Abstract
Background/Aims
Migraine is frequently accompanied by symptoms consistent with functional gastrointestinal disorders (FGIDs). This study evaluated the prevalence of functional gastrointestinal symptoms and assessed the symptoms' relationship with the concomitant functional symptoms of anxiety, depression, and headache-related disability.
Methods
This prospective study included 109 patients with migraine who were recruited from a headache clinic at a teaching hospital. The participants completed a self-administered survey that collected information on headache characteristics, functional gastrointestinal symptoms (using Rome III criteria to classify FGID), anxiety, depression, and headache-related disability.
Results
In total, 71% of patients met the Rome III criteria for at least one FGID. In patients with FGID, irritable bowel syndrome was the most common symptom (40.4%), followed by nausea and vomiting syndrome (24.8%) and functional dyspepsia (23.9%). Depression and anxiety scores were significantly higher in patients meeting the criteria for any FGID. The number of the symptoms consistent with FGID in individual patients correlated positively with depression and anxiety.
Conclusions
FGID symptoms defined by the Rome III criteria are highly prevalent in migraine. These symptoms correlate with psychological comorbidities, such as depression and anxiety.
Keywords: Gastrointestinal diseases, Migraine disorders, Prevalence, Psychological comorbidity, Headache-related disability
INTRODUCTION
Migraine is a common chronic debilitating disorder that affects 22.3% of the general population in South Korea.1 Various gastrointestinal symptoms, such as nausea, vomiting, retching, and loss of appetite are characteristic of patients with migraine. In addition, migraine can be accompanied by a sense of heaviness in the epigastric region, epigastric pain, belching, and lower gastrointestinal symptoms, such as diarrhea and flatulence.2 The positive correlation between migraine and the presenece of symptoms consistent with functional gastrointestinal disorders (FGIDs) has previously been established in many clinical observations and epidemiological studies.2-6 A recent large, population-based, cross-sectional study reported a higher prevalence of headache, including migraine, in individuals with reflux symptoms, diarrhea, constipation, or nausea than in individuals without such complaints.5 A large cohort study of 97,593 patients with irritable bowel syndrome (IBS) revealed a higher prevalence of migraine (6%) compared to that in healthy controls (2.2%).6 However, a clinical study of dyspeptic patients referred for upper gastrointestinal endoscopy revealed that the prevalence of migraine in patients with reflux-like/ulcer-like dyspepsia did not differ from that in healthy controls, and was higher only among those with dysmotility-like dyspepsia.3
Migraine has been reported to be associated with psychiatric comorbidities such as depression, anxiety, bipolar disorder, panic disorder, and suicide.7 Furthermore, migraine is a significantly more frequent symptom in various unexplained clinical conditions such as IBS, fibromyalgia, chronic fatigue syndrome, and depression.6,8 These results have prompted speculation that these disorders have a similar pathogenesis. However, there are few data on the impact of FGIDs on headache-related disability, and the association between FGIDs and psychological comorbidity in migraine.
We evaluated the prevalence of functional symptoms (using Rome III criteria to classify FGID) in a prospective, systematically acquired cohort of migraine patients attending a teaching hospital in Korea. We aimed to clarify the relationship among these concomitant functional symptoms, psychological comorbidity, and headache-related disability.
MATERIALS AND METHODS
1. Patients
The study was performed at a teaching hospital of Uijeongbu St. Mary's Hospital, The Catholic University of Korea College of Medicine from March to August, 2011. One hundred nine patients (14 male, 95 female; mean age, 41 years; age range, 16 to 74 years) with migraine were consecutively recruited from a headache clinic. All participants underwent a clinical interview and physical and neurological examinations by experienced neurologists (J.W.P. and H.E.S.). The diagnosis of migraine was made based on the operational diagnostic criteria of the International Headache Society (International Classification of Headache Disorders-II).9 Excluded subjects were those suffering from severe systemic disease; those with a history of abdominal surgery (except appendectomy); those with hepatic, biliary, pancreatic, and gastrointestinal disorders who were receiving treatment; and those who had taken analgesics for headache more than 10 times during the previous 3 months. In 42 of the 109 patients (38.5%), there were no abnormal results on upper gastrointestinal endoscopy and/or colonoscopy, both of which were performed within a year before participation in the study.
2. Questionnaire
Patients completed a structured questionnaire to provide detailed data regarding the clinical symptoms and features of their headache,10 and the Korean translation of the Rome III questionnaire to assess functional gastrointestinal symptoms.11 The Korean version of the Rome III questionnaire consists of 74 questions. The validation process for the Korean version of the Rome III questionnaire consisted of forward and backward translation, as well as confirmation of the patient's ability to understand.12 Diagnosis of functional dyspepsia (FD) was based on the presence of one or more of following symptoms for at least 3 months with symptom onset at least 6 months prior: bothersome postprandial fullness, early satiation, epigastric pain, epigastric burning with no evidence of structural disease observed on upper endoscopy, which would likely provide an explanation for the symptoms. FD was classified into postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) subtypes. To diagnose IBS, patients must have had abdominal pain or discomfort for at least 3 of the previous 6 months, with two or more of the following symptoms: pain improvement after defecation, symptoms associated with a change in stool frequency, or symptoms associated with a change in stool form. We categorized IBS into subtypes by asking participants if their stools were hard/lumpy stools or loose/watery.
All patients were given headache diaries at the initial visit. Patients were asked questions about headache characteristics and symptoms associated with each attack. Patients completed a diary at the same time each day on days in which they experienced a headache.10 Each diary covered a 4-week period. The intensity of headache pain was assessed using a visual analog scale scored from 0 to 10, where 0 indicated no pain at all and 10 indicated the worst pain imaginable.
3. Headache-related disability
We adapted the Migraine Disability Assessment Scale (MIDAS) and the Headache Impact Test-6 (HIT-6) to assess headache-related disability. The MIDAS consisted of five health-related disability items to measure the effect of migraine on functional ability in three domains: school/work; household work; and family, social or leisure activities.13 The items covered a >50% reduction in ability to attend work or school, a >50% reduction in ability to do household work, and inability to participate in nonwork activities (total score of 0 to 3 for each headache attack).10 The total score was calculated by adding the five items, with a higher score indicating increased headache-related disability. HIT-6 contained six items: pain, social functioning, role functioning, energy or fatigue, cognitive functioning, and emotional distress.14 Patients answered each of the six items using a 5-point scale comprising the categories: "never," "rarely," "sometimes," "very often," and "always," which were assigned values of 6, 8, 10, 11, and 13, respectively. The HIT-6 score was calculated by adding the six items and ranged between 36 and 78, with a higher score indicating a greater impact of headache on daily life.10 Patients completed the MIDAS and the HIT-6 questionnaire over a 4-week period.
4. Psychological comorbidity
The Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) were administered to determine psychological status. The BAI is a 21-item questionnaire developed to measure anxiety-related symptoms over the past week.15 Patients were asked to rate how much they had been bothered by each symptom on a four-point scale ranging from 0 to 3. The BDI is a 21-item self-reported scale for depression.16 Each item was scored on a scale ranging from 0 to 3 according to how the patient felt at that particular time.
5. Statistical analysis
Statistical analysis was performed with SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Categorical variables are presented as proportions, and continuous variables are presented as means±SD. Continuous data were compared using an independent sample t-test, whereas categorical data were analyzed using chi-square or Fisher exact tests. For multiple comparisons, ANOVA with post hoc comparison of all pairwise differences (with Tukey correction for multiple comparisons) was used. A value of p<0.05 was considered statistically significant for all tests.
6. Ethics statement
This study protocol was approved by the Institutional Research Ethics Board of Uijeongbu St. Mary's Hospital, The Catholic University of Korea College of Medicine (IRB No. UC11OISI0046) and adhered to the Declaration of Helsinki. All of the study subjects completed an informed consent form before participating in the study. The informed consent was approved by the IRB.
RESULTS
1. Prevalence of symptoms consistent with FGID
Demographic and headache characteristics in the migraine cohort are described in Table 1. The prevalence of FGID in the migraine cohort is shown in Table 2. Overall, 77 (70.6%) participants met the Rome III criteria for one or more FGID (Table 1), 33 (30.3%) met the criteria for one FGID, and 44 (40.3%) met the criteria for more than one FGID. In all patients with FGIDs, the frequencies of esophageal, gastroduodenal, bowel, and anorectal disorders were 23.9%, 42.2%, 57.8%, and 2.8%, respectively. The most prevalent FGID was IBS (40.4%), followed by nausea and vomiting disorders (24.8%), and FD (23.9%). Functional heartburn was the most frequently observed esophageal disorder. With regard to functional gastroduodenal disorders, nausea and vomiting disorders were the most prevalent, followed by FD, and belching disorders (3.7%). In terms of functional bowel disorders, IBS was the most prevalent, followed by functional bloating (15.6%), functional diarrhea (2.8%), and functional constipation (0.9%). With respect to functional anorectal disorders, the frequency of functional defecation disorder was 2.8%.
Table 1.
Demographic and Headache Characteristics in Migraine Patients
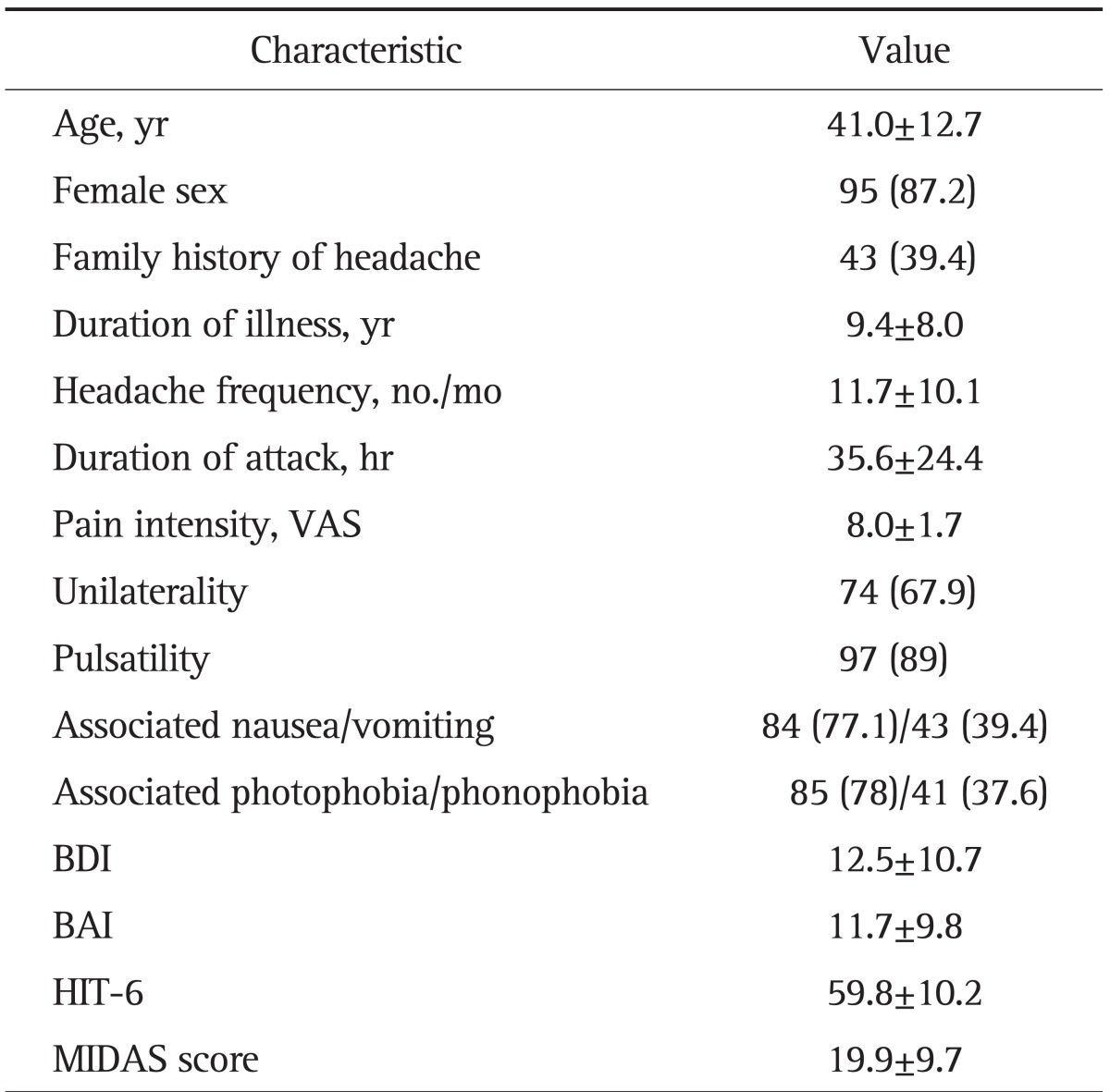
Data are presented as mean±SD or number (%).
VAS, visual analogue scale; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; HIT-6, Headache Impact Test-6; MIDAS, Migraine Disability Assessment Scale.
Table 2.
Prevalence of Functional Gastrointestinal Symptoms in 109 Migraine Patients
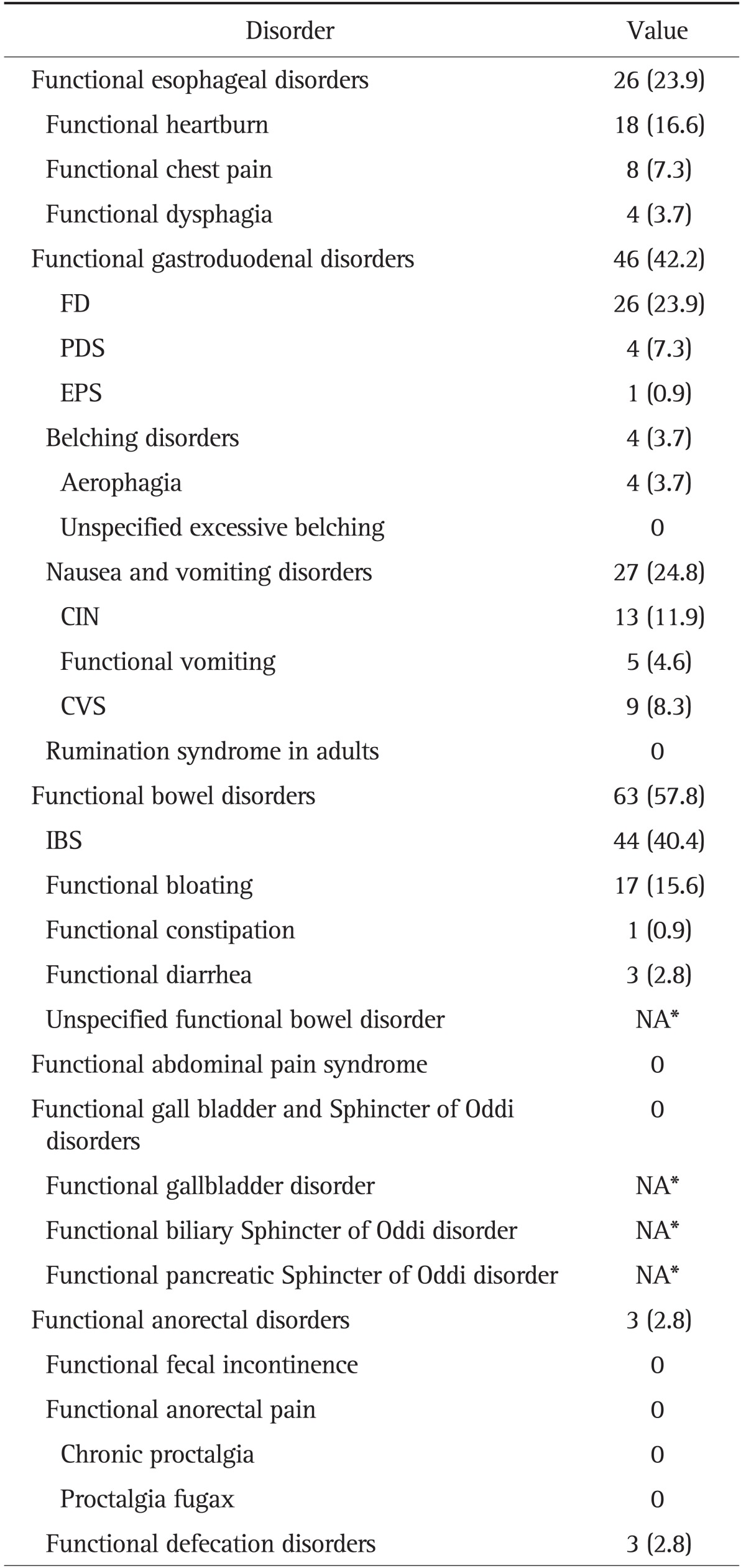
Data are presented as number (%). The percentages given might exceed 100% because of the overlap of functional gastrointestinal disorders (and subtypes) in certain patients.
FD, functional dyspepsia; PDS, postprandial distress syndrome; EPS, epigastric pain syndrome; CIN, chronic idiopathic nausea; CVS, cyclic vomiting syndrome; IBS, irritable bowel syndrome; NA, not available.
*Not possible to detect by survey only.
Among the 26 patients with FD, the following dyspeptic subgroups were identified: eight patients (7.3%) had postprandial fullness and/or early satiation (PDS subgroup), and one patient (0.9%) had epigastric pain and/or burning. Seventeen patients (15.6%) with FD met neither the PDS nor EPS criteria. Among the 27 patients with nausea and vomiting disorders, 13 (11.9%) had bothersome nausea not usually associated with vomiting (chronic idiopathic nausea, CIN subgroup), nine patients (8.3%) had cyclic vomiting syndrome (CVS), and five (4.6%) had functional vomiting. Forty-four patients (40.4%) fulfilled the Rome III criteria for the diagnosis of IBS. Of these, 11 (10.1%) were classified as IBS with constipation (IBS-C), five (4.6%) as IBS with diarrhea (IBS-D), 27 (24.8%) as mixed IBS (IBS-M), and one as nonsubtyped IBS (IBS-U). Of the IBS subtypes, IBS-M was the most common, followed by IBS-C, and IBS-D.
2. Overlap of functional gastroduodenal disorders
Of 109 patients with migraine, 44 (40.3%) had more than one FGID. FD and IBS were the most frequent combination observed (16.5%). The concomitant FGID most frequently found with FD was IBS (69.2%), followed by functional heartburn (42.3%), CIN (23.1%), CVS (11.5%), and belching disorders (7.7%). The concomitant FGID most frequently observed with IBS was FD (40.9%), followed by functional heartburn (25%), CIN (18.2%), functional bloating (6.8%), CVS (4.5%), and belching disorders (4.5%).
3. Effect of concomitant FGID symptoms on headache-related disability, anxiety, and depression
Mean BDI and BAI scores were significantly higher in patients who met the criteria for any FGID compared to patients who did not (BDI, 14.42 vs 7.78, p=0.003; BAI, 13.64 vs 7.27, p=0.001). There was no significant difference in MIDAS and HIT-6 scores between patients who met the criteria for any FGID and those that did not.
Additionally, the load of symptoms consistent with FGID in each patient (i.e., the number of FGID symptom clusters per patient) correlated with psychological comorbidity. The number of FGID symptom clusters in patients correlated positively with higher BDI and BAI scores (r=0.37, p<0.001; r=0.38, p<0.001, respectively) (Table 3). However, there were no correlations between the number of FGID symptom clusters and MIDAS or HIT-6 scores. The BDI score was positively correlated with MIDAS and HIT-6 scores (r=0.23, p=0.017; r=0.26, p=0.005, respectively). The BAI score was also positively correlated with MIDAS and HIT-6 scores (r=0.25, p=0.01; r=0.24, p=0.014, respectively).
Table 3.
Comparisons between Mean Depression, Anxiety, and Headache-Related Disability Scores Stratified by the Load of Functional Gastrointestinal Disorder Symptoms in Migraine Patients*
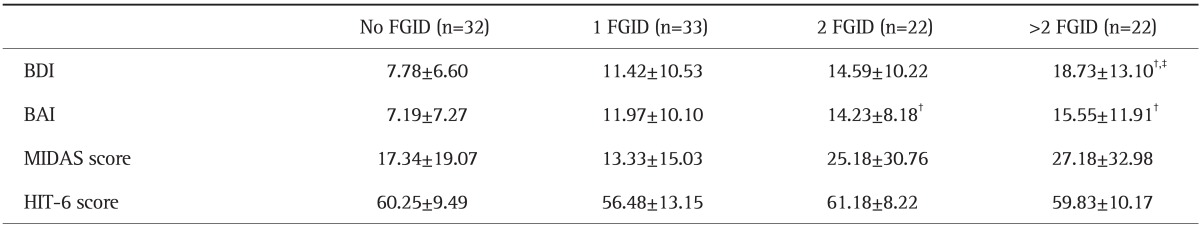
Data are presented as mean±SD.
FGID, functional gastrointestinal disorder; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; MIDAS, Migraine Disability Assessment Scale; HIT-6, Headache Impact Test-6.
*Tukey's honestly significant difference post hoc comparisons of the means of BDI, BAI, MIDAS, and HIT-6 in migraine patients with no FGID compared with 1, 2, and >2 FGID; †p<0.05 in the No FGID column; ‡p<0.05 in the 1 FGID column.
4. Associations of psychological comorbidity and headache-related disability with specific FGID symptoms
Symptoms consistent with IBS were the most prevalent of any discrete FGID subtype. Strong correlations between IBS subtype and depression/anxiety were evident (Table 4). Results of symptoms consistent with nausea and vomiting disorders were similar. However, MIDAS score correlated only with FD subtype.
Table 4.
Interactions between Mean Depression, Anxiety, and Headache-Related Disability Scores in Migraine Patients with and without Symptoms of a Particular Functional Gastrointestinal Disorder
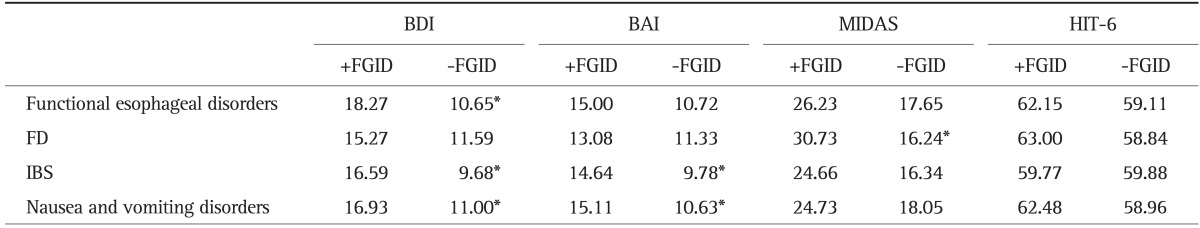
BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; MIDAS, Migraine Disability Assessment Scale; HIT-6, Headache Impact Test-6; FD, functional dyspepsia; IBS, irritable bowel syndrome.
*Denotes statistical significance at p<0.05.
DISCUSSION
In this prospectively identified, hospital-wide population of migraine patients, we showed that 70% of patients met the Rome III criteria for concurrent FGID, with 40% of the cohort meeting the criteria for more than one FGID. Furthermore, we demonstrated a clear link between coexistent FGID symptoms, psychological comorbidity and the existence of a "load effect," as anxiety/depression scores worsened with increasing numbers of FGID symptoms. Previous studies of both FGID and migraine did not show this load effect.
The positive association between migraine and IBS has been confirmed by several clinic- and population-based studies.6,8,17-20 IBS and migraine affect 6% to 30% of the general population, usually young adults.6,17,20 IBS was the most prevalent FGID in migraine patients in our study. Possible physiological pathways common to IBS and migraine may be associated with the brain-gut axis, neuroimmunity, and neuroendocrine interactions.20,21 Altered serotonin signaling is one of several hypothetical mechanisms underlying IBS and migraine.22,23 IBS and migraine show strong familial aggregation and presumably have a genetic basis. One recent study reported a familial association among patients with major depressive disorder and fibromyalgia, IBS, and migraine.24 We previously reported that polymorphisms in the promoter region of the serotonin reuptake transporter (SERT) gene (SERT deletion/deletion genotype) are associated with IBS, especially diarrhea-predominant IBS.25 In addition, SERT gene polymorphism of the variable number of tandem repeats is associated with migraine.26 Although further genetic study is needed, these results suggest that both diseases share heritable pathophysiological features. Previous studies of FGID in migraine patients did not demonstrate differences in frequencies according to IBS subtype. However, our data indicate that IBS-M was the most prevalent in migraine patients. This is similar to our clinic-based study in which IBS-M was the most common IBS among patients with chronic gastrointestinal symptoms.12
In this study, the second most common FGIDs in migraine patients were nausea and vomiting disorders. In the Rome II criteria, nausea is considered a symptom of motility-like dyspepsia.27 However, the Rome III criteria exclude patients with predominant nausea from this category, using instead new terms such as CIN.28 Another new category, cyclic vomiting in adults, was also added.28 Although CVS in children has been well-described, its prevalence in adults has been underappreciated and there is to our knowledge no study of the true incidence and prevalence of CVS in the general population. Recent clinic-based studies have demonstrated that CVS may be the cause of unexplained vomiting in 3% to 14% of referred patients assessed in tertiary gastrointestinal motility clinics for unexplained nausea/vomiting.29 Migraine headache, abdominal migraine, and CVS seem to be manifestations of a common diathesis with episodic attacks separated by symptom-free intervals.30 Patients with these disorders may progress from one disorder to another over time. This progression occurs more often in patients with pediatric onset of disease.29 A possible mechanism include the presence of mitochondrial dysfunction that leads to cellular energy deficits and contributes to autonomic nervous system dysfunction. Wang et al.31 reported similar genetic homoplasmic sequence variants in the mitochondrial DNA of CVS patients and migraine patients without aura in pediatric populations. However, a recent study that involved larger numbers of adult CVS patients demonstrated that one in four adults have a history of migraine headaches.32 In our study, a small percentage of migraine patients (8.3%) had CVS. Larger prospective studies are needed to clarify the relationship between CVS in adults and migraine.
The prevalence of FD symptoms in migraine patients was higher than in the general population in our previous study (23.9% vs 11.7%).33 However, the present study cohort exhibited a significantly lower rate of FD symptoms compared with a previous report.4 This discrepancy might relate to the different criteria used by the authors to classify FD. In the Rome III criteria, FD is defined as the presence of symptoms thought to originate in the gastroduodenal region (specifically epigastric pain or burning, postprandial fullness, or early satiation). In addition, FD is subclassified into the new diagnostic categories PDS and EPS.28 These changes were expected to prevent dyspeptic patients from being diagnosed as FD and to decrease the number of FD patients compared with the Rome II criteria. Meucci et al.3 reported that the prevalence of migraine was higher in patients with dysmotility-like dyspepsia than in healthy controls or in patients with reflux-like and ulcer-like dyspepsia. However, a substantial proportion of FD (65.4%) were not classified into subgroups such as EPS or PDS in our study. This difference may be due to the varying symptom frequency in FD and its subtypes. The Rome III criteria require "at least" weekly symptoms for the diagnosis of FD but more frequent symptoms for subtype diagnosis.28 The results may therefore differ when different criteria are applied to the same symptom frequency.
Migraine has been reported to be comorbid with mood and/or anxiety disorder in both population-based and clinic-based studies.34,35 Additionally, psychiatric comorbidity is directly correlated with the severity of FGID symptoms and degree of impairment.36 When we compared depression and anxiety between patients meeting the criteria for any FGID with patients who did not, all scores were significantly higher in the former group. In addition, an increased load of FGID symptoms correlated with a greater burden of psychological distress. The presence of psychiatric comorbidity is associated with a negative outcome in the treatment of migraine patients.37 Thus, accurate interpretation of FGID symptoms and appropriate management should be considered, and further study into their effective treatment is needed. Although MIDAS and HIT-6 are validated instruments for assessing functional impairment, FD correlated only with the MIDAS score. This may have been due to the limited number of study participants.
This study has several strengths, including neurologist confirmation of the migraine diagnosis and the use of a standardized and validated questionnaire to ascertain FGID symptoms, psychological comorbidity, and headache-related disability. However, the study also had several limitations. Although the validated questionnaire for the Rome III criteria improved the characterization of FGID symptoms, there was an important problem regarding whether gastrointestinal symptoms were functional or organic, as more than 60% of the patients did not undergo endoscopy. FGID remained primarily a clinical diagnosis. In addition, a much larger sample would be required to reliably assess the effect of FGID symptoms on the headache-related disability in migraine patients.
In conclusion, we found that FGID symptoms according to Rome III criteria were highly prevalent in migraine patients, and demonstrated that a greater burden of FGID symptoms correlated with higher rates of depression and anxiety. Treatment of FGID symptoms may have the potential to improve migraine. Further research is needed to establish the underlying pathophysiology and association between migraine and FGID.
ACKNOWLEDGEMENTS
This research was supported, in part, by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0006902).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Roh JK, Kim JS, Ahn YO. Epidemiologic and clinical characteristics of migraine and tension-type headache in Korea. Headache. 1998;38:356–365. doi: 10.1046/j.1526-4610.1998.3805356.x. [DOI] [PubMed] [Google Scholar]
- 2.Centonze V, Polito BM, Cassiano MA, et al. The dyspeptic syndrome in migraine: morphofunctional evaluation on 53 patients. Headache. 1996;36:442–445. doi: 10.1046/j.1526-4610.1996.3607442.x. [DOI] [PubMed] [Google Scholar]
- 3.Meucci G, Radaelli F, Prada A, et al. Increased prevalence of migraine in patients with uninvestigated dyspepsia referred for open-access upper gastrointestinal endoscopy. Endoscopy. 2005;37:622–625. doi: 10.1055/s-2005-870251. [DOI] [PubMed] [Google Scholar]
- 4.Kurth T, Holtmann G, Neufang-Hüber J, Gerken G, Diener HC. Prevalence of unexplained upper abdominal symptoms in patients with migraine. Cephalalgia. 2006;26:506–510. doi: 10.1111/j.1468-2982.2005.01076.x. [DOI] [PubMed] [Google Scholar]
- 5.Aamodt AH, Stovner LJ, Hagen K, Zwart JA. Comorbidity of headache and gastrointestinal complaints. The Head-HUNT Study. Cephalalgia. 2008;28:144–151. doi: 10.1111/j.1468-2982.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- 6.Cole JA, Rothman KJ, Cabral HJ, Zhang Y, Farraye FA. Migraine, fibromyalgia, and depression among people with IBS: a prevalence study. BMC Gastroenterol. 2006;6:26. doi: 10.1186/1471-230X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65–72. doi: 10.1111/j.1526-4610.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Shin HE, Park JW, Kim YI, Lee KS. Headache Impact Test-6 (HIT-6) scores for migraine patients: their relation to disability as measured from a headache diary. J Clin Neurol. 2008;4:158–163. doi: 10.3988/jcn.2008.4.4.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Park JM, Choi MG, Cho YK, et al. Functional gastrointestinal disorders diagnosed by Rome III questionnaire in Korea. J Neurogastroenterol Motil. 2011;17:279–286. doi: 10.5056/jnm.2011.17.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–S28. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 14.Bayliss MS, Dewey JE, Dunlap I, et al. A study of the feasibility of Internet administration of a computerized health survey: the headache impact test (HIT) Qual Life Res. 2003;12:953–961. doi: 10.1023/a:1026167214355. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 17.Jones R, Lydeard S. Irritable bowel syndrome in the general population. BMJ. 1992;304:87–90. doi: 10.1136/bmj.304.6819.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandvik PO, Wilhelmsen I, Ihlebaek C, Farup PG. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implications. Aliment Pharmacol Ther. 2004;20:1195–1203. doi: 10.1111/j.1365-2036.2004.02250.x. [DOI] [PubMed] [Google Scholar]
- 19.Azpiroz F, Dapoigny M, Pace F, et al. Nongastrointestinal disorders in the irritable bowel syndrome. Digestion. 2000;62:66–72. doi: 10.1159/000007780. [DOI] [PubMed] [Google Scholar]
- 20.Mulak A, Paradowski L. Migraine and irritable bowel syndrome. Neurol Neurochir Pol. 2005;39(4 Suppl 1):S55–S60. [PubMed] [Google Scholar]
- 21.Riedl A, Schmidtmann M, Stengel A, et al. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res. 2008;64:573–582. doi: 10.1016/j.jpsychores.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari MD, Odink J, Tapparelli C, Van Kempen GM, Pennings EJ, Bruyn GW. Serotonin metabolism in migraine. Neurology. 1989;39:1239–1242. doi: 10.1212/wnl.39.9.1239. [DOI] [PubMed] [Google Scholar]
- 24.Hudson JI, Mangweth B, Pope HG, Jr, et al. Family study of affective spectrum disorder. Arch Gen Psychiatry. 2003;60:170–177. doi: 10.1001/archpsyc.60.2.170. [DOI] [PubMed] [Google Scholar]
- 25.Park JM, Choi MG, Park JA, et al. Serotonin transporter gene polymorphism and irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:995–1000. doi: 10.1111/j.1365-2982.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 26.Park JW, Han SR, Yang DW, Kim YI, Lee KS. Serotonin transporter protein polymorphism and harm avoidance personality in migraine without aura. Headache. 2006;46:991–996. doi: 10.1111/j.1526-4610.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 27.Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45(Suppl 2):II37–II42. doi: 10.1136/gut.45.2008.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Hejazi RA, McCallum RW. Review article: cyclic vomiting syndrome in adults: rediscovering and redefining an old entity. Aliment Pharmacol Ther. 2011;34:263–273. doi: 10.1111/j.1365-2036.2011.04721.x. [DOI] [PubMed] [Google Scholar]
- 30.Stickler GB. Relationship between cyclic vomiting syndrome and migraine. Clin Pediatr (Phila) 2005;44:505–508. doi: 10.1177/000992280504400606. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Ito M, Adams K, et al. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am J Med Genet A. 2004;131:50–58. doi: 10.1002/ajmg.a.30323. [DOI] [PubMed] [Google Scholar]
- 32.Hejazi RA, Reddymasu SC, Namin F, Lavenbarg T, Foran P, McCallum RW. Efficacy of tricyclic antidepressant therapy in adults with cyclic vomiting syndrome: a two-year follow-up study. J Clin Gastroenterol. 2010;44:18–21. doi: 10.1097/MCG.0b013e3181ac6489. [DOI] [PubMed] [Google Scholar]
- 33.Jeong JJ, Choi MG, Cho YS, et al. Chronic gastrointestinal symptoms and quality of life in the Korean population. World J Gastroenterol. 2008;14:6388–6394. doi: 10.3748/wjg.14.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oedegaard KJ, Neckelmann D, Mykletun A, et al. Migraine with and without aura: association with depression and anxiety disorder in a population-based study. The HUNT Study. Cephalalgia. 2006;26:1–6. doi: 10.1111/j.1468-2982.2005.00974.x. [DOI] [PubMed] [Google Scholar]
- 35.Guidetti V, Galli F, Fabrizi P, et al. Headache and psychiatric comorbidity: clinical aspects and outcome in an 8-year follow-up study. Cephalalgia. 1998;18:455–462. doi: 10.1046/j.1468-2982.1998.1807455.x. [DOI] [PubMed] [Google Scholar]
- 36.Haug TT, Mykletun A, Dahl AA. Are anxiety and depression related to gastrointestinal symptoms in the general population? Scand J Gastroenterol. 2002;37:294–298. doi: 10.1080/003655202317284192. [DOI] [PubMed] [Google Scholar]
- 37.Seng EK, Holroyd KA. Psychiatric comorbidity and response to preventative therapy in the treatment of severe migraine trial. Cephalalgia. 2012;32:390–400. doi: 10.1177/0333102411436333. [DOI] [PubMed] [Google Scholar]


