA for the differentiation.
Materials and Methods
1. Patients and data collection
This study was performed at Asan Medical Center, a 2,700-bed tertiary care-affiliated teaching hospital in Seoul, Republic of Korea. We prospectively monitored patients who were admitted to the neurosurgical department and underwent neurosurgery between September 2009 and August 2010. We enrolled patients who underwent CSF analysis and had CSF pleocytosis within 14 days of neurosurgery. During the study period, the following CSF and peripheral blood tests were performed according to the protocols of the neurosurgical department: CSF white blood cell (WBC) differential count, CSF protein, CSF glucose, CSF ADA, CSF Gram stain, CSF bacterial cultures, blood WBC (B-WBC), S-PCT, and C-reactive protein (CRP). CSF cultures and the other CSF tests were performed on the same day. Blood tests were performed within 3 days of CSF cultures. CSF cultures were performed using standard techniques. CSF specimens were routinely inoculated onto blood agar and chocolate agar plates, and examined up to 7 days later. In the study center, thioglycollate broth was not routinely used for CSF specimen analysis, except for cases of CSF shunt catheters. Institutional review board approval was obtained for this study.
2. Definitions
A patient was considered to have CSF pleocytosis if the CSF WBC count was ≥ 6/mm3. To eliminate any false-positive elevations in CSF WBC count caused by CNS bleeding, corrected CSF WBC count was used, which was calculated using the following formula: [corrected CSF WBC count] = [measured CSF WBC count] -[(B-WBC count) × (CSF red blood cell count)/blood red blood cell count] [17]. Patients were defined as having PBM or PAM if they had a positive or negative CSF bacterial culture, respectively.
3. Measurement of S-PCT and CSF ADA
S-PCT was measured using the Vidas PCT assay (bioMérieux, Durham, NC, USA), according to the manufacturer's instructions. CSF ADA measurement was performed with the Biencolle ADA kit (Toyobo, Japan) using a Viva-E analyzer (Siemens, Germany).
4. Statistical analysis
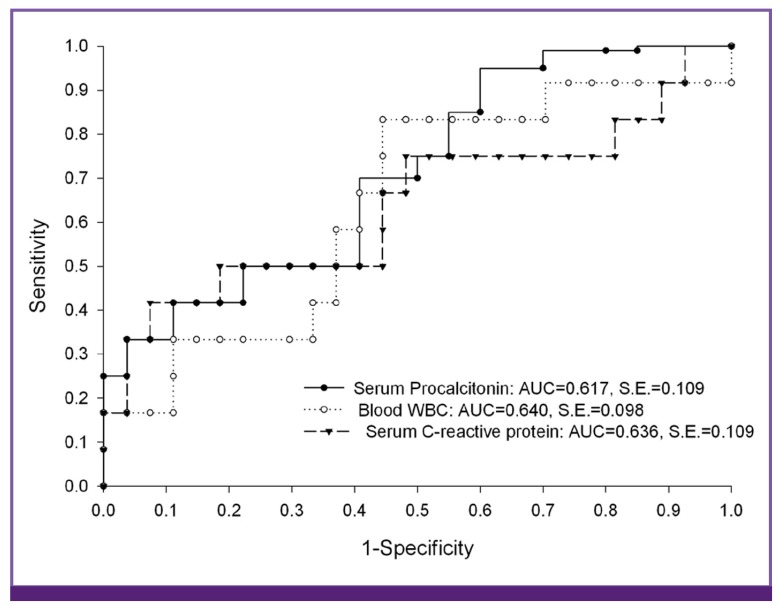
We compared age, sex, types of surgery, and laboratory test results of blood and CSF between patients with PBM and PAM. Statistical analyses were performed using SPSS software (version 12.0; SPSS, Chicago, IL, USA). Binary data were compared using a Chi-square test or Fisher's exact test, and continuous scaled data were compared using Student's t-test or the Mann-Whitney U-test. A P-value of < 0.05 (2-tailed) was considered significant. The receiver operating characteristic (ROC) curve was used to determine cutoff values of B-WBC count, CRP level, and S-PCT level in differentiating PBM from PAM (Fig. 1). Using the cutoff value, the diagnostic performance of each laboratory marker for PBM is presented as sensitivity, specificity, positive predictive value, and negative predictive value.
Figure 1.
Receiver operating characteristic curve for serum C-reactive protein, serum procalcitonin, and blood WBC for predicting postoperative bacterial meningitis in patients who underwent neurosurgery.
Results
1. Patient population
During the study period, 153 patients underwent neurosurgery, and 78 with CSF pleocytosis were included in the study analysis. Of these, 14 (17.9%) were defined as PBM cases based on a positive CSF bacterial culture. Coagulase-negative staphylococci (5/14, 35.7%) were the most common causative organisms, followed by Enterobacter aerogenes (3/14, 21.4%), Streptococcus pneumoniae (2/14, 14.3%), Acinetobacter spp. (2/14, 14.3%), Staphylococcus aureus (1/14, 7.1%), and Corynebacterium (1/14, 7.1%). Coagulase-negative staphylococci included Staphylococcus epidermidis (2/5), Staphylococcus hominis (2/5), and Staphylococcus haemolyticus (1/5). The causative organisms according to the type of surgical procedure were as follows: for craniotomy, the causative organisms were E. aerogenes, Acinetobacter baumannii, methicillin-resistant S. aureus, and S. pneumoniae; for transsphenoidal surgery, the causative organism was S. pneumoniae; for external ventricular drainage, the causative organisms were Corynebacterium, S. epidermidis, and Acinetobacter lwoffii; for ventriculoperitoneal shunt, the causative organism was S. homonis; for external lumbar drainage and ommaya insertion, the causative organisms were S. haemolyticus, S. hominis, and E. aerogenes.
2. Comparison of characteristics between PBM and PAM cases
Comparisons of the demographic, clinical, and laboratory findings between patients with PBM and patients with PAM are summarized in Table 1. Male patients tended to be more frequent in the PAM group than in the PBM group (54.7% vs. 28.6%, P = 0.08). The median age of PBM patients did not differ from that of PAM patients (52.0 years vs. 47.5 years, P = 0.11). Type of neurosurgery did not differ between patients with PBM and PAM. The most frequent procedures in both groups were craniotomy and external ventricular drainage. There was no difference in CSF profiles between the 2 groups. The median CSF ADA value of PBM cases was not different from that of PAM cases (3.2 U/L vs. 3.3 U/L, P = 0.51). There were some differences in the levels of B-WBC, CRP, and S-PCT between PBM and PAM cases.
Table 1.
Comparison of characteristics and laboratory markers between patients with postoperative bacterial meningitis and those with postoperative aseptic meningitis
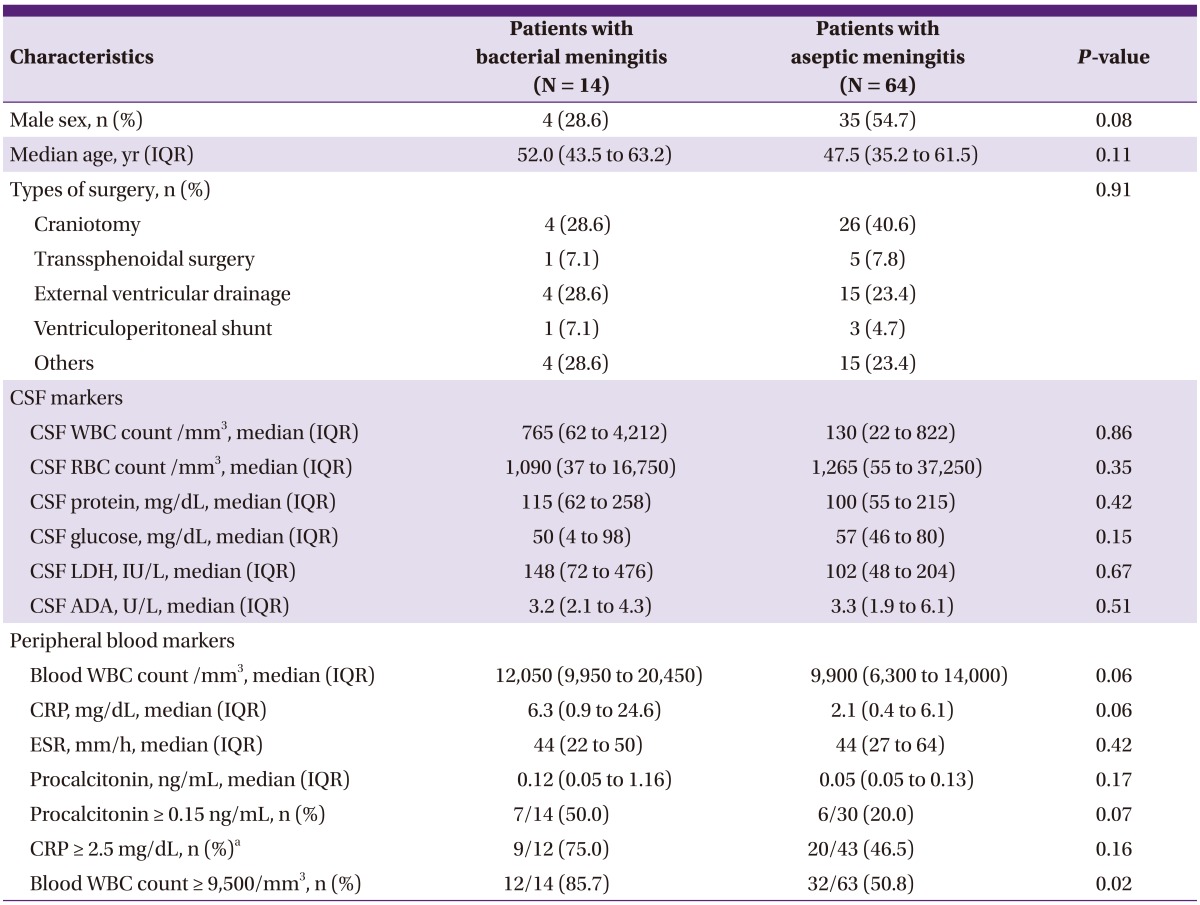
CSF, cerebrospinal fluid; WBC, white blood cell; RBC, red blood cell; LDH, lactate dehydrogenase; ADA, adenosine deaminase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range.
Data not normally distributed are presented as median (range) and were analyzed with the Mann-Whitney U-test.
Categorical data are presented as number (%) and were analyzed with the Fisher's exact test (excepta).
aanalyzed with Chi-square test.
For the laboratory markers, respective cut-off values were identified using ROC curves. An S-PCT level ≥ 0.15 ng/mL (50.0% vs. 20.0%, P = 0.07) and a CRP level ≥ 2.5 mg/dL (75.0% vs. 46.5%, P = 0.16) tended to be more frequent in patients with PBM than in those with PAM. A B-WBC count ≥ 9,500/mm3 was more frequently observed in the former than in the latter group (85.7% vs. 50.8%, P = 0.02).
3. Predictive performance of laboratory markers
We investigated the diagnostic performance of each of the 3 laboratory markers (S-PCT, CRP, and B-WBC) using the defined cut-off values, along with the performance of other criteria combining the markers (Table 2). Of the 3 laboratory markers, B-WBC count had the highest sensitivity (85.7%) and S-PCT level had the highest specificity (80.0%). The criteria including "an S-PCT level ≥ 0.15 ng/mL" were more specific than those not including it. The specificities of "a CRP level ≥ 2.5 mg/dL plus an S-PCT level ≥ 0.15 ng/mL" and "a B-WBC count ≥ 9,500/mm3 plus an S-PCT level ≥ 0.15 ng/mL" were higher than that of a "CRP level ≥ 2.5 mg/dL" and that of a "B-WBC count ≥ 9,500/mm3," respectively (81.5% vs. 53.5%; 90.0% vs.49.2%). The combined criteria of a "CRP level ≥ 2.5 mg/dL, a B-WBC count ≥ 9,500/mm3, and an S-PCT level ≥ 0.15 ng/mL" had the highest specificity (92.6%) of all the combined criteria. The criteria including "or an S-PCT level ≥ 0.15 ng/mL" were not more sensitive than those not including it. The sensitivities of "a CRP level ≥ 2.5 mg/dL or an S-PCT level ≥ 0.15 ng/mL" and "a B-WBC count ≥ 9,500/mm3 or an S-PCT level ≥ 0.15 ng/mL" were rarely different from those of "a CRP level ≥ 2.5 mg/dL" and "a B-WBC count ≥ 9,500/mm3," respectively (76.9% vs. 75.0%; 85.7% vs. 85.7%). Sensitivities of "a B-WBC count ≥ 9,500/mm3" and "a CRP level ≥ 2.5 mg/dL or a B-WBC count ≥ 9,500/mm3 or an S-PCT level ≥ 0.15 ng/mL" were the same (85.7%).
Table 2.
Diagnostic performance of the laboratory markers in predicting postoperative bacterial meningitis in patients who underwent neurosurgery
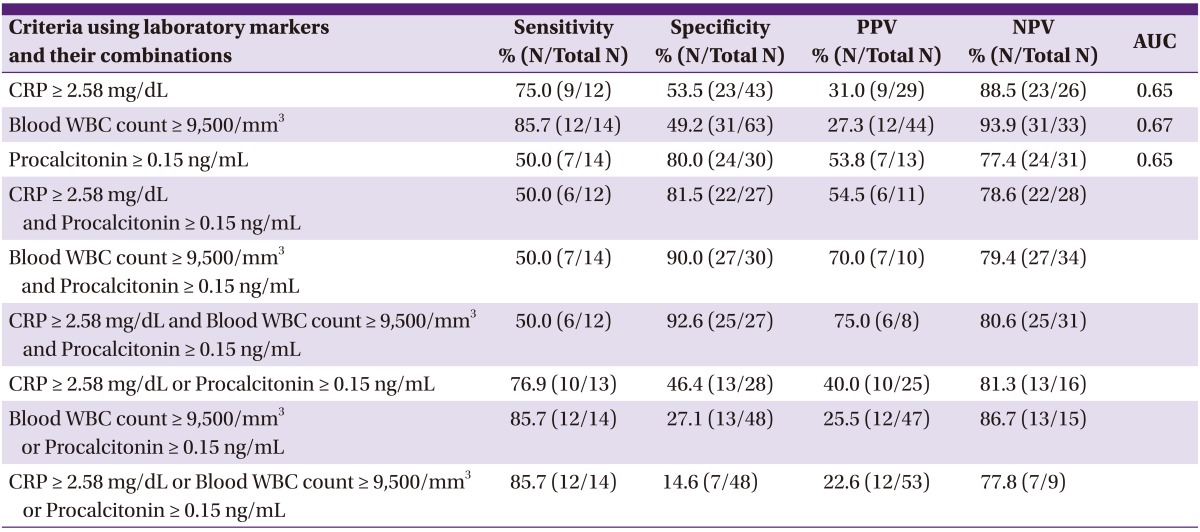
CRP, C-reactive protein; WBC, white blood cell; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
Discussion
It has been suggested that PAM results from a local inflammatory response to the breakdown products of red blood cells or to surgical materials after transection of the dura and the arachnoid [16]. PAM reportedly accounts for > 50% of all postoperative meningitis cases [6], and its clinical outcomes are favorable [3]. To the contrary, PBM has been known to be associated with fatal clinical outcomes when appropriate antimicrobial therapy is not [4, 5]. Thus, early differentiation of PBM from PAM is essential. Many clinical and laboratory parameters such as high fever, new focal neurological defect, CSF WBC, CSF protein, CSF glucose, CSF/blood glucose ratio, and CSF lactate, have been evaluated for their predictive performance for PBM. However, such studies yielded either conflicting results or included only limited clinical data [6-10]. In addition, in this study, CSF profiles did not differ between patients with PBM and PAM.
S-PCT has been regarded as a useful marker for distinguishing between community-acquired bacterial and viral infections [18-22]. Several studies have been performed to evaluate the discriminative performance of S-PCT in adult patients and children with acute meningitis [11-14]. The majority of studies on S-PCT have shown sensitivity and specificity values of > 80-90% for the diagnosis of bacterial meningitis using variable cut-off values. To the best of our knowledge, ours is the first study to evaluate the diagnostic performance of S-PCT in a relatively large series of patients who underwent neurosurgery. In this study, S-PCT had a specificity of 80.0%, but a low sensitivity of 50.0%, for the differential diagnosis of PBM from PAM. Hoffmann et al. [23] reported low sensitivity for S-PCT in 12 adult patients with bacterial meningitis. They observed normal values of S-PCT in 3 patients who had bacterial meningitis after neurosurgery; these were false-negative cases suggestive of a low sensitivity for S-PCT. This finding is consistent with our results of a low sensitivity for S-PCT. Based only on S-PCT, some PBM cases would be misdiagnosed as PAM. A low S-PCT level may result from the lower number of pathogenic organisms causing nosocomial meningitis than those causing community-acquired meningitis, or from inflammatory responses more confined to the CNS in nosocomial meningitis. However, the specificity of S-PCT was high, and the specificities of some laboratory criteria were improved by the inclusion of S-PCT level. Thus, a high S-PCT level may be helpful in confirming the diagnosis of PBM when used together with other clinical or laboratory discriminative markers of PBM. Further studies of S-PCT may be needed to evaluate its usefulness in the context of postoperative meningitis.
The predictive performance of B-WBC count and CRP level for PBM has rarely been evaluated in previous studies. This may be because of a lack of research interest in these laboratory markers, because they may be indirect markers for meningitis. In one study, however, by Ross et al. [7], B-WBC level was compared between PBM and PAM cases. The mean B-WBC count was higher in patients with PBM than in those with PAM (14,906/mm3 vs. 10,517/mm3, P < 0.01). The sensitivity and specificity values of a B-WBC count >12,000/mm3 for the prediction of PBM were 75% and 74%, respectively. In our study, the B-WBC and CRP levels were higher in patients with PBM than in those with PAM. Their sensitivity values were relatively good (B-WBC, 85.7%; CRP, 75.0%), but their specificities were poor (B-WBC, 49.2%; CRP, 53.5%). There was considerable overlap between the values for patients with PBM and those with PAM. Although B-WBC count and CRP level are thought to be neither sensitive nor specific enough for the diagnosis of PBM, accumulation of more clinical data may be needed to draw conclusions on their usefulness.
A few studies have shown the CSF ADA level to be higher in bacterial meningitis than in viral or aseptic meningitis [15, 16]. In a study by Choi et al. [15], the mean CSF ADA values (standard deviation) of bacterial and viral/aseptic meningitis cases were 7.4 (3.3) U/L and 2.6 (2.4) U/L, respectively; in a study by Sun et al. [16], the values were 9.6 (5.5) U/L and 4.3 (2.5) U/L, respectively. However, in this study, there was no difference in the CSF ADA values between PBM and PAM cases. For reasons similar to those for the low S-PCT level in PBM, CSF ADA levels might also remain relatively low in PBM cases and might not show any difference from those in PAM. CSF ADA may prove to be of little value in the diagnosis of PBM.
No ideal discriminatory marker was identified in this study. The differentiation between PBM and PAM has been reported to depend only on CSF culture results [6]. The British Society of Antimicrobial Chemotherapy recommends presumptive antimicrobial therapy for all patients with postoperative meningitis, and that therapy should be continued or stopped based on CSF culture results after 2 or 3 days [24]. This approach may be more acceptable in current clinical practice than decisions based on any clinical or laboratory markers for PBM.
Our study has several limitations. First, the number of patients was small. Second, patients with various CNS diseases undergoing various neurosurgeries were included in this study, resulting in heterogeneity of the study patients. Third, because our definitions were based only on positive CSF culture results, we may have included CSF culture contaminations in the PBM group. However, even if we had used other clinical factors, we still might not have been able to clearly categorize the patients as PBM or PAM cases because of the obscurity in the clinical manifestations of these conditions. Fourth, among individual cases, we did not perform follow-up measurements of S-PCT after neurosurgery. Serial S-PCT data might provide valuable information on the normal kinetics of S-PCT after neurosurgery, enabling the differentiation of infection-related increases in S-PCT from stress-induced increases in S-PCT. Further studies may be needed on the changes in S-PCT after neurosurgery. Fifth, we did not present any information on antibiotic therapy before and after neurosurgery; such therapy might have influenced the clinical and laboratory characteristics of PBM and PAM cases. However, all of the study patients had antibiotic prophylaxis before surgery, and nearly all had postsurgical antibiotic therapy for more than a few days.
In conclusion, S-PCT showed limited performance for the diagnosis of postoperative meningitis. However, it could be a useful adjunct for the improvement of diagnostic sensitivity when used in combination with other inflammatory markers.
Acknowledgement
This work was supported by the 2009 Korean Society of Infectious Diseases - Pfizer research grant.
Footnotes
No conflicts of interest.
References
- 1.Korinek AM The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord; Service Epidémiologie Hygiène et Prévention. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. Neurosurgery. 1997;41:1073–1079. doi: 10.1097/00006123-199711000-00010. discussion 1079-81. [DOI] [PubMed] [Google Scholar]
- 2.Blomstedt GC. Postoperative aseptic meningitis. Acta Neurochir (Wien) 1987;89:112–116. doi: 10.1007/BF01560375. [DOI] [PubMed] [Google Scholar]
- 3.Carmel PW, Greif LK. The aseptic meningitis syndrome: a complication of posterior fossa surgery. Pediatr Neurosurg. 1993;19:276–280. doi: 10.1159/000120744. [DOI] [PubMed] [Google Scholar]
- 4.Buckwold FJ, Hand R, Hansebout RR. Hospital-acquired bacterial meningitis in neurosurgical patients. J Neurosurg. 1977;46:494–500. doi: 10.3171/jns.1977.46.4.0494. [DOI] [PubMed] [Google Scholar]
- 5.Federico G, Tumbarello M, Spanu T, Rosell R, Iacoangeli M, Scerrati M, Tacconelli E. Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scand J Infect Dis. 2001;33:533–537. doi: 10.1080/00365540110026557. [DOI] [PubMed] [Google Scholar]
- 6.Zarrouk V, Vassor I, Bert F, Bouccara D, Kalamarides M, Bendersky N, Redondo A, Sterkers O, Fantin B. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007;44:1555–1559. doi: 10.1086/518169. [DOI] [PubMed] [Google Scholar]
- 7.Ross D, Rosegay H, Pons V. Differentiation of aseptic and bacterial meningitis in postoperative neurosurgical patients. J Neurosurg. 1988;69:669–674. doi: 10.3171/jns.1988.69.5.0669. [DOI] [PubMed] [Google Scholar]
- 8.Leib SL, Boscacci R, Gratzl O, Zimmerli W. Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Dis. 1999;29:69–74. doi: 10.1086/520184. [DOI] [PubMed] [Google Scholar]
- 9.Forgacs P, Geyer CA, Freidberg SR. Characterization of chemical meningitis after neurological surgery. Clin Infect Dis. 2001;32:179–185. doi: 10.1086/318471. [DOI] [PubMed] [Google Scholar]
- 10.Tavares WM, Machado AG, Matushita H, Plese JP. CSF markers for diagnosis of bacterial meningitis in neurosurgical postoperative patients. Arq Neuropsiquiatr. 2006;64:592–595. doi: 10.1590/s0004-282x2006000400012. [DOI] [PubMed] [Google Scholar]
- 11.Dubos F, Moulin F, Gajdos V, De Suremain N, Biscardi S, Lebon P, Raymond J, Breart G, Gendrel D, Chalumeau M. Serum procalcitonin and other biologic markers to distinguish between bacterial and aseptic meningitis. J Pediatr. 2006;149:72–76. doi: 10.1016/j.jpeds.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Dubos F, Korczowski B, Aygun DA, Martinot A, Prat C, Galetto-Lacour A, Casado-Flores J, Taskin E, Leclerc F, Rodrigo C, Gervaix A, Leroy S, Gendrel D, Bréart G, Chalumeau M. Serum procalcitonin level and other biologic markers to distinguish between bacterial and aseptic meningitis in children: a European multicenter case cohort study. Arch Pediatr Adolesc Med. 2008;162:1157–1163. doi: 10.1001/archpedi.162.12.1157. [DOI] [PubMed] [Google Scholar]
- 13.Viallon A, Desseigne N, Marjollet O, Birynczyk A, Belin M, Guyomarch S, Borg J, Pozetto B, Bertrand JC, Zeni F. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Crit Care. 2011;15:R136. doi: 10.1186/cc10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alkholi UM, Al-Monem NA, Abd El-Azim AA, Sultan MH. Serum procalcitonin in viral and bacterial meningitis. J Glob Infect Dis. 2011;3:14–18. doi: 10.4103/0974-777X.77290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi SH, Kim YS, Bae IG, Chung JW, Lee MS, Kang JM, Ryu J, Woo JH. The possible role of cerebrospinal fluid adenosine deaminase activity in the diagnosis of tuberculous meningitis in adults. Clin Neurol Neurosurg. 2002;104:10–15. doi: 10.1016/s0303-8467(01)00159-7. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Sha W, Xiao HP, Tian Q, Zhu H. Evaluation of cerebrospinal fluid adenosine deaminase activity for the differential diagnosis of tuberculosis and nontuberculous meningitis. Am J Med Sci. 2012;344:116–121. doi: 10.1097/MAJ.0b013e318238fee3. [DOI] [PubMed] [Google Scholar]
- 17.Tunkel AR. Approach to the patient with central nervous system infection. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. Philadelphia: Churchil Livingstone; 2009. [Google Scholar]
- 18.Kaufman BA, Tunkel AR, Pryor JC, Dacey RG., Jr Meningitis in the neurosurgical patient. Infect Dis Clin North Am. 1990;4:677–701. [PubMed] [Google Scholar]
- 19.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gendrel D, Raymond J, Assicot M, Moulin F, Iniguez JL, Lebon P, Bohuon C. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis. 1997;24:1240–1242. doi: 10.1086/513633. [DOI] [PubMed] [Google Scholar]
- 21.van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis. 2004;4:620–630. doi: 10.1016/S1473-3099(04)01146-6. [DOI] [PubMed] [Google Scholar]
- 22.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann O, Reuter U, Masuhr F, Holtkamp M, Kassim N, Weber JR. Low sensitivity of serum procalcitonin in bacterial meningitis in adults. Scand J Infect Dis. 2001;33:215–218. doi: 10.1080/00365540151060905. [DOI] [PubMed] [Google Scholar]
- 24.Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy. The management of neurosurgical patients with postoperative bacterial or aseptic meningitis or external ventricular drain-associated ventriculitis. Br J Neurosurg. 2000;14:7–12. doi: 10.1080/02688690042834. [DOI] [PubMed] [Google Scholar]