Abstract
Background/Aims
Seasonal variation may influence the development and exacerbation of inflammatory bowel disease (IBD). However, most epidemiologic studies on this topic have been conducted in Western countries. The purpose of this study was to determine whether birth dates and symptom flares follow a seasonal pattern in Korean patients with IBD.
Methods
Patients with a diagnosis of IBD established between January 2003 and December 2010 were investigated at six university hospitals in Korea. The expected births and flares, with a uniform distribution during the year and considering differences in the number of days in the months of 1 year, were calculated.
Results
A total of 411 patients with ulcerative colitis (UC) and 316 patients with Crohn disease (CD) were included in the study. Birth during the winter period, and especially in January and February, was associated with an increased risk of IBD, especially in UC patients. The symptom flares of CD patients occurred most frequently in the spring, with a nadir in the autumn. However, no disease flare seasonality was noted for UC patients.
Conclusions
Our data suggest that seasonally varying environmental factors during pregnancy and the postpartum period are associated with a susceptibility to IBD later in life and that exacerbations of CD are influenced by seasonal factors.
Keywords: Inflammatory bowel diseases, Seasonality, Birth month, Symptom flares
INTRODUCTION
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC), and Crohn disease (CD), is a chronic relapsing inflammatory disorder of the gastrointestinal tract. The exact cause of IBD remains unclear, but it has generally been thought that immune abnormalities, genetic factors, and environmental factors may play a significant role in its etiology and pathogenesis.1 It is currently thought that intestinal inflammation in IBD is caused by altered immunological function resulting from interactions between genetic and environmental factors.1 There have been many studies in recent decades seeking to identify environmental factors that affect the course of IBD, including seasonal variation. Some studies have demonstrated significant associations between seasonality and birth month, onset of symptoms, or flares in IBD, whereas other studies have failed to show such a relationship.2-17 However, most of these studies were performed in Western countries. Although the incidence and prevalence of IBD in Asia have been rapidly increasing in recent years,18 there have been few studies regarding the seasonal patterns of IBD in Asian populations. To date, only one study has examined seasonal variation in flares and month of birth in patients with UC in an Asian population, in this case a Chinese population.19
Several studies have revealed that there is some heterogeneity among ethnic groups in the clinical phenotypes of IBD, which may be due to differences in genetic and environmental backgrounds.18,20 The clinical course of IBD in Korean patients seems milder than in patients in Western countries, as indicated by the lower rates of surgical intervention and better responses to medical management.21-23 In addition, Korean CD patients differ from Western patients in gender distribution, disease location, and perianal fistula occurrence.23 Based on this, we speculate that Korean IBD patients may show different seasonal patterns from those of Western patients. The aim of this study was to investigate the seasonal and monthly variations of birth dates and symptom flares among Korean patients with UC and CD.
MATERIALS AND METHODS
1. Study subjects
This was a retrospective and multicenter study conducted at six university hospitals (Kangbuk Samsung Hospital, Samsung Medical Center, Kyung Hee University Medical Center, Dongguk University Ilsan Hospital, Soonchunhyang University Cheonan Hospital, and Hanyang University Guri Hospital) in Seoul, Korea. Patients with a diagnosis of IBD established between January 2003 and December 2010 were included and investigated for associations between birth month and symptom flares. UC and CD diagnoses were confirmed by previously established international criteria based on clinical, endoscopic, histopathological, and radiological findings.24 We included only patients who were able to accurately indicate the exact month when symptoms appeared or became aggravated. The following patients were excluded from the study: 1) patients who had been on systemic corticosteroids or immunosuppressive agents for concomitant diseases other than IBD, such as rheumatologic disease, asthma, or chronic obstructive pulmonary disease, as these drugs may reduce IBD flares; 2) patients who had no IBD flares identified during follow-up; and 3) patients with less than 1 year of follow-up time.
2. Identification of flares
Symptom flares were established if at least one of the following criteria were met: 1) the appearance of typical symptoms, such as diarrhea, abdominal pain, weight loss, perianal fistula, perianal abscess, or rectal bleeding; 2) receipt of a new prescription or increasing dose of immunosuppressants, corticosteroids, cyclosporine, or infliximab in existing medications; or 3) hospitalization or surgery due to the worsening of symptoms or the development of complications.
We defined the seasons as follows: spring included March, April, and May; summer included June, July, and August; autumn included September, October, and November; and winter included December, January, and February.
This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital, which confirmed that the study was in accordance with the ethical guidelines of the Declaration of Helsinki.
3. Statistical analysis
The software program SPSS version 12 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The expected number of births and flares were calculated assuming a uniform distribution during the year and taking into account the differences in the number of days in a month and compared to the number and distribution of births and flares observed. Statistical analysis was performed using the chi-square test and p-values <0.05 were considered statistically significant.
RESULTS
1. Clinical characteristics of study subjects
A total of 727 patients with IBD (411 patients with UC and 316 patients with CD) were enrolled in the study. A total of 698 symptom flares in UC patients and 587 symptom flares in CD patients were identified. Table 1 shows the clinical characteristics of all enrolled patients. There was a predominance of males for both UC and CD (58.9% and 73.1%, respectively). The median age at diagnosis of UC and CD was 42 years (range, 18 to 81 years) and 27 years (range, 14 to 78 years), respectively. The median follow-up durations for UC patients and CD patients after diagnosis were 5.5 years (range, 1.0 to 13.0 years) and 5.5 years (range, 1.0 to 16.2 years), respectively. The number of patients who had experienced two or more flare-ups was 144 (35.0%) for UC and 142 (44.9%) for CD. Bloody stool and abdominal pain were the most common symptoms of flares in UC and CD, respectively. Twenty-six patients with UC (3.7%) and 93 patients with CD (15.8%) underwent surgical intestinal resection for treatment of symptom flare-ups.
Table 1.
Clinical Characteristics of Inflammatory Bowel Disease Patients
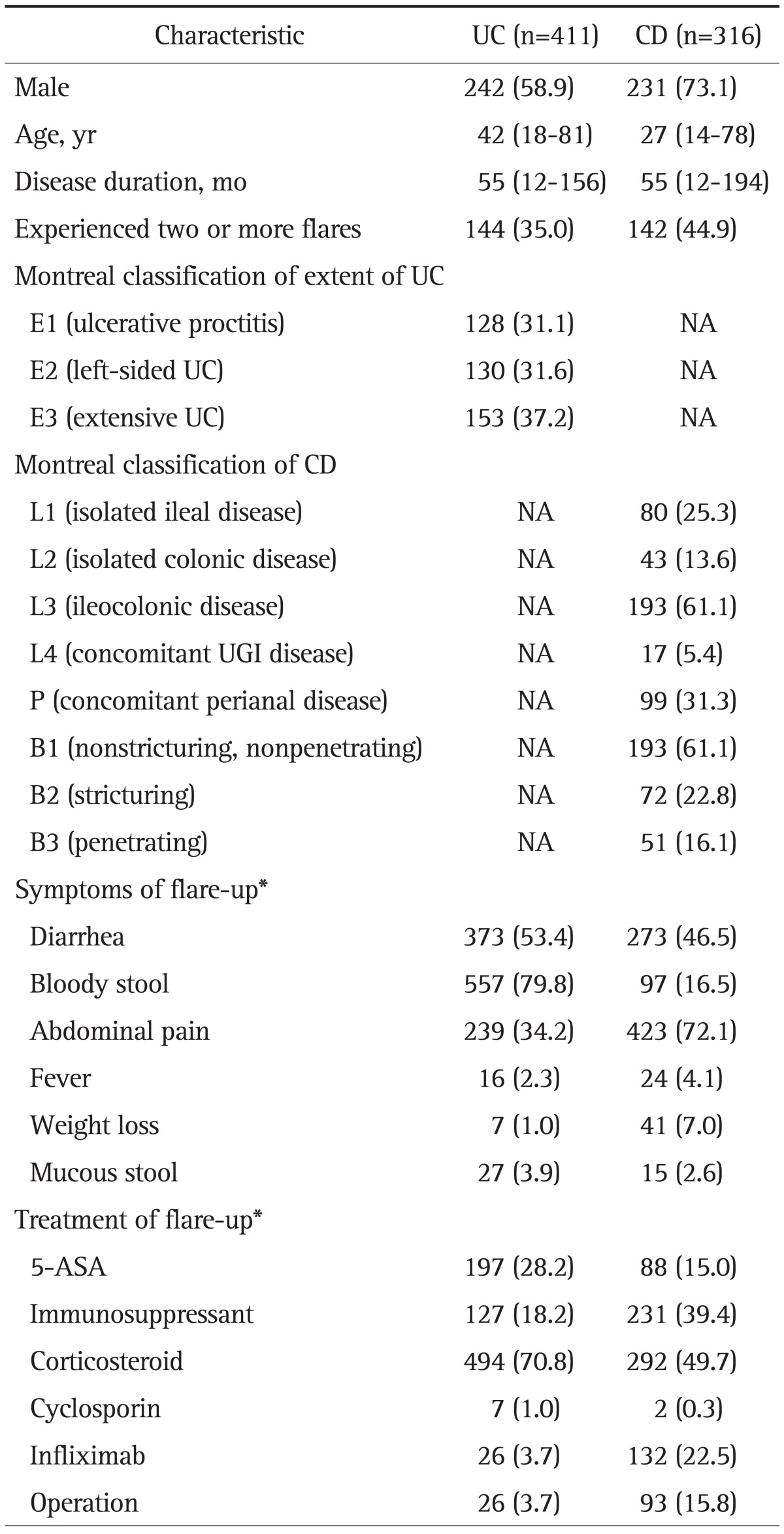
Data are presented as number (%) or median (range).
UC, ulcerative colitis; CD, Crohn disease; NA, not applicable; UGI, upper gastrointestinal; ASA, aminosalicylic acid.
*A total of 1,285 symptom flares (698 flares in UC and 587 flares in CD) were analyzed.
2. Monthly and seasonal distributions of birth dates
The observed and expected frequency of birth month in patients with IBD by month and season are shown in Tables 2 and 3, respectively. IBD patients as a whole presented monthly (χ2(11 df)=21.772, p=0.026) and seasonal variations (χ2(3 df)=9.434, p=0.024) in births. The peak birth dates for IBD patients as a whole occurred during the winter, especially in January and February, while the nadir occurred in the autumn. This seasonal variation was also observed for UC (χ2(3 df)=9.668, p=0.022). A similar trend of monthly variation, although not statistically significant, was observed for UC (χ2(11 df)=18.264, p=0.076). For CD, birth dates occurred most frequently in winter, especially in January and February, but this difference did not reach statistical significance (χ2(3 df)=1.512, p=0.679 and χ2(11 df)=14.726, p=0.195).
Table 2.
Observed versus Expected Number of Births for Every Month in 727 Inflammatory Bowel Disease Patients
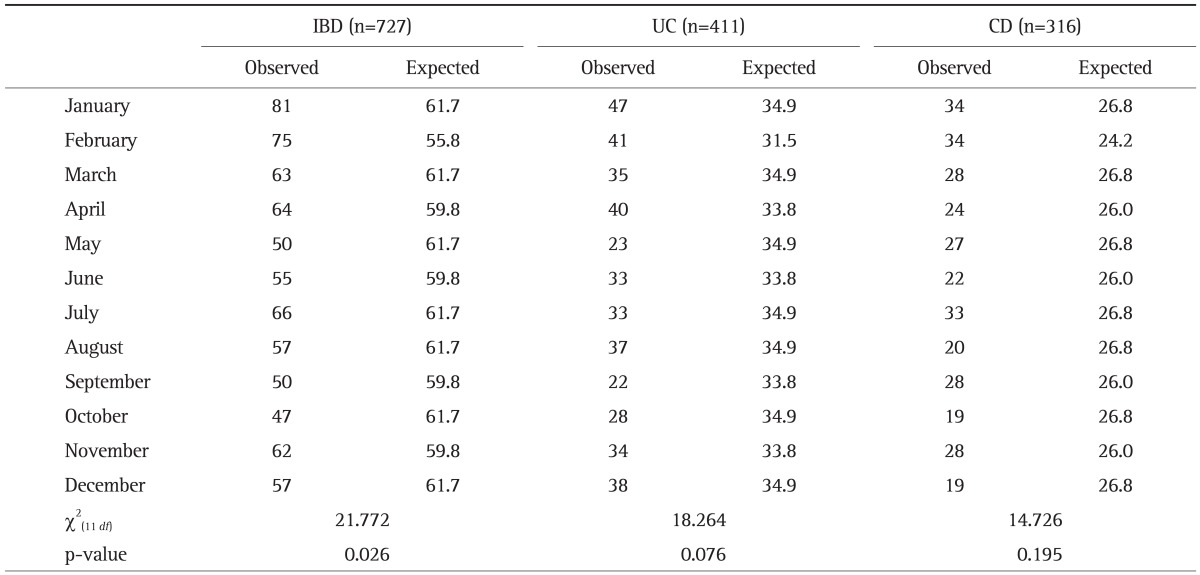
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn disease.
Table 3.
Observed versus Expected Number of Births for Every Season in 727 Inflammatory Bowel Disease Patients
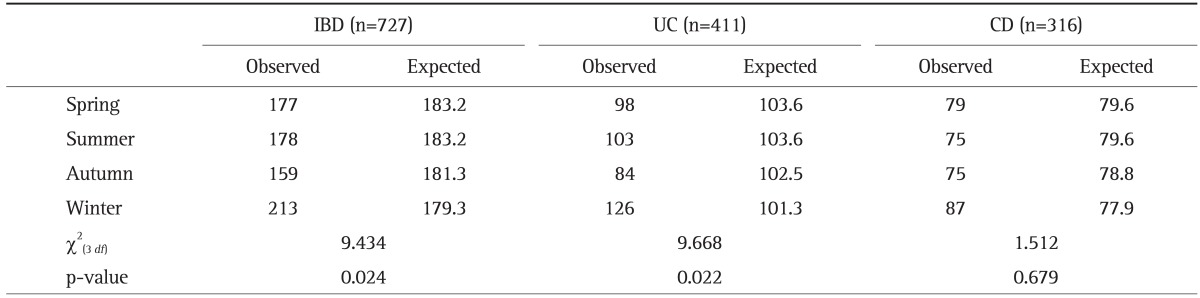
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn disease.
3. Monthly and seasonal distribution of disease flares
Tables 4 and 5 show the observed and expected frequency of symptom flares in patients with IBD per month and season, respectively. There was no statistical difference in disease flares according to month in UC (p=0.968) or CD (p=0.117). When the season during which disease flares occurred was included, a significant departure from a uniform distribution was seen among CD patients. The peak of disease flares was shown to be in spring, and the nadir in autumn (χ2(3 df)=10.584, p=0.014). However, we found no statistical differences in the seasonal distribution of disease flares among UC patients (p=0.963). When we analyzed only patients who had experienced two or more flare-ups, disease flares in both UC and CD were unaffected by any monthly (p=0.561 and p=0.198, respectively) or seasonal variation (p=0.325 and p=0.234, respectively).
Table 4.
Observed versus Expected Monthly Frequency of Disease Flares in 727 Inflammatory Bowel Disease Patients (Total of 1,285 Symptom Flares: 698 Flares in Ulcerative Colitis and 587 Flares in Crohn Disease)
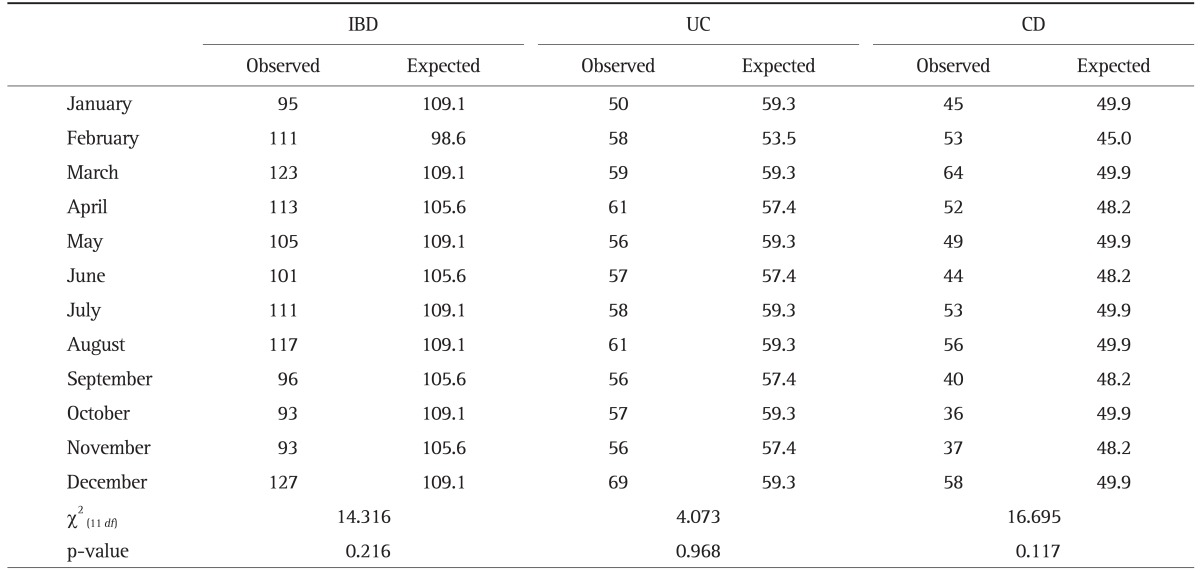
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn disease.
Table 5.
Observed versus Expected Seasonal Frequency of Disease Flares in 727 Inflammatory Bowel Disease Patients (Total of 1,285 Symptom Flares: 698 Flares in Ulcerative Colitis and 587 Flares in Crohn Disease)
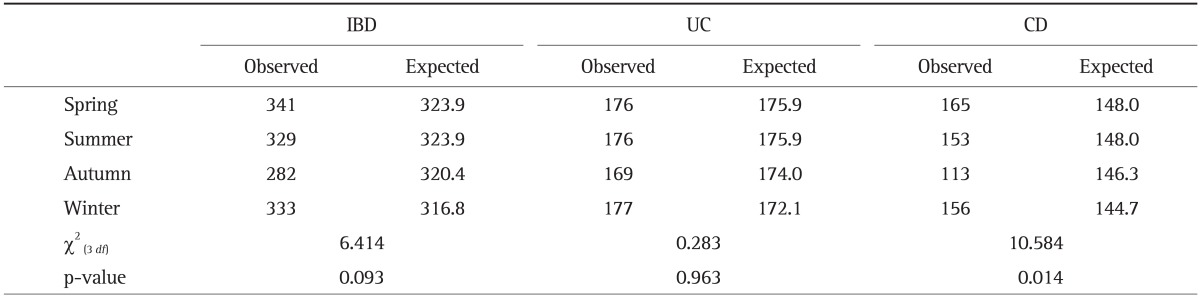
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn disease.
DISCUSSION
Environmental exposures change with the seasons. These environmental exposures may contribute to the inducement of IBD and may influence the clinical course of IBD. The relationship between seasonality and birth month or symptom flares among IBD patients has been investigated in a number of studies. However, these studies have reported inconsistent results.
Several previous studies found a significant association between the month or season of birth and later development of IBD. A Danish study demonstrated a peak in birth dates occurring in August and a trough in March among 627 CD patients aged less than 21.15 According to a study from Slovakia, the birth rate of children with later childhood-onset CD was highest in summer.3 In a Belgian cohort, a significantly reduced risk for development of CD was observed for people born in June.17 Another study in an Israeli population revealed that birth during the winter period was associated with an increased risk for development of CD, whereas no such seasonal variation was noted for UC patients.14 More recently, a study from Italy showed a significant association between the occurrence of CD and birth in the months of July.13 On the other hand, three publications from the United Kingdom demonstrated no seasonal variation in the birth month of patients who subsequently developed IBD.12,16,25
In the current study, we found a high frequency of births in the winter, especially in January and February, among IBD patients as a whole. These monthly and seasonal patterns were more pronounced for UC than for CD. Our results regarding births are roughly similar to those of the study in an Israeli population, but they are not consistent with those from Western populations. These heterogenous results among study populations may reflect the distinct genetic backgrounds of the different races and variation in environmental triggering factors by geographic area. Seasonal changes in immune function are mediated by the pineal hormone melatonin.26 This hormone induces proinflammatory action by enhancing the release of T helper cell type 1 cytokines and by modulating the release of glucocorticoids.26 An increase in the blood concentration of adrenocortical steroids in response to winter stressors can compromise immune function.27 Perinatal exposure to seasonal factors such as hormonal changes during the maturation of the immune system may play a role in development of IBD and predispose individuals to increased risk later in life.
Conflicting data have also been reported regarding the seasonal variation in IBD symptom flares. Some studies have demonstrated increased rates of flares in the spring in patients with UC,8,9 whereas other studies have found increased rates of flares in the autumn or winter.2,6,28 One study reported that symptom flares occurred more frequently in autumn and winter in patients with CD.11 Still other studies did not find a seasonal pattern of flares among patients with IBD.7,10,29
The present study demonstrated that CD symptom flares were higher during spring and lower during autumn. A few studies have identified an association between respiratory tract infection and relapse of IBD and have suggested that respiratory tract infection may predispose patients to exacerbation of IBD.30,31 In Korea, most respiratory tract viral infections show a seasonal pattern of occurrence with a peak in the spring and lower occurrence in autumn.32-34 This characteristic seasonal pattern of viral infection in Korea may suggest a link between viral infection and CD flares. Another possible explanation could be that temperature change may facilitate CD flares in the spring. Warmer temperatures may promote the locomotion of human neutrophilic leukocytes and thereby induce the release of inflammatory mediators such as interleukin-6.35 IBD patients adapted to cold weather may be more vulnerable to a rise in temperature rather than the high temperature itself, explaining why summer is not the season with the greatest number of flares. Further investigations are needed to elucidate the mechanisms behind the seasonality of flares in patients with CD. Our results showed a significant seasonality in symptom flares in CD patients but not in UC patients. The reasons for this discrepancy are not clear, but our findings suggest that CD flares may be more influenced by environmental factors such as seasonality than UC flares.
To date, little data on seasonal patterns of birth month and IBD flares have been collected from Asian populations. Only one study has investigated the seasonal variation in flares and birth month in an Asian population, and this was done in a Chinese population.19 According to that study, the peak number of UC symptom flares occurred during spring and summer, while there was no statistical difference by seasonal birth distribution, although the births of UC patients occurred more frequently in the autumn-winter period than in the spring-summer period. However, the seasonality of CD patients was not examined. Our study is the first to evaluate the seasonality of both CD and UC in an Asian population.
We confirmed that the seasonal patterns in births and symptom flares in Korean IBD patients were different from those found in Chinese and Western populations. These differences in the seasonality of IBD between Asian nations emphasize the importance of genetic and environmental factors in different geographic areas in the development and clinical course of IBD.
Our study had several limitations. First, we analyzed symptom flares retrospectively and thus it is possible that some flares may have gone undocumented, as symptoms may not have been very severe and thus not reported. Second, smoking status and medications such as antibiotics, nonsteroidal anti-inflammatory drugs, and oral contraceptives, which could potentially influence IBD relapse, were not investigated. Failure to adjust for these factors may produce bias in our results. Third, the observed births were compared to the expected births with a uniform distribution during the year, rather than the actual births of an age-and sex-matched control group. Fourth, we did not include patients who had no IBD flares identified in analysis of association between birth months and seasonality. Finally, our study was not population-based, but rather hospital-based. Therefore, there may be a referral-center bias with the inclusion of more severe cases in our cohort. However, IBD is still an uncommon disease in Korea, and IBD patients are generally diagnosed and managed by gastroenterology specialists in a university hospital. Furthermore, we only analyzed data from patients first diagnosed in the study hospitals, excluding those referred by other physicians. In addition, our study was performed in six university hospitals located in rural as well as urban areas. Therefore, it is not unreasonable that our cohort adequately represented Korean IBD patients.
In conclusion, this study was the first of its kind to be performed in Korea, and found a significant association between the occurrence of IBD and birth in the winter season, especially in January and February. In addition, this study showed that the symptom flares of CD patients occurred mainly in the spring, whereas a seasonal pattern was not observed in symptom flares of UC patients. These results suggest that seasonal environmental variation may be involved in the development of IBD and the clinical course of CD. Further studies should be performed in Asian populations to evaluate the numerous potential links between the pathogenesis of IBD and environmental factors.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Myszor M, Calam J. Seasonality of ulcerative colitis. Lancet. 1984;2:522–523. doi: 10.1016/s0140-6736(84)92600-x. [DOI] [PubMed] [Google Scholar]
- 3.Mikulecký M, Cierna I. Seasonality of births and childhood inflammatory bowel disease. Wien Klin Wochenschr. 2005;117:554–557. doi: 10.1007/s00508-005-0391-2. [DOI] [PubMed] [Google Scholar]
- 4.Moum B, Aadland E, Ekbom A, Vatn MH. Seasonal variations in the onset of ulcerative colitis. Gut. 1996;38:376–378. doi: 10.1136/gut.38.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aratari A, Papi C, Galletti B, et al. Seasonal variations in onset of symptoms in Crohn's disease. Dig Liver Dis. 2006;38:319–323. doi: 10.1016/j.dld.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Sellu DP. Seasonal variation in onset of exacerbations of ulcerative proctocolitis. J R Coll Surg Edinb. 1986;31:158–160. [PubMed] [Google Scholar]
- 7.Lewis JD, Aberra FN, Lichtenstein GR, Bilker WB, Brensinger C, Strom BL. Seasonal variation in flares of inflammatory bowel disease. Gastroenterology. 2004;126:665–673. doi: 10.1053/j.gastro.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Tysk C, Järnerot G. Seasonal variation in exacerbations of ulcerative colitis. Scand J Gastroenterol. 1993;28:95–96. doi: 10.3109/00365529309096052. [DOI] [PubMed] [Google Scholar]
- 9.Karamanolis DG, Delis KC, Papatheodoridis GV, Kalafatis E, Paspatis G, Xourgias VC. Seasonal variation in exacerbations of ulcerative colitis. Hepatogastroenterology. 1997;44:1334–1338. [PubMed] [Google Scholar]
- 10.Vergara M, Fraga X, Casellas F, Bermejo B, Malagelada JR. Seasonal influence in exacerbations of inflammatory bowel disease. Rev Esp Enferm Dig. 1997;89:357–366. [PubMed] [Google Scholar]
- 11.Zeng L, Anderson FH. Seasonal change in the exacerbations of Crohn's disease. Scand J Gastroenterol. 1996;31:79–82. doi: 10.3109/00365529609031631. [DOI] [PubMed] [Google Scholar]
- 12.Card TR, Sawczenko A, Sandhu BK, Logan RF. No seasonality in month of birth of inflammatory bowel disease cases: a prospective population based study of British under 20 year olds. Gut. 2002;51:814–815. doi: 10.1136/gut.51.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelucci E, Cocco A, Cesarini M, et al. Monthly and seasonal birth patterns and the occurrence of Crohn's disease. Am J Gastroenterol. 2009;104:1608–1609. doi: 10.1038/ajg.2009.107. [DOI] [PubMed] [Google Scholar]
- 14.Chowers Y, Odes S, Bujanover Y, Eliakim R, Bar Meir S, Avidan B. The month of birth is linked to the risk of Crohn's disease in the Israeli population. Am J Gastroenterol. 2004;99:1974–1976. doi: 10.1111/j.1572-0241.2004.40058.x. [DOI] [PubMed] [Google Scholar]
- 15.Sørensen HT, Pedersen L, Nørgård B, Fonager K, Rothman KJ. Does month of birth affect risk of Crohn's disease in childhood and adolescence? BMJ. 2001;323:907. doi: 10.1136/bmj.323.7318.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenberg A. Date of birth in the occurrence of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:206–211. doi: 10.1002/ibd.20730. [DOI] [PubMed] [Google Scholar]
- 17.Van Ranst M, Joossens M, Joossens S, et al. Crohn's disease and month of birth. Inflamm Bowel Dis. 2005;11:597–599. doi: 10.1097/01.mib.0000163697.34592.d4. [DOI] [PubMed] [Google Scholar]
- 18.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134–147. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 19.Bai A, Guo Y, Shen Y, Xie Y, Zhu X, Lu N. Seasonality in flares and months of births of patients with ulcerative colitis in a Chinese population. Dig Dis Sci. 2009;54:1094–1098. doi: 10.1007/s10620-008-0453-1. [DOI] [PubMed] [Google Scholar]
- 20.Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review. Am J Gastroenterol. 2009;104:2100–2109. doi: 10.1038/ajg.2009.190. [DOI] [PubMed] [Google Scholar]
- 21.Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010;4:1–14. doi: 10.5009/gnl.2010.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SH, Kim YM, Yang SK, et al. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis. 2007;13:278–283. doi: 10.1002/ibd.20015. [DOI] [PubMed] [Google Scholar]
- 23.Ye BD, Yang SK, Cho YK, et al. Clinical features and long-term prognosis of Crohn's disease in Korea. Scand J Gastroenterol. 2010;45:1178–1185. doi: 10.3109/00365521.2010.497936. [DOI] [PubMed] [Google Scholar]
- 24.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 25.Haslam N, Mayberry JF, Hawthorne AB, Newcombe RG, Holmes GK, Probert CS. Measles, month of birth, and Crohn's disease. Gut. 2000;47:801–803. doi: 10.1136/gut.47.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson RJ, Drazen DL. Melatonin mediates seasonal changes in immune function. Ann N Y Acad Sci. 2000;917:404–415. doi: 10.1111/j.1749-6632.2000.tb05405.x. [DOI] [PubMed] [Google Scholar]
- 27.Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- 28.Riley SA, Mani V, Goodman MJ, Lucas S. Why do patients with ulcerative colitis relapse? Gut. 1990;31:179–183. doi: 10.1136/gut.31.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Don BA, Goldacre MJ. Absence of seasonality in emergency hospital admissions for inflammatory bowel disease. Lancet. 1984;2:1156–1157. doi: 10.1016/s0140-6736(84)91590-3. [DOI] [PubMed] [Google Scholar]
- 30.Kangro HO, Chong SK, Hardiman A, Heath RB, Walker-Smith JA. A prospective study of viral and mycoplasma infections in chronic inflammatory bowel disease. Gastroenterology. 1990;98:549–553. doi: 10.1016/0016-5085(90)90272-3. [DOI] [PubMed] [Google Scholar]
- 31.Mee AS, Jewell DP. Factors inducing relapse in inflammatory bowel disease. Br Med J. 1978;2:801–802. doi: 10.1136/bmj.2.6140.801-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MR, Lee HR, Lee GM. Epidemiology of acute viral respiratory tract infections in Korean children. J Infect. 2000;41:152–158. doi: 10.1053/jinf.2000.0715. [DOI] [PubMed] [Google Scholar]
- 33.Yun BY, Kim MR, Park JY, Choi EH, Lee HJ, Yun CK. Viral etiology and epidemiology of acute lower respiratory tract infections in Korean children. Pediatr Infect Dis J. 1995;14:1054–1059. doi: 10.1097/00006454-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Ahn KM, Chung SH, Chung EH, et al. Clinical characteristics of acute viral lower respiratory tract infections in hospitalized children in Seoul, 1996-1998. J Korean Med Sci. 1999;14:405–411. doi: 10.3346/jkms.1999.14.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maes M, Stevens W, Scharpé S, et al. Seasonal variation in peripheral blood leukocyte subsets and in serum interleukin-6, and soluble interleukin-2 and -6 receptor concentrations in normal volunteers. Experientia. 1994;50:821–829. doi: 10.1007/BF01956463. [DOI] [PubMed] [Google Scholar]


