Abstract
Background
Mucormycosis is an uncommon and life-threatening fungal infection. The clinical predictors of outcome were evaluated in patients with invasive mucormycosis.
Materials and Methods
We retrospectively reviewed histologically proven cases of invasive mucormycosis in our institution from 1996 to 2012.
Results
A total of 64 patients were analyzed. The median age was 59 years (interquartile range [IQR], 50-67), and 32 patients (50%) were male. The most common underlying diseases were diabetes mellitus (67%), hematologic malignancy (22%), and solid cancer (19%). The most common infection sites were the rhino-orbito-cerebral area (56%) and the lungs (31%). The 180-day all-cause mortality was 33%. Disseminated infection was associated with increased mortality (hazard ratio [HR]: 169.74, 95% confidence interval [CI]: 6.41 to 4492.64; P = 0.002). Pulmonary infection (HR: 0.08, 95% CI: 0.01 to 0.66; P = 0.02) and complete surgical removal of infected tissue (HR: 0.12, 95% CI: 0.02 to 0.64; P = 0.01) were associated with decreased mortality.
Conclusions
These results suggest that patients with mucormycosis had a lower risk of mortality if they developed a pulmonary infection, rather than a disseminated infection and with complete debridement of infected tissue.
Keywords: Risk factor, Mortality, Mucormycosis
Introduction
Mucormycosis is an uncommon, life-threatening infection caused by filamentous fungi of the order Mucorales and class Zygomycetes. These ubiquitous organisms can be found in bread mold, soil, manure, and decaying vegetation. Along with candidiasis and aspergillosis, mucormycosis is one of the primarily opportunistic invasive mycoses that frequently develop in immunocompromised patients who have received hematopoietic stem cell or solid organ transplantations or who have hematologic malignancies [1-4]. Moreover, mucormycosis can occur in immunocompetent patients with diabetes mellitus, subcutaneous tissue injury, and iron overload [5], and who are undergoing deferoxamine therapy [6]. The site of infection varies, and can occur in the lungs, the skin and soft tissue, the rhino-orbito-cerebral region, and the gastrointestinal tract. Mucormycosis infection may also appear as a disseminated disease that infects more than one noncontiguous site [4].
The mortality rate depends on the patient's underlying diseases and the infection site [7]. Treatment strategy involves timely diagnosis, aggressive surgical debridement combined with high-dose amphotericin B, and reversal of underlying predisposing factors whenever possible [8]. Factors associated with poor outcome have not been well defined. In addition, the only published data available on mucormycosis in Korea consists of case reports. Therefore, we conducted a retrospective study to specifically evaluate clinical predictors of outcome in patients with mucormycosis.
Materials and Methods
This retrospective study was performed at the Asan Medical Center, a 2,700-bed tertiary care teaching hospital in Seoul, Korea. The pathology database was electronically searched to identify all cases of mucormycosis from patients admitted between April 1996 and November 2012. Data was collected on patients' demographics, underlying conditions, concomitant immunosuppressive medications, laboratory data, radiologic findings, clinical features, antifungal treatment, surgical procedures, and outcomes.
Patients were included in the study if they met the criteria for proven invasive mucormycosis based on the revised definitions of invasive fungal disease of the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) [9]. A diagnosis of mucormycosis was based on histopathological demonstration of broad, ribbon-like, wide-angled branching, non-septate hyphae even in the absence of positive cultures, and accompanying tissue invasion by fungal hyphae [10, 11]. The mucormycosis genus was determined by morphological examination of conidia, hyphae, and whole colonies.
Infection sites were classified as rhino-orbito-cerebral, pulmonary, gastrointestinal, cutaneous, or disseminated mucormycosis. The rhino-orbito-cerebral involvements were classified as rhinocerebral, sino-orbital, or isolated sinusitis [4], while the pulmonary infections were subcategorized into potentially resectable cases and definitively non-resectable cases. The day of diagnosis was defined as the day on which a procedure (biopsy, surgery, or culture) leading to a mucormycosis diagnosis was conducted. The outcome was assessed by 180-day all-cause mortality after a diagnosis of invasive mucormycosis.
Data were analyzed using IBM SPSS for Windows (version 19.0; IBM Corp., Armonk, NY, USA). Categorical variables were analyzed using either the Chi-square test or Fisher's exact test. Continuous variables were analyzed using the Student's t-test or Mann-Whitney U-test, as appropriate. The primary objective of the study was to identify independent predictors of 180-day mortality in invasive mucormycosis. Therefore, a Cox proportional hazards regression model for multivariate analyses was built using a forward stepwise method. Covariates with a P < 0.2 were used in the stepwise method. All tests were 2-tailed and differences were considered significant when P < 0.05.
Results
1. Patient characteristics and clinical outcomes
During the study period, 64 patients with histologically proven invasive mucormycosis were identified. Mycological diagnosis was performed in only 7 patients (11%); 4 patients were infected with Rhizopus species (6%) and 3 patients were infected with Mucor species (5%). The median age was 59 years (nterquartile range [IQR], 50-67), and 32 patients (50%) were male. The majority of patients had an underlying disease (63/64, 98%), the most common of which was diabetes mellitus (67%), followed by hematologic malignancy (22%) and solid cancer (19%). The most common infection site was the rhino-orbito-cerebral area (36 cases, 56%), followed by the lung (20 cases, 31%) and the gastrointestinal tract (4 cases, 6%). All 64 mucormycosis patients received antifungal therapy; the majority of patients (57/64, 89%) received amphotericin B (conventional or liposomal) as the first-line antifungal agent. Surgery was performed on 46 patients (46/64, 72%), with complete removal of the infected site in 22 cases (22/46, 48%). In cases of rhino-orbito-cerebral mucormycosis, surgical debridement was performed in 89% (32/36) of the patients, and the majority was for rhino-cerebral lesions (17/19), followed by sinusitis (12/14) and sino-orbital lesions (3/3). Eight patients (8/20, 40%) received surgical resection for pulmonary mucormycosis. The 180-day all-cause mortality after diagnosis of invasive mucormycosis was 33% (21/64).
2. Risk factors for mortality
Demographic and clinical characteristics of the 43 surviving patients and the 21 patients who died within 180 days after diagnosis of invasive mucormycosis are listed in Table 1. Gastrointestinal (2% vs. 24%, P = 0.01) and disseminated mucormycosis (0% vs. 14%, P = 0.03) were less common in surviving patients than in patients who did not survive. Complete surgery was done more commonly in surviving patients than in patients who did not survive (44% vs. 14%, P = 0.04), while incomplete surgical removal (28% vs. 57%, P = 0.046) was more common in patients who did not survive. Cases with pulmonary mucormycosis were also more common in survived patients than in patients who died (40% vs. 14%, P = 0.08).
Table 1.
Comparison of clinical characteristics between patients with invasive mucormycosis who survived and died within 180 days after diagnosis
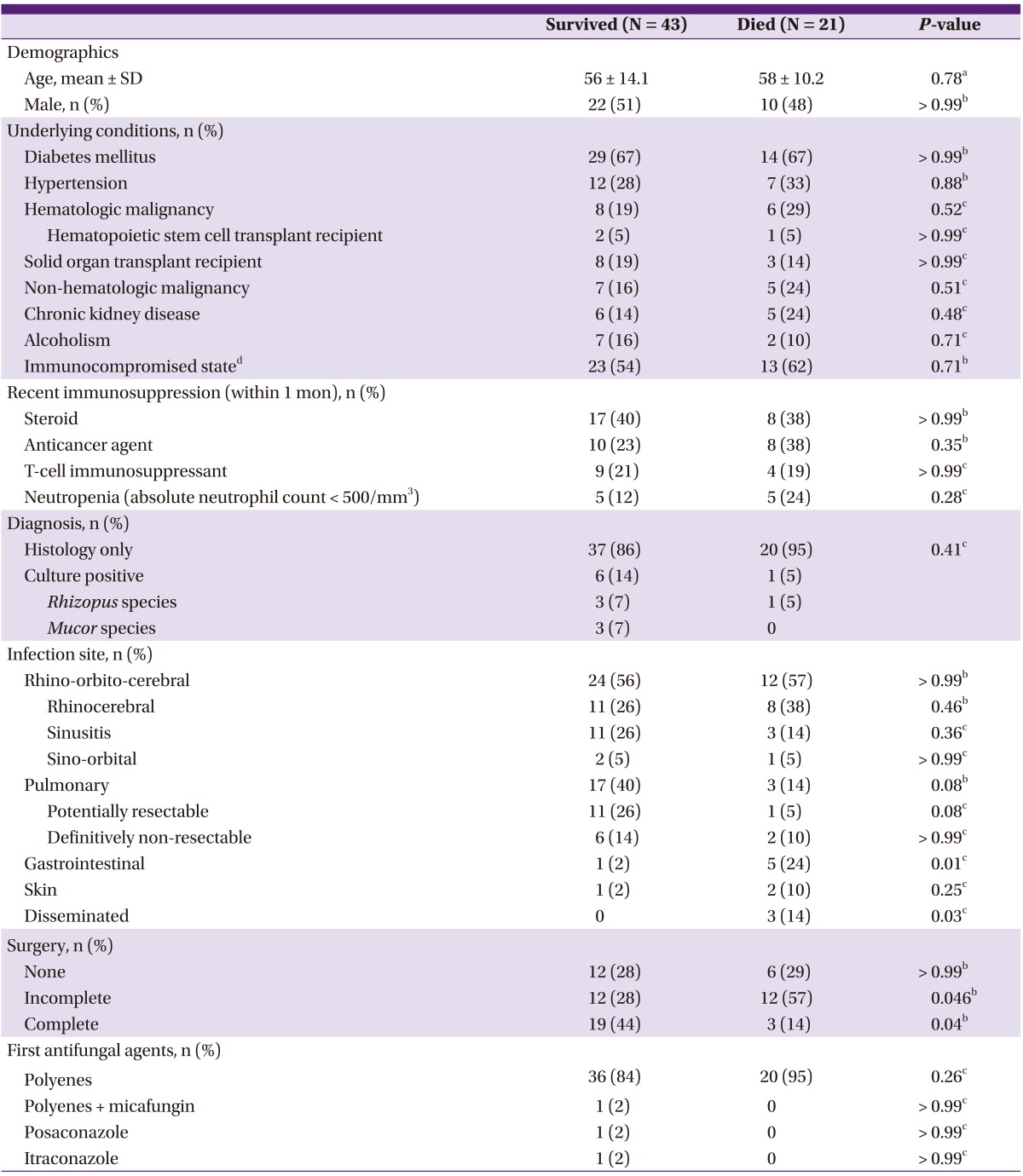
IQR, interquartile range.
aStudent's t-test was used.
bChi-square test was used.
cFisher's exact test was used.
dThe immunocompromised state was verified if the patients (i) had daily administration of corticosteroids, (ii) were solid organ or hematopoietic stem cell transplant recipients, and (iii) had received treatment with chemotherapy for an underlying malignancy during the 6 months prior to hospital admission.
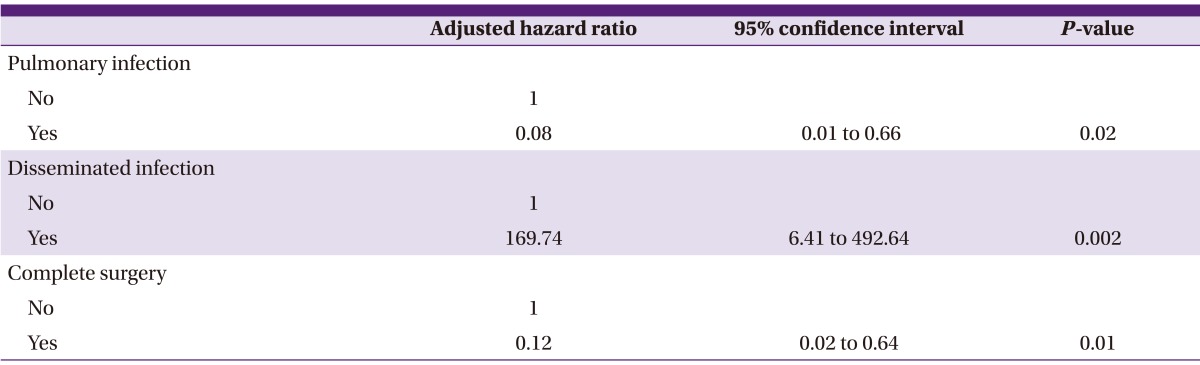
Univariate analysis was performed using a Cox proportional hazards model to determine clinical variables significantly associated with 180-day all-cause mortality (Table 2). Our analyses revealed that gastrointestinal mucormycosis (P = 0.02) and disseminated mucormycosis (P = 0.01) were significantly associated with mortality. In subsequent multivariate analysis (Table 3), disseminated infection was independently associated with an increased risk of 180-day mortality (hazard ratio [HR]: 169.74, 95% confidence interval [CI]: 6.41 to 4492.64; P = 0.002). Pulmonary infection (HR: 0.08, 95% CI: 0.01 to 0.66; P = 0.02) and complete surgical removal of lesions (HR: 0.12, 95% CI: 0.02 to 0.64; P = 0.01) were associated with improved survival at 180 days post-diagnosis.
Table 2.
Cox regression analysis with single clinical variable associated with 180-day all-cause mortality from invasive mucormycosis
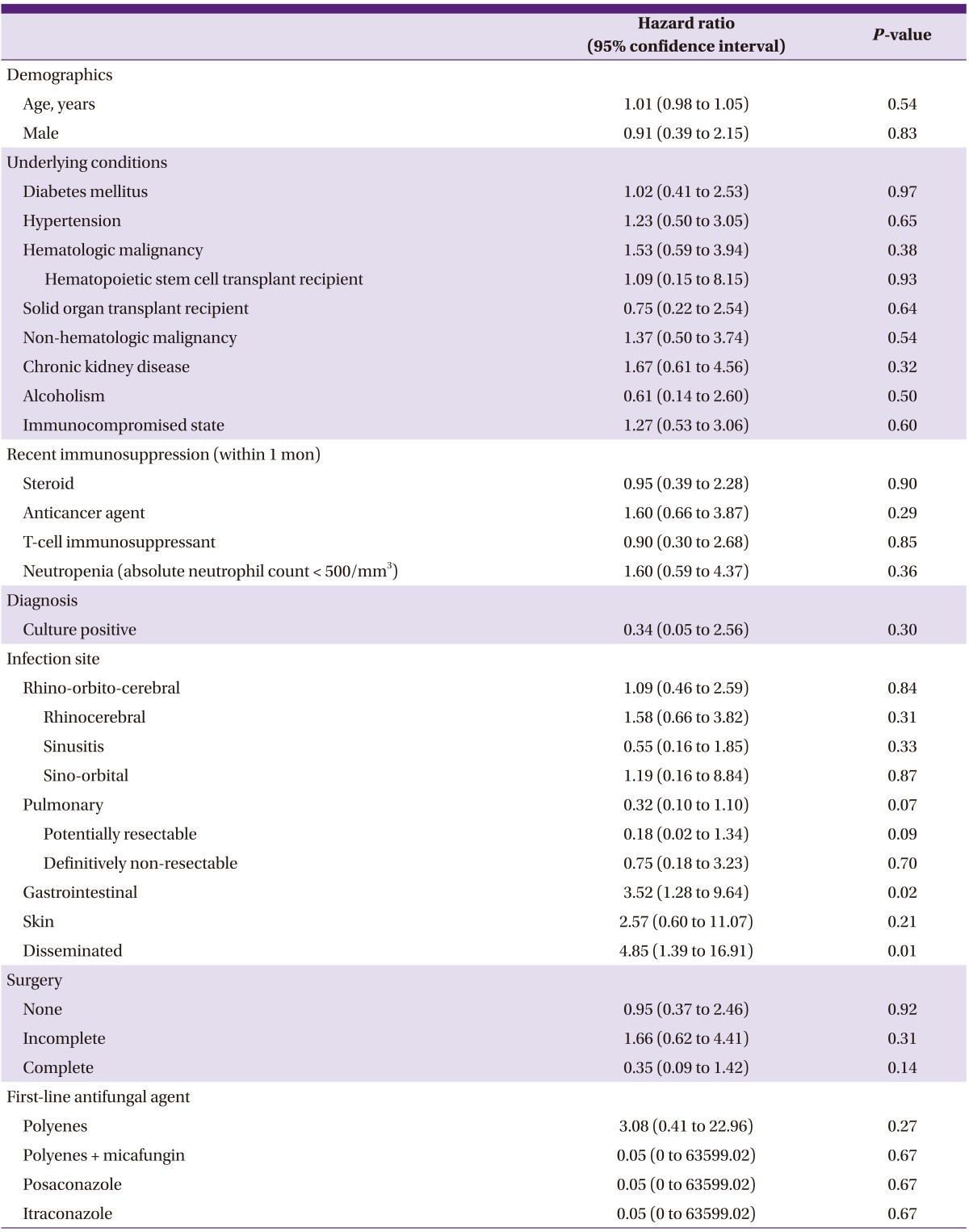
Table 3.
Cox regression with analysis multiple clinical variables associated with 180-day all-cause mortality from invasive mucormycosis
Discussion
This study suggests that the greatest risk for mortality from invasive mucormycosis was associated with disseminated infection, and complete surgical removal of the infection site or pulmonary infection was associated with decreased mortality.
Mucormycosis is rare but often highly fatal. Our results show the mucormycosis mortality rate to be 33%, which is lower than the previously reported rates in mucormycosis (44-80%) [4, 7, 12-14]. This may be explained the differences in patients' characteristics, resectable lesions of infection site, and therapeutic strategies. In the present study, the proportion of patients with diabetes mellitus as an underlying disease was much higher than in other recent reports (67% vs. 17-23%) [12, 13, 15], whereas the frequency of patients with underlying hematologic malignancy was lower (22% vs. 44-50%). Since rapid correction of underlying predisposing factors is critical to outcomes, a relatively higher proportion of patients with more easily controlled diabetes mellitus than hematologic malignancy, could have a favorable effect on outcomes in our cases. Additionally, compared to the previous epidemiologic studies (44-61%), a large proportion of patients in this study (72%) underwent surgery as part of therapy [4, 12, 13], which may have contributed to relatively high survival rates in our patients. Many patients had more resectable lesions and tended to undergo aggressive surgical debridement despite deep or multiple lesions. Among 36 patients with rhino-orbito-cerebral mucormycosis, 14 patients had isolated sinusitis with more resectable lesions; 89% of the patients (32/36) received surgical management. Twelve patients out of 20 with pulmonary mucormycosis had potentially resectable lesions and 2 patients out of 8 patients with definitively non-resectable lesions received surgical management.
Several previous studies have investigated risk factors for mucormycosis [4, 7, 12, 13]. In agreement with our results, Roden et al. [4] reported disseminated infection to be an independent risk factor for increased mortality (odds ratio [OR]: 11.2, 95% CI: 5.79 to 21.73). They found, however, that pulmonary infection presented a relatively lower 180-day mortality rate (15%, 3/20) and was associated with greater survival (HR: 0.08, 95% CI: 0.01 to 0.66). Spellberg et al. [7] reported that the 90-day mortality rate for patients with pulmonary infection was 75% (6/8). Pulmonary involvement was associated with lower Kaplan-Meier survival compared to non-pulmonary infection (pulmonary [n = 8] vs. non-pulmonary [n =12]). The reported survival differences could be explained by more resectable lesions in our study population, which may have contributed to the favorable outcomes of pulmonary involvement in our study (180-day mortality rate of potentially resectable, 8% [1/12] vs. definitively non-resectable, 25% [2/8]; P = 0.54). Except for the frequency of surgical intervention, no underlying medical conditions were significantly different between potentially resectable lesions and definitively non-resectable lesions. Considering the relatively small number of patients with pulmonary mucormycosis in our study, further investigation with larger study populations is required to delineate the relationship between pulmonary involvement and mortality in mucormycosis.
Effective mucormycosis treatment is challenging, involving extensive debridement, high-dose amphotericin B, and correction of any underlying diseases [4, 13, 16]. Our study indicates that complete removal of infected lesions significantly improves outcomes (HR: 0.18, 95% CI: 0.03 to 1.05). Roden et al. [4] also reported surgery to be associated with a decreased risk of mortality (OR: 0.24, 95% CI: 0.15 to 0.37). Similarly, the mortality rate in pulmonary mucormycosis patients treated with both surgery and antifungal agents was 11%, significantly lower than the 68% mortality rate for patients treated with only antifungal agents (P = 0.0004) [14]. These results suggest that extensive debridement of infected tissue may be necessary to minimize mortality. In contrast to previous reports, we did not find amphotericin B to be associated with survival (HR: 3.08, 95% CI: 0.41 to 22.96). Further, because amphotericin B was used in the majority of patients that received an antifungal agent (89%), we could not evaluate the comparative effectiveness of various antifungal agents.
Our study has several limitations. First, the study was performed at a single center. Second, our study was based on retrospective observation, which limits the amount and type of information that could be gathered. Third, the number of cases of mucormycosis identified by culture was small (7/64; 11%), so associations between mucormycosis genus/species and mortality could not be evaluated.
Despite these limitations, our results suggest that patients with mucormycosis had reduced mortality risk if they had a pulmonary infection, if their infection was not disseminated, and if extensive and complete surgical debridement of the infected tissue was performed. With the increasing incidence of mucormycosis [17], further investigation based on data gathered at multiple centers will be necessary to characterize the prevalence and risk factors for mortality in mucormycosis.
References
- 1.Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000;30:851–856. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 3.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt HW, Schneider YJ. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest. 1993;91:1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spellberg B, Kontoyiannis DP, Fredricks D, Morris MI, Perfect JR, Chin-Hong PV, Ibrahim AS, Brass EP. Risk factors for mortality in patients with mucormycosis. Med Mycol. 2012;50:611–618. doi: 10.3109/13693786.2012.669502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, Lortholary O, Petrikkos GL European Conference on Infections in Leukemia. Diagnosis and treatment of mucormycosis in patients with haematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3) Haematologica. 2013;98:492–504. doi: 10.3324/haematol.2012.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE European Organization for Research and Treatment of Cancer/Invasive Fun; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med. 2001;125:375–378. doi: 10.5858/2001-125-0375-HFOZ. [DOI] [PubMed] [Google Scholar]
- 11.Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine(Baltimore) 1986;65:113–123. doi: 10.1097/00005792-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, Bitar D, Dromer F, Lortholary O French Mycosis Study Group. A global analysis of mucormycosis in France: the RetroZygo Study (2005-2007) Clin Infect Dis. 2012;54(Suppl 1):S35–S43. doi: 10.1093/cid/cir880. [DOI] [PubMed] [Google Scholar]
- 13.Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, Lass-Florl C, Bouza E, Klimko N, Gaustad P, Richardson M, Hamal P, Akova M, Meis JF, Rodriguez-Tudela JL, Roilides E, Mitrousia-Ziouva A, Petrikkos G European Confederation of Medical Mycology Working Group on Zygomycosis. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17:1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- 14.Cinque P, Bossolasco S, Vago L, Fornara C, Lipari S, Racca S, Lazzarin A, Linde A. Varicella-zoster virus (VZV) DNA in cerebrospinal fluid of patients infected with human immunodeficiency virus: VZV disease of the central nervous system or subclinical reactivation of VZV infection? Clin Infect Dis. 1997;25:634–639. doi: 10.1086/513754. [DOI] [PubMed] [Google Scholar]
- 15.Rüping MJ, Heinz WJ, Kindo AJ, Rickerts V, Lass-Flörl C, Beisel C, Herbrecht R, Roth Y, Silling G, Ullmann AJ, Borchert K, Egerer G, Maertens J, Maschmeyer G, Simon A, Wattad M, Fischer G, Vehreschild JJ, Cornely OA. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother. 2010;65:296–302. doi: 10.1093/jac/dkp430. [DOI] [PubMed] [Google Scholar]
- 16.Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J, Jr, Ibrahim AS. Recent advances in the management of mucormycosis : from bench to bedside. Clin Infect Dis. 2009;48:1743–1751. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitar D, VanCauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, Desenclos JC, Lortholary O. Increasing incidenceof zygomycosis (mucormycosis), France, 1997- 2006. Emerg Infect Dis. 2009;15:1395–1401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]


