Abstract
Background/Aims
We investigated the effects of sorafenib monotherapy on advanced hepatocellular carcinoma (HCC) and portal vein tumor thrombosis (PVTT) in a clinical setting.
Methods
In total, 143 consecutive patients with unresectable HCC were treated with sorafenib. Among these patients, 30 patients with advanced HCC and PVTT (Vp3 or 4) were treated with sorafenib monotherapy.
Results
All patients had a performance status of 1 to 2 (Eastern Cooperative Oncology Group 1/2, 20/10) and Child-Pugh class A or B (A/B, 17/13). Eleven patients had modified Union for International Cancer Control stage IVA tumors, whereas 19 had stage IVB tumors. All patients had PVTT (Vp3, 6; Vp4, 24). Following sorafenib monotherapy, three patients (10.0%) had a partial response with PVTT revascularization, and nine (30.0%) had stable disease, with a disease control rate of 33.3%. The median overall survival was 3.1 months (95% confidence interval [CI], 2.70 to 3.50), and the median progression-free survival was 2.0 months (95% CI, 1.96 to 2.05). Fatigue and hand-foot skin reactions were the most troublesome side effects.
Conclusions
A limited proportion of patients with advanced HCC and PVTT exhibited a remarkable outcome after sorafenib monotherapy, although the treatment results in this type of patient is extremely poor. Further studies to predict good responders to personalized therapy are warranted.
Keywords: Carcinoma, hepatocellular; Portal vein; Thrombosis; Sorafenib
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide, behind only lung and stomach cancer.1 Early-stage HCC (solitary or up to three nodules ≤3 cm in size) with preserved liver function can be effectively treated by resection, liver transplantation, or percutaneous ablation with the possibility of a long-term cure, and 5-year survival figures range from 50% to 75%.2 However, only about 30% of patients are indicated for such radical therapies.3 HCC that is diagnosed at an advanced stage has a dismal prognosis due to the underlying liver disease and lack of effective treatment options. Sorafenib is an oral multikinase inhibitor that blocks tumor-cell proliferation and angiogenesis and increases the rate of apoptosis.4 Sorafenib targets the serine-threonine kinases, Raf-1 and B-Raf, the receptor tyrosine kinase of vascular endothelial growth factor (VEGF) receptor,5 and platelet-derived growth factor receptor-β.4,6 Sorafenib is the only efficacious systemic treatment and is well-tolerated in patients with advanced HCC.7
Advanced HCC with tumor thrombosis in the portal vein (Vp3, 4) has an extremely poor prognosis.8-10 In particular, there are few treatment options for advanced HCC with portal vein tumor thrombosis (PVTT). Arterial or systematic infusion of chemotherapeutic agents has been used to eliminate PVTT,11,12 however, results have been disappointing. Radiation is sometimes effective, but the indication is often limited by the extent of the lesion or impaired liver function.13 In the Sorafenib Hepatocellular Carcinoma Assessment Randomised Protocol (SHARP) study7 and multicenter study in Asian-Pacific region,14 sorafenib was shown to be efficacious and well-tolerated in patients with advanced HCC. A subgroup analysis for macroscopic vascular invasions in two studies showed a survival benefit for sorafenib over placebo.7,14
However, the concrete data of sorafenib treatment for patients with advanced HCC and PVTT (Vp3, 4) in clinical field is rare. This study was conducted to assess the effect of sorafenib therapy on patients with advanced HCC and PVTT (Vp3, 4) in clinical practice settings.
MATERIALS AND METHODS
1. Patients
Between May 2008 and May 2011, 143 consecutive patients with advanced HCC were treated with sorafenib at Soonchunhyang University Hospital, Seoul, Korea, Soonchunhyang University Bucheon Hospital, Bucheon, Korea, and Soonchunhyang University Cheonan Hospital, Cheonan, Korea. From that group, 30 patients with PVTT (Vp3, 4) were treated with sorafenib monotherapy. HCC was diagnosed either by histology or from characteristic radiographic findings and elevated serum α-fetoprotein (AFP) levels.15 All patients had advanced HCC that was not suitable for surgical resection, liver transplantation, or nonsurgical interventions including percutaneous ethanol injection, radiofrequency ablation, or transcatheter arterial chemoembolization because of diffuse HCC and major portal vein invasion. Other eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, Child-Pugh class A or B, preserved organ function (serum creatinine level ≤1.5 mg/dL and alanine aminotransferase ≤5 times the institutional upper limit of normal), acceptable blood cell counts (absolute neutrophil count of ≥1,500 cells/mm3, platelet count ≥75,000 cells/mm3, hemoglobin ≥10 g/dL), and at least one unidimensionally measurable lesion. Patients were ineligible if they had other primary malignancies, had any other concurrent serious medical condition(s), or had undergone systemic chemotherapy previously. This study was carried out according to the principles set out in the Declaration of Helsinki 1964 and was approved by the Ethics Review Boards of Soonchunhyang University Hospital, Soonchunhyang University Bucheon Hospital, and Soonchunhyang University Cheonan Hospital.
2. Treatment
An initial dose of 400 mg sorafenib was administered twice daily. The dose was reduced to 400 mg/day (200 mg twice daily) if there were drug-related grade 3/4 toxicities or at the discretion of the treating physician until recovery from the adverse effects. Although the dose reduction was continued if symptoms did not improve, sorafenib was discontinued until the patient recovered from the adverse event. Dose modifications for a hand-foot skin reaction were based on prescribing information and 2008 consensus panel recommendations.16 The side effects of sorafenib were determined using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 3.0. Treatment continued until disease progression or intolerable toxicities appeared, or until a patient refused further treatment.
3. Assessments
Dynamic spiral computed tomography (CT) or contrast-enhanced dynamic magnetic resonance imaging (MRI) and determination of AFP level were performed at baseline. The response to treatment was evaluated every 2 months by means of a CT or earlier with clinical signs of progression. Tumor response was assessed until disease progression. The tumor response was evaluated according to the modified Response Evaluation Criteria in Solid Tumors.17 Patients who died before their first radiographic evaluation were assessed as having progressive disease. Malignant portal vein thrombosis (PVT) in HCC was defined as expansion of the thrombosis within the involved portal vein and enhancement of the thrombus itself on contrast-enhanced CT or MRI.18,19
4. Treatment outcome and statistical analysis
The primary study objective was to assess overall survival (OS). Secondary objectives were to evaluate overall response rate, progression-free survival (PFS), and toxicity. OS was defined as the time interval between the initiation of sorafenib and death. PFS was defined as the time interval from the first cycle of sorafenib to the date when disease progression or any cause of death was first observed. Survival curves were calculated by the Kaplan-Meier method and compared with the log-rank test. A Cox-regression hazard model was used for multivariate Cox proportional analysis. A p<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software package version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Patients
There were 21 male patients and nine female patients with a mean age of 58 years (range, 41 to 84 years). The most common cause of liver disease was hepatitis B virus (80.0%). The ECOG performance status score was 1 (n=20), or 2 (n=10). All patients had Barcelona Clinic Liver Cancer stage C tumors. According to the modified Union for International Cancer Control (UICC) TNM staging system,20 19 patients (63.3%) had stage IVB cancers. All patients had Vp3 or Vp4 PVTT. Vp3 invasion occurred in six patients, and Vp4 invasion occurred in 24 patients. Nineteen patients (63.3%) showed extrahepatic involvement (i.e., nodal invasion and/or distant metastases). Distant metastases were most frequently observed in the lungs (n=14, 46.7%), followed by the lymph nodes (n=9, 30.0%), bone (n=3, 10.0%), and adrenal glands (n=1, 3.3%). Nine patients (30.0%) had peritoneal lymph node metastases, one patient had a right supraclavicular lymph node metastasis, and one had a thoracoabdominal lymph node metastasis. Half of patients (n=15) presented with tumors that had not been previously treated, and locoregional therapy had failed in the remaining 15 patients (Table 1).
Table 1.
Baseline Characteristics (n=30)
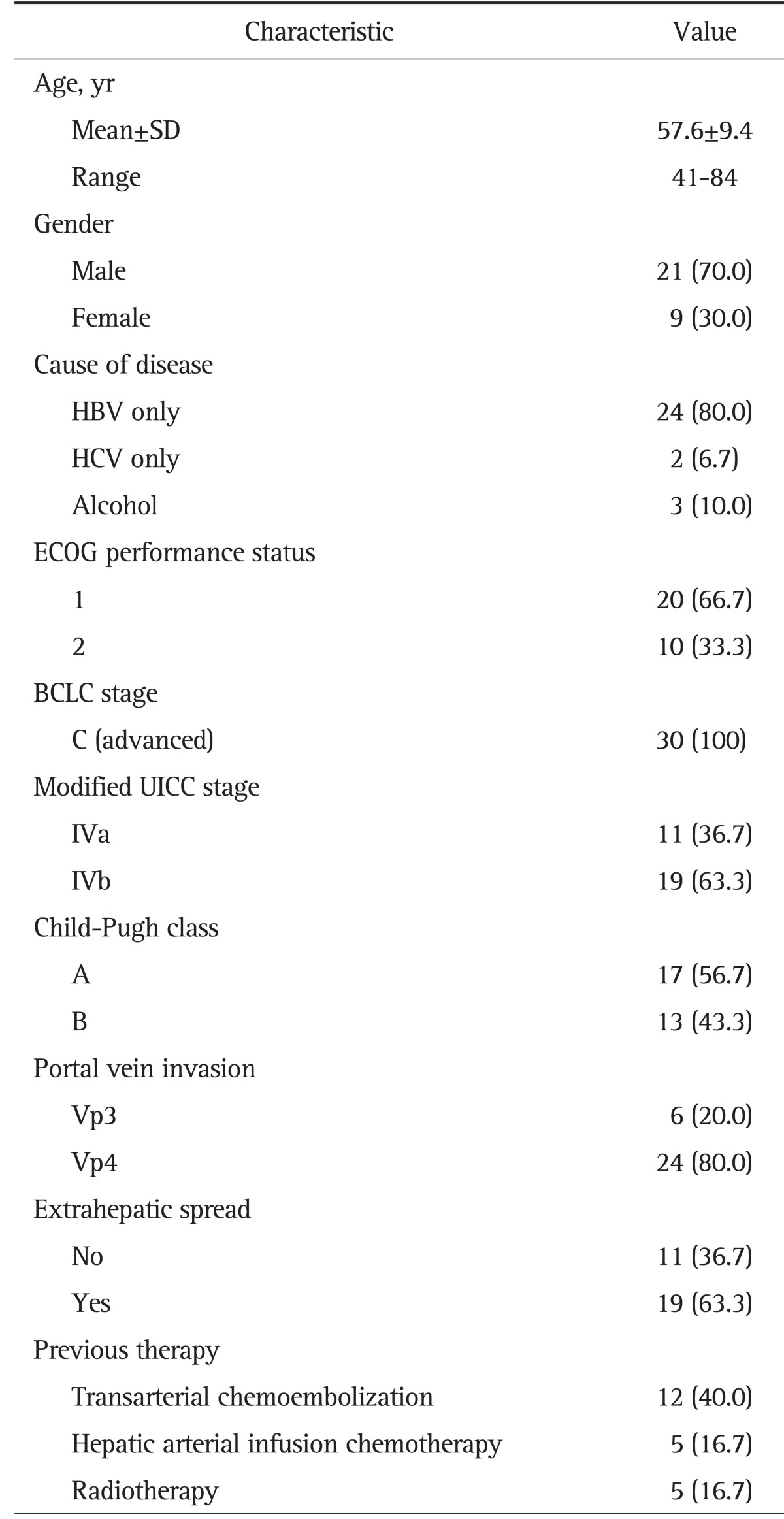
Data are presented as number (%).
SD, standard deviation; HBV, hepatitis B virus; HCV, hepatitis C virus; ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer; UICC, Union for International Cancer Control.
2. Efficacy and survival
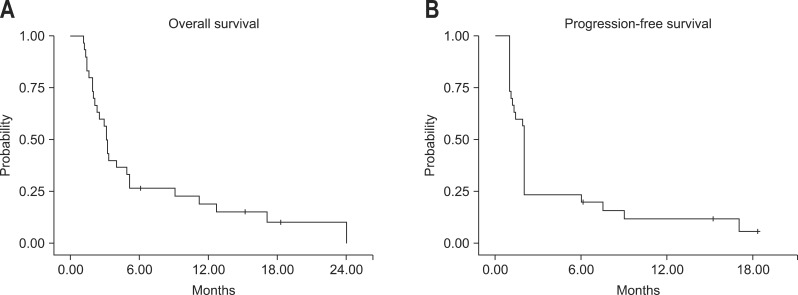
Median duration of sorafenib therapy was 5.1 weeks. Those of Child A and Child B patients were 61 and 28 days, respectively. Twenty-seven patients (90.0%) died at the last follow-up (hepatic failure, 59.3%; hepatorenal syndrome, 22.2%; respiratory failure, 7.4%; variceal hemorrhage, 3.7%; pneumonia, 3.7%; and hemorrhagic brain metastasis, 3.7%). The median OS duration of the patients was 3.1 months (95% confidence interval [CI], 2.70 to 3.50) and median PFS was 2 months (95% CI, 1.96 to 2.05) (Fig. 1).
Fig. 1.
Kaplan-Meier analysis of overall survival and time to progression. (A) The median overall survival was 3.1 months. (B) The median time to progression was 2 months.
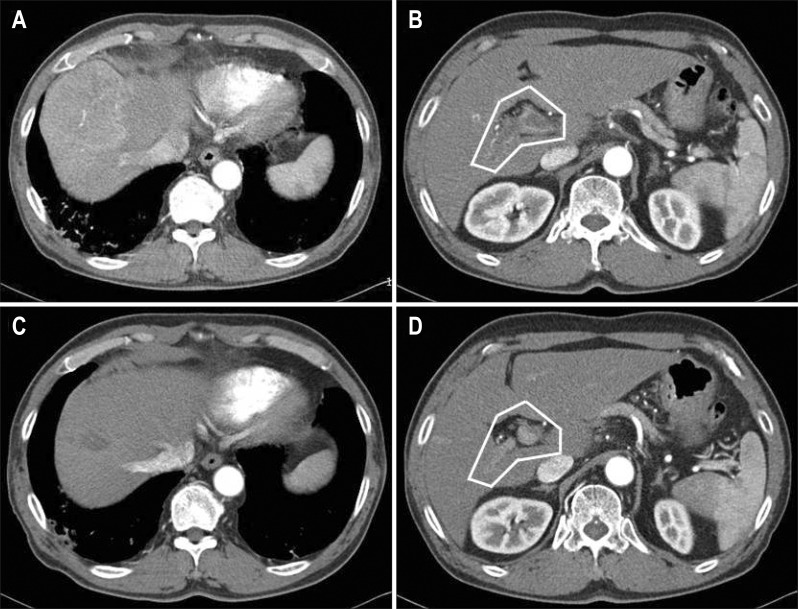
Three patients (10.0%) had a partial response (PR) and nine (30.0%) had stable disease (SD), making the disease control rate (DCR) 33.3% (Table 2). Three patients with a PR achieved marked PVTT revascularization after 4 months of sorafenib monotherapy (Fig. 2). In the efficacy and survival according to previous therapy, median OS was 4.0 months (95% CI, 1.48 to 6.53) for patients with previous therapy and 3.1 months (95% CI, 2.11 to 4.09) for naïve patients (p=0.663). Median PFS was 2.0 months (95% CI, 1.94 to 2.06) versus 1.4 months (95% CI, 0.83 to 1.97) for patients with previous therapy and naïve patients, respectively (p=0.570). DCR for both group were 33.3% (p=0.650). The presence of previous therapy before sorafenib did not show significance in efficacy and survival.
Table 2.
Summary of Efficacy
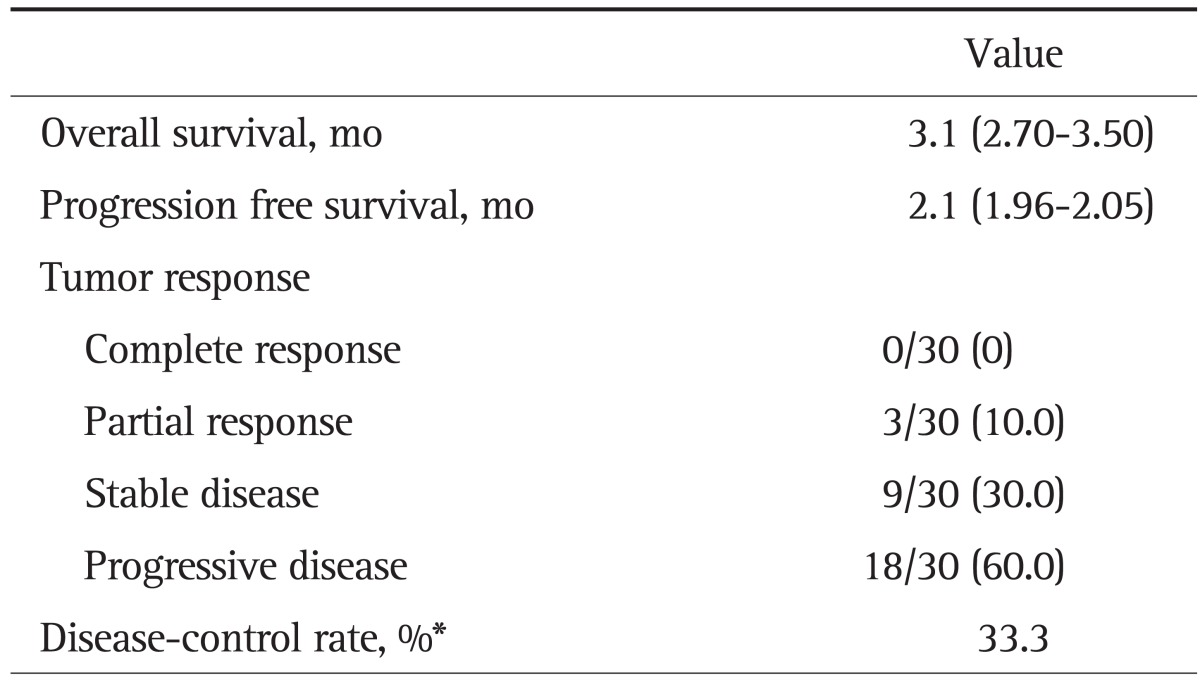
Data are presented as median (95% CI) or number (%).
CI, confidence interval.
*The proportion of patients who had a best response rating of a complete response, partial response, or stable disease that was maintained ≥4 weeks from the first manifestation of that rating.
Fig. 2.
Decreased hepatocellular carcinoma and portal vein tumor thrombosis (PVTT) revascularization after sorafenib monotherapy. (A, B) Before treatment. (C, D) Four months after sorafenib monotherapy. (A) A 7.0×6.9-cm-sized well-enhanced mass in the hepatic dome. (B) The white line indicates enhanced and expanded PVTT in the main and right portal veins. (C) Markedly decreased size and enhancement of the mass. (D) The white line indicates the markedly decreased size and enhancement of PVTT.
By univariate and multivariate analysis, a longer median OS was achieved by those with good liver function (Child-Pugh A; p=0.040), AFP <400 ng/mL (p=0.005), and tumor response (PR or SD; p=0.032) (Table 3).
Table 3.
Univariate and Multivariate Analyses for Overall Survival
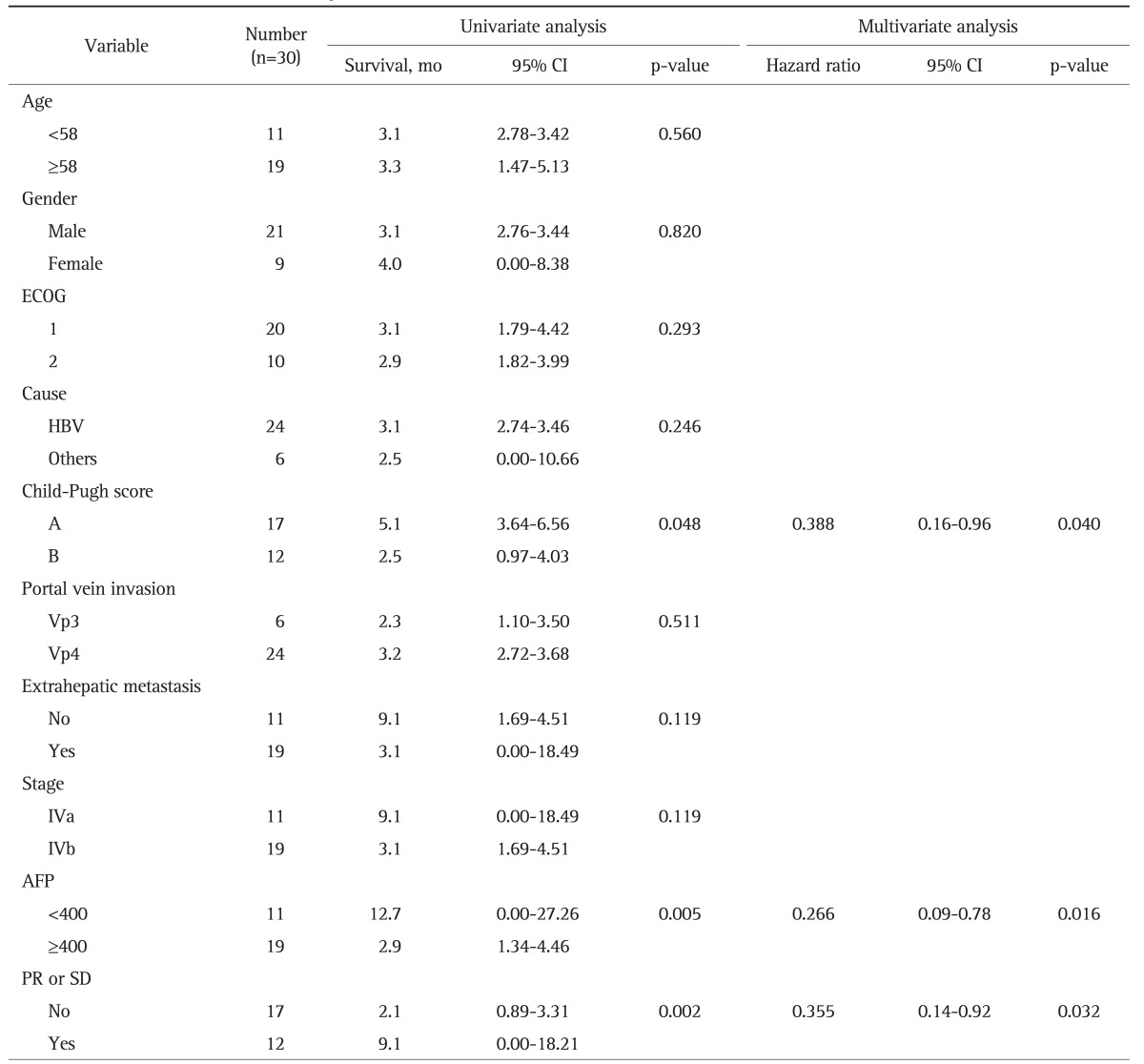
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; AFP, α-fetoprotein; PR, partial response; SD, stable disease.
3. Toxicity
The overall incidence of treatment-related adverse events was 90.0%. The reported adverse events were predominantly grades 1 or 2 in severity and constitutional, dermatological, or gastrointestinal in nature. Nine patients (30%) of total patients showed several adverse events together. Fatigue and hand-foot skin reactions were the most troublesome side effects, occurring in 13 (43.3%) and nine patients (30.0%), respectively. Other frequent toxicities were diarrhea (n=6, 20.0%), anorexia (n=6, 20.0%), rash or desquamation of the skin (n=3, 10.0%), and nausea (n=2, 6.7%). Esophageal variceal bleeding and liver dysfunction occurred in one patient (3.0%) each. Grade 3 toxicities included fatigue (n=3, 10.0%), hand-foot skin reaction (n=1, 3.3%), and liver dysfunction (n=1, 3.3%, severe hyperbilirubinemia). Grade 4 toxicities were not observed. The incidence of adverse events of Child A and B patients were 16 patients (94.1%), and 11 patients (84.6%), respectively. The incidence of adverse events in Child A and B patients were as follows: fatigue (7 and 6), hand-foot skin reaction (7 and 2), diarrhea (5 and 1), anorexia (2 and 4), rash or desquamation (3 and 0), nausea (0 and 2), esophageal variceal bleeding (0 and 1), severe hyperbilirubinema (0 and 1), respectively. The percentage of hand-foot skin reaction, diarrhea, rash or desquamation of Child A patients were higher than Child B because of long duration of treatment. The percentage of fatigue, anorexia, nausea, variceal bleeding, and severe hyperbilirubinemia were high in Child B patients.
Twenty-one patients (70.0%) had to reduce their daily sorafenib dose. Thirteen patients required a dose reduction due to fatigue, five from hand-foot syndrome, five from diarrhea, two from nausea, one from skin rash, and one from skin desquamation. The rate of discontinuation of sorafenib due to adverse events was 36.7% (n=11). The most frequent adverse events leading to discontinuation were fatigue (n=6, 20.0%), nausea (n=2, 6.7%), hand-foot skin reaction (n=1, 3.3%), esophageal variceal bleeding (n=1, 3.3%), and liver dysfunction (n=1, 3.3%) (Table 4). Besides adverse events, sixteen patients stopped the sorafenib by disease progression.
Table 4.
Incidence of Adverse Events
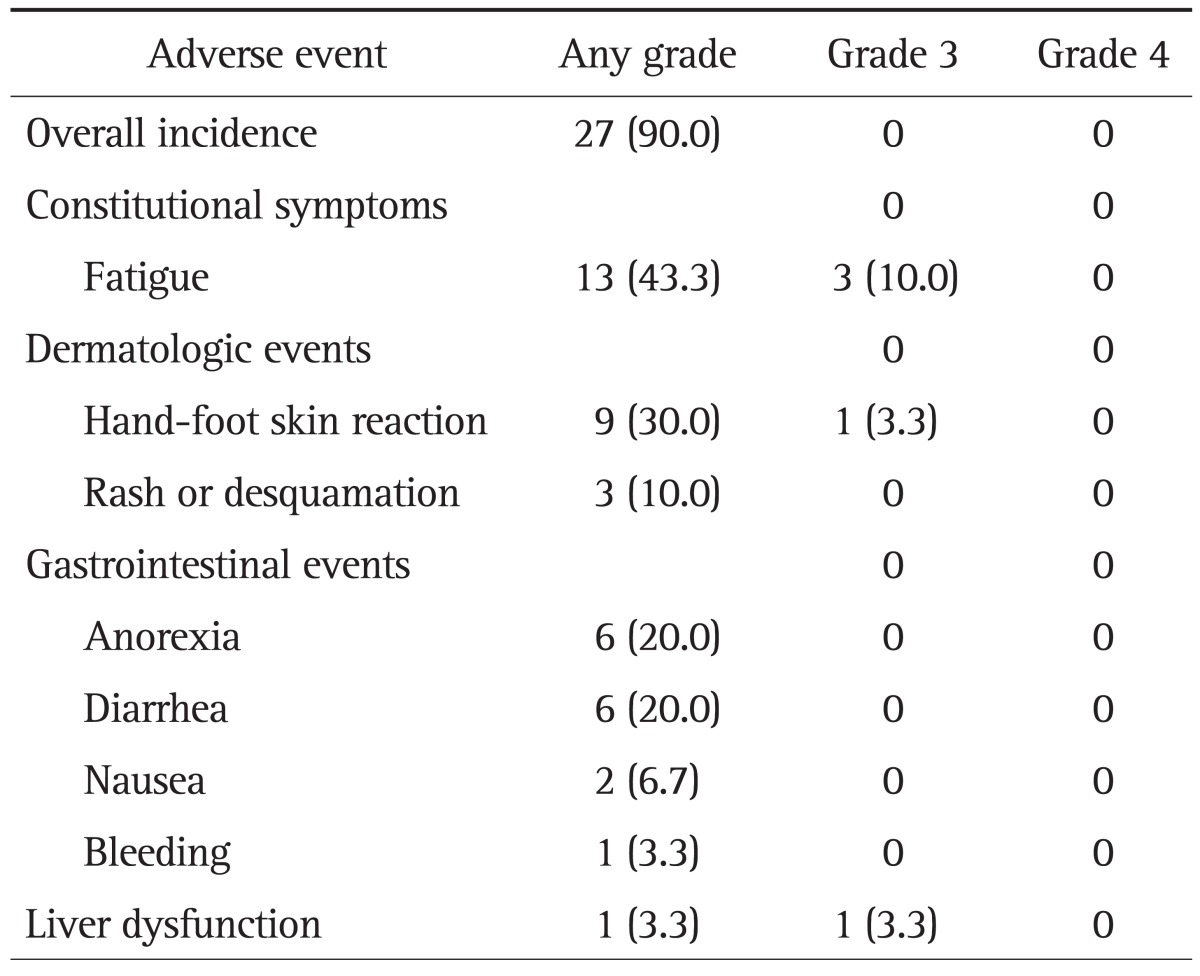
Data are presented as number (%).
DISCUSSION
The aim of this study was to analyze the effect of sorafenib monotherapy in patients with advanced HCC and PVTT. Although sorafenib has been recommended for patients with HCC and PVTT, the clinical data of sorafenib in those patients was rare. In this study, 10% of patients showed a PR and 30% of patients showed SD, making the DCR 33.3%. The median OS duration was 3.1 months, and the median PFS was 2 months. Ten percent of patients showed PR with PVTT revascularization, and responsive patients showed a significant prolongation in OS compared with that in nonresponsive patients. Moreover, marked regression of PVTT was noted between 3 and 4 months after sorafenib monotherapy in the three responsive patients. The mechanisms underlying the antithrombotic effect of sorafenib have not been clarified. However, VEGF may play a pivotal role both in HCC angiogenesis and malignant PVT onset and evolution.21 Sorafenib may exert a beneficial effect on malignant PVT by inhibiting the VEGF pathway. The PVTT revascularization by sorafenib is a remarkable finding, because there is no definite cure for advanced HCC with PVTT.
SD and DCR in this study were lower than SHARP trial (SD, 71%; DCR, 43%) and Asia-Pacific population study (SD, 54%; DCR, 35.3%), although the evaluation methods were different (modified RECIST vs RECIST). Because the percentage of patients with Child-Pugh A in this study was 56.7% lower than SHARP trial (Child-Pugh A, 95%) and Asia-Pacific population study (Child-Pugh A, 97.3%). Also, all patients in this study had advanced HCC with Vp3 or Vp4 PVTT comparing with SHARP trial (macroscopic vascular invasion, 36%) and Asia-Pacific population study (macroscopic vascular invasion, 36%). The low SD and DCR may reflect the abysmal prognosis of patients in this study rather than lack of effect of the sorafenib. SHARP trial showed a significant effect with a DCR of 43% in the sorafenib group and 32% in the placebo group. Therefore, deaths related to advanced liver disease might have masked any significant activity of sorafenib in this study. In SHARP trial and Asia-Pacific trial, a subgroup analysis for macroscopic vascular invasions showed a survival benefit for sorafenib over placebo.
The benefit of sorafenib was also consistent among all prespecified stratification groups, including patients with the worst prognosis, such as those with an ECOG performance status of 1 or 2 or with macroscopic vascular invasion or extrahepatic spread.
We also investigated whether the previous treatment may affect the treatment outcome of sorafenib monotherapy. The percentage of patients with previous treatments was 50% in this study and it was similar to over half of SHARP trial. The relation with previous treatment showed no significance in efficacy and survival of sorafenib treatment like SHARP trial.
In this study, we also analyzed prognostic factors impacting survival. Child-Pugh A, AFP <400 ng/mL, and tumor response (PR or SD) were favorable parameters relative to PFS and OS; these findings are similar to those in other reports.22-24 The adverse events in this study were similar to those in previous clinical trials,7,14 with dermatological and gastrointestinal symptoms being common adverse events. However, the most prominent adverse event in this study was fatigue and general weakness. Thirteen patients required a dose reduction because of fatigue, and it was the most frequent adverse event leading to sorafenib discontinuation. The high percentage of discontinuation due to fatigue and general weakness might have come from the advanced HCC and the patient's weakened condition.
Besides the clinical results of advanced HCC and PVTT of sorafenib, we can induce three important points in this study. The first is the chemosensitivity of sorafenib, the second is the resistance, and the final point is individualized therapy. HCC is a complex and heterogeneous disease at the molecular level, and different pathways can be aberrantly activated in distinct patient subgroups. With such heterogeneity of pathway activation, an individualized approach based on detecting predictive markers for sensitivity and resistance will improve treatment efficacy. Zhang et al.25 reported that phosphorylated extracellular signal-regulated kinase could be a useful biomarker predicting sensitivity to sorafenib in HCC tumor cells in an in vitro study. Recently, sorafenib potentiated irradiation effect in HCC in vitro and in vivo.26 Sequential treatment with radiation followed by sorafenib had the greatest in vivo antitumor effect.27 Bhoori et al.28 also showed a personalized approach to molecular targeted therapy for an advanced recurrent HCC in the clinical transplant setting using a combination of sorafenib and the mammalian target of rapamycin inhibitor everolimus, although data were limited to a single case. However, there are no clear predictive factors, and limited data are available on resistance. Hoshida et al.29 suggested that a new class of genomic information, microRNA dysregulation and epigenetic alterations, will provide insight for more precise understanding of HCC mechanism and expand the opportunity of biomarker/therapeutic target discovery. Further study to investigate predictive factors and resistance to sorafenib for personalized therapy is warranted. This study has several limitations, including the small number of patients, retrospective design and a single arm study without a control group. Most patients had advanced HCC with Vp3 or Vp4 PVTT and/or extrahepatic metastases, so they could not maintain long-term sorafenib treatment. Therefore, a per-protocol evaluation in an adequate number of assessable patients could not be achieved and treatment duration was too short to assess the efficacy of sorafenib. Further study with a large number of patients treated with long duration will be necessary to assess the efficacy.
In conclusion, sorafenib treatment showed that 10% of patients with advanced HCC and PVTT showed remarkable outcomes with PR and PVT revascularization, although the treatment results of this type of patients is extremely poor. A sensitivity investigation to predict good responders for personalized therapy is warranted.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 6.Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561–574. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Yamakado K, Tanaka N, Nakatsuka A, Matsumura K, Takase K, Takeda K. Clinical efficacy of portal vein stent placement in patients with hepatocellular carcinoma invading the main portal vein. J Hepatol. 1999;30:660–668. doi: 10.1016/s0168-8278(99)80197-4. [DOI] [PubMed] [Google Scholar]
- 9.The Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 10.Nihon Kangan Kenkyukai. The general rules for the clinical and pathological study of primary liver cancer. 5th ed. Tokyo: Kanehara Shuppan; 2008. [Google Scholar]
- 11.Ando E, Yamashita F, Tanaka M, Tanikawa K. A novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal vein. Cancer. 1997;79:1890–1896. doi: 10.1002/(sici)1097-0142(19970515)79:10<1890::aid-cncr8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Iwamiya T, Sawada S, Ohta Y. Repeated arterial infusion chemotherapy for inoperable hepatocellular carcinoma using an implantable drug delivery system. Cancer Chemother Pharmacol. 1994;33(Suppl):S134–S138. doi: 10.1007/BF00686685. [DOI] [PubMed] [Google Scholar]
- 13.Chen SC, Lian SL, Chang WY. The effect of external radiotherapy in treatment of portal vein invasion in hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33(Suppl):S124–S127. doi: 10.1007/BF00686683. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 15.Park JW Korean Liver Cancer Study Group and National Cancer Center. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004;10:88–98. [PubMed] [Google Scholar]
- 16.Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 17.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah ZK, McKernan MG, Hahn PF, Sahani DV. Enhancing and expansile portal vein thrombosis: value in the diagnosis of hepatocellular carcinoma in patients with multiple hepatic lesions. AJR Am J Roentgenol. 2007;188:1320–1323. doi: 10.2214/AJR.06.0134. [DOI] [PubMed] [Google Scholar]
- 19.Rossi S, Ghittoni G, Ravetta V, et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur Radiol. 2008;18:1749–1756. doi: 10.1007/s00330-008-0931-z. [DOI] [PubMed] [Google Scholar]
- 20.Ueno S, Tanabe G, Nuruki K, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395–403. doi: 10.1016/s1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Xu B, Fu L, Hao XS. Correlation of four vascular specific growth factors with carcinogenesis and portal vein tumor thrombus formation in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2006;25:403–409. [PubMed] [Google Scholar]
- 22.Leung TW, Tang AM, Zee B, et al. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer. 2002;94:421–427. doi: 10.1002/cncr.10236. [DOI] [PubMed] [Google Scholar]
- 23.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217–2222. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2217::AID-CNCR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist. 2009;14:717–725. doi: 10.1634/theoncologist.2009-0038. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Zhou X, Shen H, Wang D, Wang Y. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Med. 2009;7:41. doi: 10.1186/1741-7015-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu W, Gu K, Yu Z, et al. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2013;329:109–117. doi: 10.1016/j.canlet.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Plastaras JP, Kim SH, Liu YY, et al. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007;67:9443–9454. doi: 10.1158/0008-5472.CAN-07-1473. [DOI] [PubMed] [Google Scholar]
- 28.Bhoori S, Toffanin S, Sposito C, et al. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010;52:771–775. doi: 10.1016/j.jhep.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis. 2010;30:35–51. doi: 10.1055/s-0030-1247131. [DOI] [PMC free article] [PubMed] [Google Scholar]