Abstract
Background/Aims
An impaired oxidative/antioxidative status plays an important role in the pathogenesis of many diseases, including cancer. The aim of this study was to evaluate the levels of the novel marker ischemia-modified albumin (IMA) and albumin-adjusted IMA (Adj-IMA) in patients with colorectal cancer (CRC) and look for the associations of these with the total antioxidant status (TAS), total oxidant status (TOS), and oxidative stress index (OSI).
Methods
Forty patients with CRC (19 females and 21 males; mean age, 56.5±2.1 years) and 39 age- and sex-matched healthy people (22 females and 17 males; mean age, 56.0±1.7 years) were included in this study. Serum levels of IMA, TAS, and TOS were analyzed, and the OSI was calculated.
Results
Serum IMA, TOS, and OSI levels were significantly higher in patients with CRC than in controls (p<0.0001), whereas TAS levels were significantly lower in CRC patients (p=0.03). There was no significant difference in serum Adj-IMA levels between groups (p=0.32).
Conclusions
In this study, the oxidative/antioxidant status was impaired in favor of oxidative stress in CRC patients. This observation was not confirmed by IMA measurement. Further studies are needed to establish the relationship between IMA and oxidative stress parameters in CRC and other cancers.
Keywords: Colorectal neoplasms, Ischemia-modified albumin, Oxidative stress
INTRODUCTION
Ischemia-modified albumin (IMA) is a new sensitive biomarker of myocardial ischemia, which has a negative predictive value and is highly sensitive.1,2 Acute ischemic conditions induces oxidative stress which alters the N-terminal region of albumin.3 IMA is indicated as a marker of ischemia and oxidative stress originating as a consequence of tissue hypoxia.4-6 However, IMA is influenced by serum albumin concentration. Albumin-adjusted IMA (Adj-IMA) is better correlated than IMA when the patients are in low levels of serum albumin.7
In recent years, different studies have described the role of IMA as a marker for diseases related to inflammation. Several reports proved that ischemia induces a cascade of proinflammatory reactions that lead to the generation of reactive oxygen species (ROS).8,9 There have also been studies describing the relationship between inflammation and cancer. This relationship may include both an extrinsic and an intrinsic pathway. The extrinsic pathway is related to inflammatory circumstances, which increase cancer risk, whereas the intrinsic pathway is maintained by genetic changes that cause inflammation, which may also induce oncogenesis.10,11
Oxidative stress could be involved in the development of many cancers, including colorectal cancer (CRC). There is still a controversy on whether oxidative stress is a causative for tumor development or a phenomenon of the pathophysiology of cancer. Some results have indeed demonstrated that ROS plays a mutagenic role causing DNA damage, suppressing apoptosis, and promoting proliferation, invasiveness and metastasis.12 Prospective evidence for an association between biomarkers of oxidative stress and CRC risk is limited.13 However, IMA levels are higher in very inflammatory and oxidative stress-associated disease.3 Serum IMA levels were found to be increased in patients with gastric, prostate, soft tissue cancer, and neuroblastoma.14-16 There is no published report about serum concentrations of IMA in patients with CRC.
Serum levels of different oxidant species can be measured separately in laboratories. These measurements are time-consuming, expensive to perform, and require complicated equipment. Recently, serum lipid peroxidation levels were observed by determining total oxidant status (TOS).17 Furthermore, total antioxidant status (TAS) is a useful indicator of the activity of antioxidants in serum.18 Therefore, measurements of TAS and TOS may show data about body's overall serum oxidative stress index (OSI), which may include antioxidants and oxidants that are not yet known or easily measured.19
Due to the fact that cancer and oxidative stress belong to an associated event, and based on the fact that albumin may be modified in situations associated with oxidative stress, the aim of this study was to evaluate the levels of the novel marker IMA in patients with CRC as well as its association with TAS, TOS, and OSI.
MATERIALS AND METHODS
1. Subjects
Forty patients (19 females, 21 males; mean age, 56.5±2.1 years) admitted to an outpatient clinic of surgery were prospectively included in the study. Thirty-nine age- and sex-matched healthy control subjects (22 females, 17 males; mean age, 56.0±1.7 years) were also enrolled for comparison.
The patients (newly diagnosed-having no treatment) and healthy volunteers gave informed consent before the study. The following data were recorded for each subject: age, sex, chronic illness, treatments, alcohol consumption, smoking status, family history of gastrointestinal malignancy. All the participants were from the same ethnic group and had comparable socioeconomic status. The patients with CRC, or subjects in the control group with an unstable medical conditions (e.g., cardiovascular, hepatic, renal, or endocrine disorder) within the past 2 years or the ones using any medications that may influence the results (e.g., lipid-lowering agents, antioxidant drugs) were excluded. Final diagnosis of each patient was confirmed by the microscopic evaluation of colonoscopic biopsy samples, followed by total excision of tumors.
Physical examinations were performed. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. This study was performed in accordance with the ethical standards set by the Declaration of Helsinki and was approved by the local ethics committee.
Blood samples were obtained after an overnight of fasting. Serum samples were then separated from cells by centrifugation at 3,000 rpm for 10 minutes. Albumin was analyzed from fresh serum samples. Albumin concentrations were determined with a commercially available kit (Abbott, Abbott Park, IL, USA) based on the bromocresol green method in our routine clinical biochemistry laboratory. Albumin level was expressed as g/dL. Serum portions were stored at -80℃ for analyzing IMA, TOS, and TAS.
2. Analytical methods
1) Measurement of the IMA
Reduced cobalt to albumin-binding capacity (IMA level) was measured using the rapid and colorimetric method developed by Bar-Or et al.20 Briefly, 200 mL of patient serum was transferred into glass tubes and 50 mL of 0.1% CoCl2*6H2O (Sigma-Aldrich Lot: S38901-248; Sigma-Aldrich, St Louis, MO, USA) added. After gentle shaking, the mixture was incubated for 10 minutes to ensure sufficient cobalt albumin binding. Then, 50 mL of 1.5 mg/mL dithiothreitol (DTT) (Sigma-Aldrich Lot: D5545-1G; Sigma-Aldrich) was added as a coloring agent. After 2 minutes, 1 mL of 0.9% NaCl was added to halt the binding between the cobalt and albumin. A blank was prepared for every specimen. At the DTT addition step, 50 mL of distilled water was used instead of 50 mL of 1.5 mg/mL DTT to obtain a blank without DTT. The absorbances were recorded at 470 nm with a spectrophotometer (Shimadzu UV1601; Shimadzu Scientific, Tokyo, Japan). Color formation in specimens with DTT was compared with color formation in the blank tubes, and the results were expressed as absorbance units. The formula suggested by Lippi et al.7 was used for calculation of Adj-IMA levels expressed as individual serum albumin concentration/median albumin concentration of the population × IMA value.
The interassay variability of IMA method in our laboratory was calculated from serum samples of 20 healthy subjects and 20 patients with acute coronary syndrome. The within-day coefficient of variation was 1.23% for healthy subjects (mean, 0.484; standard deviation, 0.006) and 0.92% for acute coronary syndrome (mean, 0.647; standard deviation, 0.006). All serum samples were analyzed within 3 days.
2) Measurement of serum TOS
Serum TOS levels were analyzed by using a novel automated colorimetric measurement method for TOS developed by Erel.17 In this method, oxidants in the sample oxidize the ferrous ion chelator complex to ferric ion which makes a colored complex with a chromogen in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (µmol H2O2 Equiv./L).
3) Measurement of serum TAS
Serum TAS levels were analyzed by using a novel automated colorimetric measurement method for TAS developed by Erel.18 In this method, antioxidants in the sample reduce dark blue-green colored 2, 2'-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical to colorless reduced ABTS form. The change of absorbance at 660 nm is related with total antioxidant level of the sample. This method determines the antioxidative effect of the sample against the potent free radical reactions initiated by the produced hydroxyl radical. The results are expressed as micromolar trolox equivalent per liter.
3. OSI
The percentage ratio of TOS level to TAS level was suggested as OSI.19 For calculation, the resulting micromolar unit of TAS was changed to millimoles per liter, and the OSI value was calculated according to the following formula: OSI (arbitrary unit)=TOS (micromolar hydrogen peroxide equivalent per liter)/TAS (micromolar trolox equivalent per liter).
4. Statistical analysis
Statistical analyses were carried out using the statistical software version 11.5.1.0 (MedCalc, Mariakerke, Belgium). In normally distributed groups, the results were presented with mean±SD, otherwise with medians. The significance of the differences between groups was determined by Student unpaired t-test for normal distributions, and by the Mann-Whitney U test in abnormal distribution. Pearson correlation coefficient and Spearman correlation coefficient were used to test the strength of any associations between different variables. The p-values less than 0.05 was accepted as the significance level.
RESULTS
There was no significant difference in age or male/female ratio between patients and controls (p>0.05). Smokers were more common in CRC patients (35%) than controls (20.5%), but the difference was not significant (p=0.23). BMI was similar in both groups (p=0.43). Nineteen of CRC patients had a family history. Demographic and clinical data obtained from CRC patients and controls are summarized in Table 1.
Table 1.
Demographic and Clinical Data for the Patients with Colorectal Carcinoma and the Controls
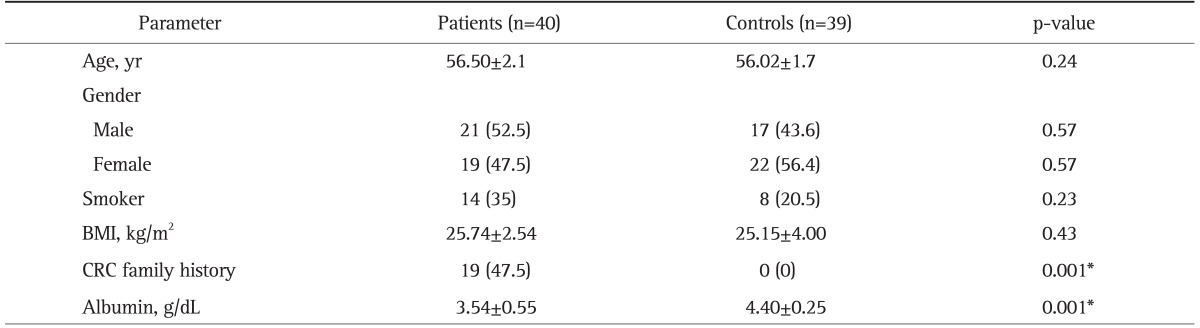
Data are presented as mean±SD or number (%). The parameters were similar, except for CRC family history and albumin levels.
BMI, body mass index; CRC, colorectal carcinoma.
*Statistically significant.
Serum IMA, TOS levels, and OSI index were significantly higher in patients with CRC compared to controls (p<0.0001), whereas TAS were significantly lower in CRC patients (p=0.03). There was no significant difference for serum Adj-IMA levels between groups (p=0.32) (Table 2).
Table 2.
Serum Ischemia-Modified Albumin (IMA), Albumin-Adjusted IMA, Total Antioxidant Status, Total Oxidant Status, and Oxidative Stress Index Levels in Colorectal Carcinoma Patients Compared with Controls
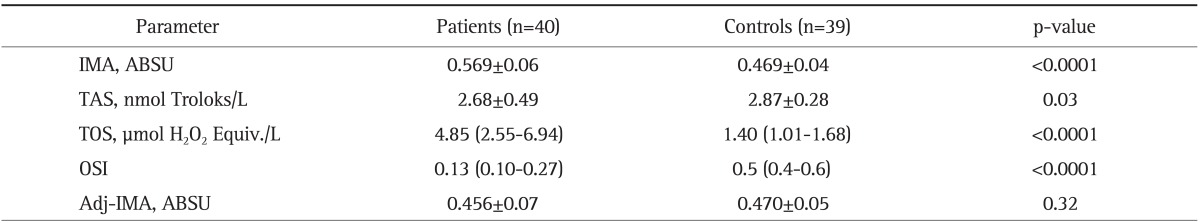
Data are presented as mean±SD or median (95% confidence interval for the median).
ABSU, absorbance unit; TAS, total antioxidant status; TOS, total oxidant status; OSI, oxidative stress index; Adj-IMA, albumin-adjusted IMA.
The final pathological examination of samples were evaluated, and subjects were divided into three groups according to the tumor grades. There were statistically significant differences for serum TOS levels and OSI index (Table 3).
Table 3.
Serum Total Oxidant Status Levels and the Oxidative Stress Index Were Significantly Different according to Grade
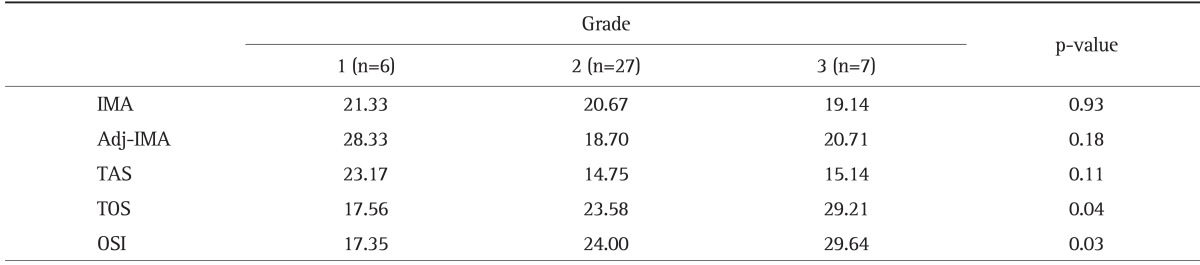
Average rank for the abnormal distribution.
IMA, ischemia-modified albumin; Adj-IMA, albumin-adjusted IMA; TAS, total antioxidant status; TOS, total oxidant status; OSI, oxidative stress index.
There were negative correlations between serum IMA and albumin levels (r=-0.59, p<0.0001), IMA and TAS (r=-0.37, p=0.001), and positive correlations between IMA and TOS (r=0.42, p=0.0004), IMA and OSI (r=0.43, p=0.0001) levels. There was a positive correlation between Adj-IMA and albumin (r=0.54, p<0.0001). There was no statistically significant correlation between Adj-IMA levels and TAS (r=0.05, p=0.61), TOS (r=0.06, p=0.55), OSI (r=0.07, p=0.58) (Table 4).
Table 4.
Relationship between Ischemia-Modified Albumin (IMA) and Albumin-Adjusted IMA and Oxidative Stress Markers (n=79)
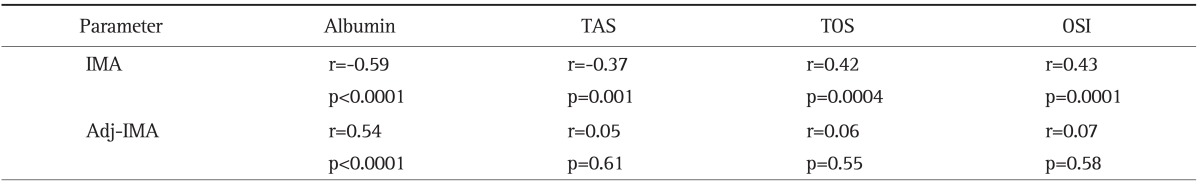
When there was a strong correlation between IMA and oxidative stress parameters, there was no correlation with Adj-IMA.
TAS, total antioxidant status; TOS, total oxidant status; OSI, oxidative stress index; Adj-IMA, albumin-adjusted IMA.
DISCUSSION
Our results showed increased oxidative stress markers (TAS, TOS) and IMA in the patients with CRC compared to healthy controls. Unlike IMA, adj-IMA was similar between CRC and healthy controls. We found significant correlation between IMA and other oxidative markers. Adj-IMA does not seem to be correlated with oxidative stress markers in CRC. Correlation of IMA with oxidative stress markers does not seem to be clinically significant for CRC.
The Albumin Cobalt Binding test or IMA was the first United States Food and Drug Administration cleared assay to detect myocardial ischemia. Condition of myocardial ischemia results in structural changes to the N-terminus of the serum albumin, which reduces its binding capacity for cobalt cations. It has been suggested that these changes are related to the production of ROS during ischemia and/or hypoxia, acidosis, and reperfusion.20 However, increased IMA levels do not seem to depend only on myocardial involvement, thus IMA concentrations may not be specific for cardiac ischemia. There are various studies on IMA in patients with different states with ischemia of noncardiac origin such as systemic sclerosis, peripheral vascular disease, skeletal muscle ischemia, glaucoma, and diabetes mellitus.21-24 Also, serum IMA levels increased in patients with gastric, prostate, soft tissue cancer, and neuroblastoma.14-16
We analyzed adj-IMA levels using a formula suggested by Lippi et al.7 according to individual and median population albumin levels. Serum IMA levels were significantly higher in patients with CRC compared to controls, whereas serum albumin levels were significantly lower. There was no statistically significant difference for serum Alb-Adj IMA levels between groups. Our results were in close agreement with those previously reported. Studies evaluating the relationship between cancer and serum IMA levels are not too many. Fidan et al.14 showed that serum IMA levels were increased in patients with gastric cancer. Mastella et al.15 found increased serum IMA levels in patients with prostate cancer, but this increase was not statistically significant. Stachowicz-Stencel et al.16 found that serum IMA levels were statistically higher in pediatric patients with neuroblastoma and soft tissue sarcomas. IMA levels were inversely related to serum albumin concentrations,25-27 in these studies, which sufficiently disclosed the interrelation between IMA and albumin. The impact of serum albumin on IMA levels is still an important factor even within the normal range.28 Therefore correction for albumin concentrations is essential in populations with wide variations in albumin levels. It has been demonstrated that each 1 g/dL change in albumin within the physiologic range of albumin, produces an opposite change of 2.6% in IMA levels, presenting a negative correlation.26 This proposes the exact need to evaluate IMA values together with those of albumin to avoid possible false-positive or false-negative values in individuals with hypoalbuminemia or hyperalbuminemia. Our results clearly figures out the situation; as statistical analysis showed a strong correlation between oxidative stress markers and unadjusted IMA, but failed to repeat such a relation for adj-IMA.
Cachexia and malnutrition in cancer patients are important problems due to a variety of mechanisms involving the tumor, the host response to the tumor, and anticancer therapies.29 Serum albumin show a simple method of estimating visceral protein function. Malnutrition and inflammation suppress albumin synthesis.30 In an adult, the normal range of serum albumin is determined as 3.5 to 5.0 g/dL and levels <3.5 g/dL is named hypoalbuminemia.31,32 The reverse correlation between body weight index and albumin synthesis in cancer patients supports the likelihood of a compensatory enhanced albumin synthesis in these metabolically influenced patients. In the further stages of disease, malnutrition and inflammation suppress albumin synthesis.33 Depending on these changes in albumin, serum albumin levels should be considered in IMA studies.
Oxidative stress is defined as an imbalance between the production of free radicals and reactive metabolites, known as oxidants or ROS, and the elimination of these radicals and metabolites by protective mechanisms, via agent known as antioxidants.34 Proteins, lipids, and DNA are also significant targets of oxidative stress, and modification of these molecules can increase the risk of mutagenesis.35 Under a sustained environmental stress, ROS are produced over a long time, and thus significant damage may occur to cell functions and structure, which may induce somatic mutations and neoplastic transformation. Essentially, the development of cancers and their progression have been linked to DNA mutations and cell proliferation caused by oxidative stress.36,37 Indeed, In recent years investigators raced to confirm the impaired oxidative/antioxidative balance was the absolute impact in the pathogenesis of many human disease, including the cancer.38
Serum (or plasma) concentrations of different oxidants and antioxidants can be measured separately in laboratories, but these measurements are time-consuming, labor-intensive and costly. As measuring different oxidants and antioxidant molecules separately is not practical, and since the effects of the molecules are additive, in order to acquire a simple understanding of the oxidative stress in patients, it is sufficient to only assess the level of TAS and TOS and to calculate the OSI. As mentioned before, increased production of reactive species decreases TAS while increasing TOS in vivo. In our study, we found that serum TOS levels and OSI index were significantly higher, while serum TAS levels were significantly lower, in patients with CRC compared to the control group. Our results showed increased serum TOS levels and OSI index in patients with high grade CRC. This findings suggest that serum TOS levels and OSI index might be important factors for the prognosis.
In conclusion, increased serum TOS levels and OSI index with decreased serum TAS levels were found in patients with CRC, and these findings strongly favor an impairment of oxidant-antioxidant balance. This observation was not confirmed by IMA measurement. However, our IMA versus adj-IMA results must be considered to be a reminder that unadjusted IMA values might be quite deceptive, and may lead to inappropriate considerations. One major limitation of the study is the small number of samples. Obviously larger studies are needed to establish the relationship of IMA and oxidative stress parameters in CRC and relationship of IMA and other cancers.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Christenson RH, Duh SH, Sanhai WR, et al. Characteristics of an Albumin Cobalt Binding Test for assessment of acute coronary syndrome patients: a multicenter study. Clin Chem. 2001;47:464–470. [PubMed] [Google Scholar]
- 2.Sinha MK, Roy D, Gaze DC, Collinson PO, Kaski JC. Role of "Ischemia modified albumin", a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J. 2004;21:29–34. doi: 10.1136/emj.2003.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy D, Quiles J, Gaze DC, Collinson P, Kaski JC, Baxter GF. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart. 2006;92:113–114. doi: 10.1136/hrt.2004.049643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Montagnana M. Ischemia-modified albumin in ischemic disorders. Ann Thorac Cardiovasc Surg. 2009;15:137. [PubMed] [Google Scholar]
- 5.Seneş M, Kazan N, Coşkun O, Zengi O, Inan L, Yücel D. Oxidative and nitrosative stress in acute ischaemic stroke. Ann Clin Biochem. 2007;44(Pt 1):43–47. doi: 10.1258/000456307779596057. [DOI] [PubMed] [Google Scholar]
- 6.Can M, Demirtas S, Polat O, Yildiz A. Evaluation of effects of ischaemia on the albumin cobalt binding (ACB) assay in patients exposed to trauma. Emerg Med J. 2006;23:537–539. doi: 10.1136/emj.2005.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G, Montagnana M, Salvagno GL, Guidi GC. Standardization of ischemia-modified albumin testing: adjustment for serum albumin. Clin Chem Lab Med. 2007;45:261–262. doi: 10.1515/CCLM.2007.039. [DOI] [PubMed] [Google Scholar]
- 8.Kotur-Stevuljevic J, Memon L, Stefanovic A, et al. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin Biochem. 2007;40:181–187. doi: 10.1016/j.clinbiochem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Vassalle C, Pratali L, Boni C, Mercuri A, Ndreu R. An oxidative stress score as a combined measure of the pro-oxidant and anti-oxidant counterparts in patients with coronary artery disease. Clin Biochem. 2008;41:1162–1167. doi: 10.1016/j.clinbiochem.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz N, Aydin O, Yegin A, Tiltak A, Eren E, Aykal G. Impaired oxidative balance and association of blood glucose, insulin and HOMA-IR index in migraine. Biochem Med (Zagreb) 2011;21:145–151. doi: 10.11613/bm.2011.023. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Leufkens AM, van Duijnhoven FJ, Woudt SH, et al. Biomarkers of oxidative stress and risk of developing colorectal cancer: a cohort-nested case-control study in the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol. 2012;175:653–663. doi: 10.1093/aje/kwr418. [DOI] [PubMed] [Google Scholar]
- 14.Fidan E, Mentese A, Kavgaci H, et al. Increased ischemia-modified albumin levels in patients with gastric cancer. Neoplasma. 2012;59:393–397. doi: 10.4149/neo_2012_051. [DOI] [PubMed] [Google Scholar]
- 15.Mastella AK, Moresco RN, da Silva DB, et al. Evaluation of ischemia-modified albumin in myocardial infarction and prostatic diseases. Biomed Pharmacother. 2009;63:762–766. doi: 10.1016/j.biopha.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Stachowicz-Stencel T, Synakiewicz A, Owczarzak A, et al. Ischemia-modified albumin as a biochemical marker in children with neuroblastoma and soft tissue sarcomas. J Clin Lab Anal. 2011;25:255–258. doi: 10.1002/jcla.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003;133:563–566. doi: 10.4414/smw.2003.10397. [DOI] [PubMed] [Google Scholar]
- 20.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med. 2000;19:311–315. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 21.Montagnana M, Lippi G, Volpe A, et al. Evaluation of cardiac laboratory markers in patients with systemic sclerosis. Clin Biochem. 2006;39:913–917. doi: 10.1016/j.clinbiochem.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Roy D, Quiles J, Sharma R, et al. Ischemia-modified albumin concentrations in patients with peripheral vascular disease and exercise-induced skeletal muscle ischemia. Clin Chem. 2004;50:1656–1660. doi: 10.1373/clinchem.2004.031690. [DOI] [PubMed] [Google Scholar]
- 23.Chang D, Sha Q, Zhang X, et al. The evaluation of the oxidative stress parameters in patients with primary angle-closure glaucoma. PLoS One. 2011;6:e27218. doi: 10.1371/journal.pone.0027218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piwowar A, Knapik-Kordecka M, Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus: preliminary report. Dis Markers. 2008;24:311–317. doi: 10.1155/2008/784313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Refaai MA, Wright RW, Parvin CA, Gronowski AM, Scott MG, Eby CS. Ischemia-modified albumin increases after skeletal muscle ischemia during arthroscopic knee surgery. Clin Chim Acta. 2006;366:264–268. doi: 10.1016/j.cca.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Zapico-Muñiz E, Santaló-Bel M, Mercé-Muntañola J, Montiel JA, Martínez-Rubio A, Ordóñez-Llanos J. Ischemia-modified albumin during skeletal muscle ischemia. Clin Chem. 2004;50:1063–1065. doi: 10.1373/clinchem.2003.027789. [DOI] [PubMed] [Google Scholar]
- 27.Van der Zee PM, Verberne HJ, van Straalen JP, et al. Ischemia-modified albumin measurements in symptom-limited exercise myocardial perfusion scintigraphy reflect serum albumin concentrations but not myocardial ischemia. Clin Chem. 2005;51:1744–1746. doi: 10.1373/clinchem.2005.054635. [DOI] [PubMed] [Google Scholar]
- 28.van Rijn BB, Franx A, Sikkema JM, van Rijn HJ, Bruinse HW, Voorbij HA. Ischemia modified albumin in normal pregnancy and preeclampsia. Hypertens Pregnancy. 2008;27:159–167. doi: 10.1080/10641950701885147. [DOI] [PubMed] [Google Scholar]
- 29.Von Meyenfeldt M. Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs. 2005;9(Suppl 2):S35–S38. doi: 10.1016/j.ejon.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32(6 Suppl 4):S118–S125. doi: 10.1016/s0272-6386(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 31.Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol. 2007;102:2557–2563. doi: 10.1111/j.1572-0241.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 32.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 33.Ballmer PE, Ochsenbein AF, Schütz-Hofmann S. Transcapillary escape rate of albumin positively correlates with plasma albumin concentration in acute but not in chronic inflammatory disease. Metabolism. 1994;43:697–705. doi: 10.1016/0026-0495(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 34.Duracková Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 35.Schraufstätter I, Hyslop PA, Jackson JH, Cochrane CG. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988;82:1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 2009;12:240–245. [PubMed] [Google Scholar]
- 38.Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]


