Abstract
Background/Aims
We investigated the efficacy of continuous long-term entecavir 0.5 mg treatment in naïve chronic hepatitis B patients showing a partial virologic response (PVR).
Methods
A total of 227 patients were included. PVR was defined as a more than 1 log10 IU/mL decline in detectable serum hepatitis B virus (HBV) DNA by polymerase chain reaction (PCR; ≥20 IU/mL) at week 48. A complete virologic response (CVR) was defined as undetectable serum HBV DNA by PCR (<20 IU/mL) at week 48.
Results
At week 48, the rate of the PVR was 64/227 (28.2%). Among patients with PVR, the cumulative rates of virologic response (serum HBV DNA <20 IU/mL) at weeks 96 and 144 were 45.2% and 73.8%, respectively. The cumulative rates of genotypic resistance were not significantly different between patients with a PVR and patients with a CVR (p=0.057). However, the cumulative rates of virologic breakthrough were higher in patients with PVR than in patients with CVR (4% vs 0% and 11.2% vs 0% at weeks 96 and 144, respectively; p<0.001).
Conclusions
Long-term continuous entecavir 0.5 mg treatment in patients with a PVR resulted in an additional virologic response without a significant increase in genotypic resistance. However, the rate of virologic breakthrough was higher in the partial responders.
Keywords: Hepatitis B, Chronic, Entecavir, Partial, Response
INTRODUCTION
The primary goal of antiviral therapy in chronic hepatitis B (CHB) patients is long-term suppression of hepatitis B virus (HBV) replication.1,2 The emergence of antiviral resistance and elevated viral load during long-term antiviral therapy are associated with increased risk of adverse liver outcomes, including progress to cirrhosis, hepatocellular carcinoma, and death.3 The importance of early viral suppression to reduce the risk of drug resistance to nucleos(t)ide analogues (NAs) has been addressed in several studies.4-6 Recently the 2012 European Association for the Study of the Liver (EASL) HBV Clinical Practice Guidelines defined partial virologic response (PVR) as a decrease in HBV DNA of more than 1 log10 IU/mL but still detectable after at least 6 months of therapy.1 Indeed, in patients with a low genetic barrier to resistance who are receiving NAs such as lamivudine (LAM) or telbivudine (LdT), or a moderately potent NA such as adefovir (ADV), PVR is associated with a well-defined risk for subsequent development of antiviral resistance.4-6 Therefore, early treatment adaptation with switching to more potent NAs should be considered in patients showing PVR to LAM, LdT or ADV.1,2
Entecavir (ETV) is a cyclopentyl guanosine analogue that has shown superior biochemical, virological, and histological efficacy compared with LAM in large phase III trials.7,8 Moreover, because of its potent antiviral suppression and a high genetic barrier to resistance, emergence of resistance to ETV is rare, with a 1.2% of cumulative probability over 6 years.9,10 In clinical trials for treatment-naïve patients on ETV monotherapy, 10% of hepatitis B e antigen (HBeAg)-negative patients and 33% of HBeAg-positive patients showed a PVR at week 48.7,8 However, there were limited data for the virologic response to long-term continuous ETV therapy in patients with a PVR. Furthermore, there is no clear evidence that patients showing PVR are likely to develop resistance in the subsequent years during extended ETV therapy. Thus, it is debatable whether treatment adaptation needs to be recommended for patients with PVR. The aim of this study was to evaluate the prevalence and factors associated with PVR in naïve-CHB patients receiving ETV and to investigate the virologic response and antiviral resistance of long-term continuous ETV therapy in patients with PVR.
MATERIALS AND METHODS
1. Patients
This retrospective study included naïve CHB patients who were treated with 0.5 mg ETV daily for more than 48 weeks between March 2007 and June 2011. Patients were eligible if they were 18 to 70 years of age and had CHB or compensated liver cirrhosis. CHB was defined as a detectable serum hepatitis B surface antigen (HBsAg) level for more than 6 months, serum HBV DNA level ≥20,000 IU/mL for HBeAg-positive or ≥2,000 IU/mL for HBeAg-negative patients, and elevated serum alanine aminotransferase (ALT) levels. Patients with a history of previous NAs treatment, antibodies against hepatitis C virus, or human immunodeficiency virus, or those with hepatic decompensation associated with jaundice, ascites, encephalopathy, or gastrointestinal bleeding were excluded. Additional exclusion criteria included a history of liver transplantation and hepatocellular carcinoma. During the study period, 408 patients took ETV for at least 48 weeks at Chonbuk National University Hospital. After excluding 137 patients who had been previously treated with NAs and 44 patients who had not met inclusion or exclusion criteria, 227 patients were included in the analysis. This study was conducted in compliance with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee at our institution.
2. Evaluation of treatment efficacy and definitions
Serum HBV DNA was quantified by real-time polymerase chain reaction (PCR) assay using the COBAS Taq-Man HBV quantitative test (Roche Molecular Systems Inc., Branchburg, NJ, USA), which had a lower limit of quantification of 20 IU/mL. Serum ALT was measured with an enzymatic assay. Serum HBsAg, antibodies to HBsAg, HBeAg, and antibodies to HBeAg were detected by electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany).
Mean reductions of serum HBV DNA levels from baseline, virologic response, serum ALT normalization, HBeAg loss or seroconversion, virologic breakthrough, and genotypic resistance were analyzed at week 48. Complete virologic response (CVR) was defined as undetectable serum HBV DNA by PCR (<20 IU/mL) at week 48.2 PVR was defined as more than 1 log10 IU/mL decline of viremia from baseline but detectable serum HBV DNA by PCR (≥20 IU/mL) at week 48.1 Cumulative rates of virological response, virologic breakthrough, and genotypic resistance during the treatment period were compared between patients with CVR and those with PVR. A virologic response was defined as undetectable DNA by real-time PCR assay (<20 IU/mL) during the treatment period.2 Normalization of serum ALT was defined as a serum ALT level <40 IU/L. Primary nonresponse was defined as a decrease in serum HBV DNA of less than 2 log10 IU/mL after at least 24 weeks of therapy.2 Virologic breakthrough was defined as an increase in the serum HBV DNA level of more than 1 log10 IU/mL from the nadir during continued treatment.2 In those cases, restriction fragment mass polymorphism (RFMP) technology was used to analyze the drug resistance mutation and genotypic resistance.11
3. Statistical analysis
Results are reported as means±SD. HBV DNA levels were logarithmically transformed for analysis. Continuous variables were compared using the 2-tailed Student t-test. Categorical data were analyzed using the chi-square test or Fisher exact test. Mean reductions of serum HBV DNA levels from baseline were compared by repeated measures analysis of variance. Cumulative rates for virologic response, virologic breakthrough, and genotypic resistance were evaluated by Kaplan-Meier analysis. Factors associated with development of PVR were analyzed by univariate and multivariate log-rank analysis. The p-values of <0.05 were considered statistically significant. Data was collected in Microsoft EXCEL (Microsoft Excel 2007; Microsoft Corp., Seattle, WA, USA) and analyzed using SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics of study patients
The mean age of the 227 patients included in the study was 48.5 years (Table 1). There were 152 men (67%) and 64 patients (28.2%) with cirrhosis. The median baseline serum ALT level was 115 IU/L and the serum HBV DNA level was 6.3 log10 IU/mL. The median treatment duration was 23.4 months (range, 12 to 54 months). Of the total 227 patients, 147 patients (64.7%) were HBeAg-positive and 80 patients (35.3%) were HBeAg-negative. HBeAg-positive patients had higher baseline serum HBV DNA levels compared to HBeAg-negative patients (6.5±1.5 log10 IU/mL vs 6.0±0.9 log10 IU/mL; p=0.001), but other baseline characteristics were not significantly different.
Table 1.
Baseline Characteristics of Study Subjects
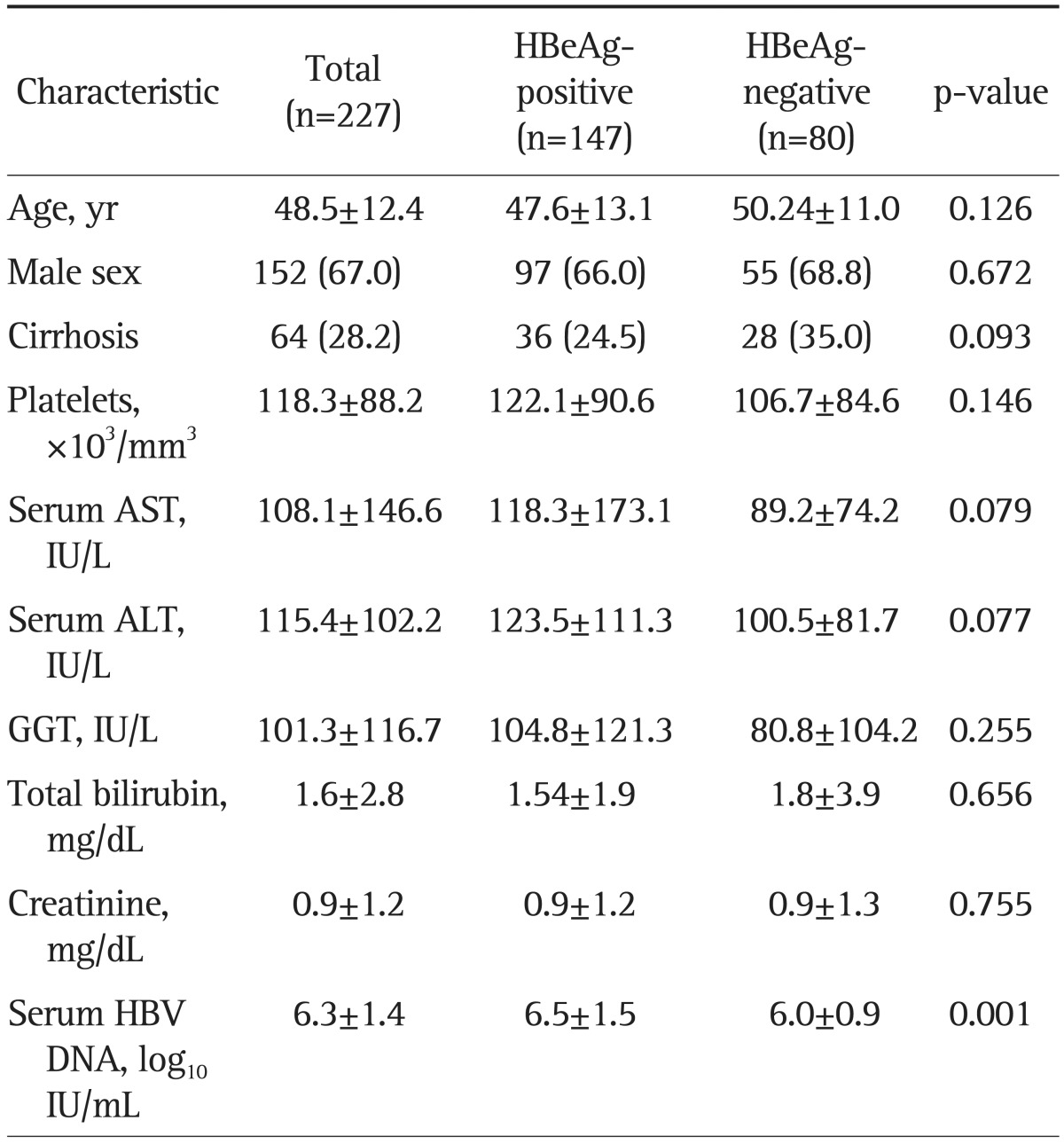
Data are presented as mean±SD or number (%).
HBeAg, hepatitis B e antigen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ glutamyl transpeptidase; HBV, hepatitis B virus.
2. PVR and treatment outcomes at week 48
Of the 227 patients, 163 patients (71.8%) had CVR, but 64 (28.2%) had PVR at week 48 (Table 2). HBeAg-positive patients had a significantly lower rate of CVR compared to HBeAg-negative patients (61.2% vs 91.3%; p<0.001), but a significantly higher rate of PVR (38.8% vs 8.8%; p<0.001). Overall mean reductions in serum HBV DNA levels from baseline were -4.7 log10 IU/mL at week 48. HBeAg-positive patients showed significantly greater reductions in serum HBV DNA levels from baseline compared to HBeAg-negative patients (-5.1 log10 IU/mL vs -4.1 log10 IU/mL; p<0.001). The rate of serum ALT normalization was 81.5% at week 48 and it was not significantly different between HBeAg-positive and -negative patients (83.0% vs 78.8%; p=0.338). Among 147 HBeAg-positive patients, 37 patients (25%) showed HBeAg loss or HBeAg seroconversion at week 48. Two patients showed virologic breakthrough during the 48 week therapy. However, two patients with virologic breakthrough were associated with poor medical adherence and there was no genotypic resistance to ETV by RFMP analysis.
Table 2.
Treatment Outcomes of 48-Week Entecavir Therapy
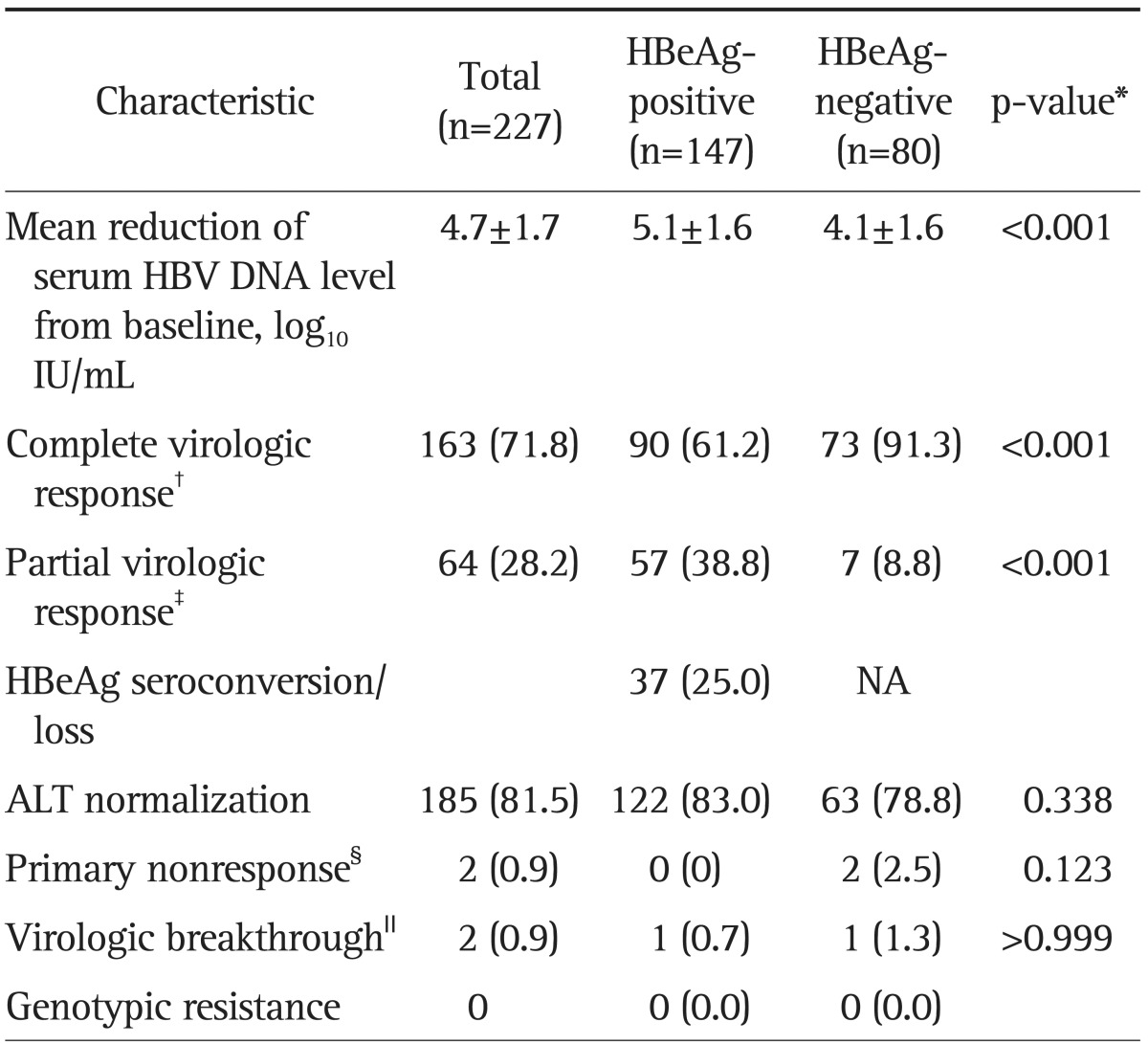
Data are presented as mean±SD or number (%).
HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; NA, not applicable; ALT, alanine aminotransferase.
*Comparison between HBeAg-positive and HBeAg-negative patients; †A complete virologic response is defined as undetectable serum HBV DNA by polymerase chain reaction (PCR) (≤20 IU/mL) at week 48; ‡A partial virologic response is defined as having more than 1 log10 IU/mL decline in viremia compared with baseline but still having detectable serum HBV DNA by PCR (≥20 IU/mL) at week 48; §A primary nonresponse was defined as a decrease in serum HBV DNA by less than 2 log10 IU/mL after at least 24 weeks of therapy; ∥A virologic breakthrough was defined as an increase in the serum HBV DNA level by more than 1 log10 IU/mL from the nadir during continuous treatment.
3. Factors associated with PVR
When we compared patients with CVR and PVR, HBeAg positivity (57.7% vs 79.7%; p=0.002), baseline serum HBV DNA level ≥8 log10 IU/mL (9.8% vs 46.9%; p<0.001), serum HBV DNA at week 12 ≥2,000 IU/mL (15.3% vs 50.0%; p<0.001), serum HBV DNA at week 24 ≥2,000 IU/mL (3.7% vs 20.3%; p<0.001), detectable serum HBV DNA (≥20 IU/mL) at week 24 (28.8% vs 87.5%; p<0.001), and poor adherence (medication <90%) (3.7% vs 15.6%; p=0.003) were significantly associated with PVR (Table 3). However, by multivariate analysis, baseline serum HBV DNA level ≥8 log10 IU/mL (odds ratio [OR], 8.67; 95% confidence interval [CI], 1.54 to 8.75; p=0.032), serum HBV DNA at week 12 ≥2,000 IU/mL (OR, 3.14; 95% CI, 1.49 to 6.63; p=0.003), and detectable serum HBV DNA (≥20 IU/mL) at week 24 (OR, 26.94; 95% CI, 0.01 to 0.15; p<0.001) were significantly associated with PVR (Table 4).
Table 3.
Comparison of Clinical Features between Patients with a Complete Virologic Response and Patients with a Partial Virologic Response at 48 Weeks

Data are presented as mean±SD or number (%).
CVR, complete virologic response; PVR, partial virologic response; HBeAg, hepatitis B e antigen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HBV, hepatitis B virus.
Table 4.
Factors Associated with Partial Virologic Response during Entecavir Therapy (Multivariate Analysis)
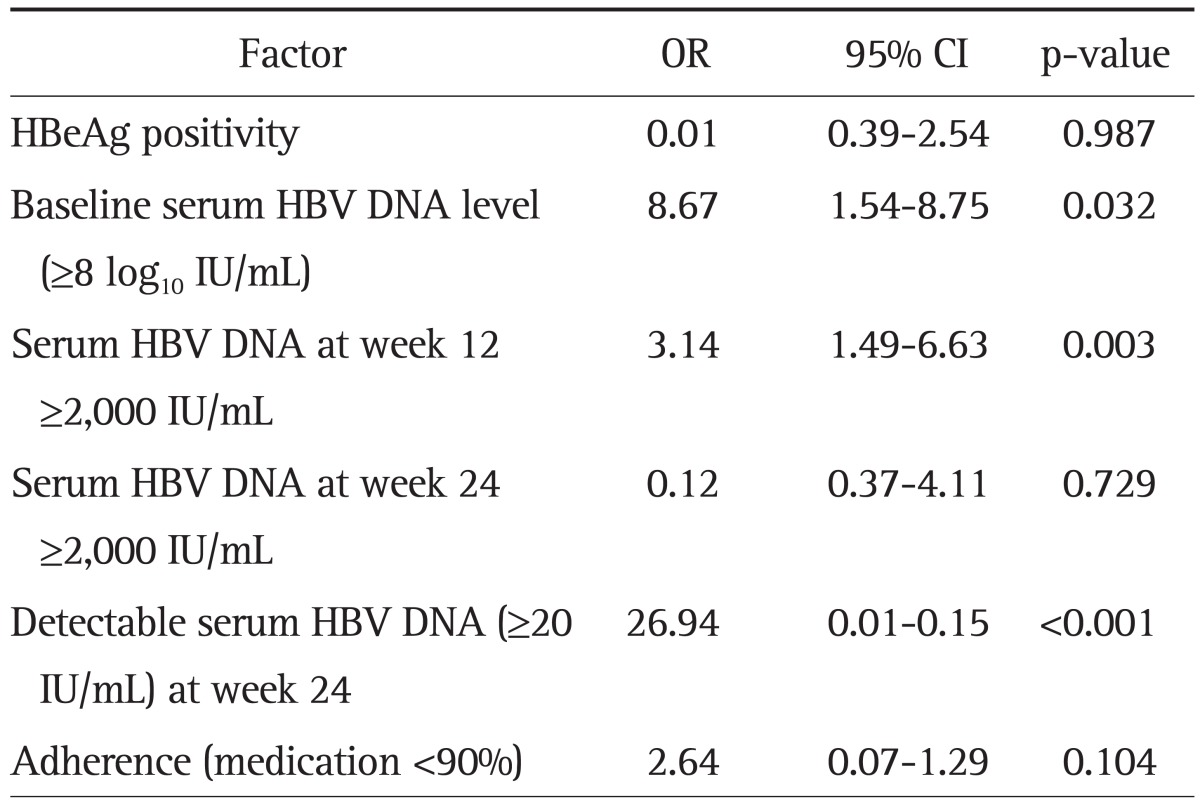
OR, odds ratio; CI, confidence interval; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus.
4. Cumulative rates of virologic response and resistance during long-term continuous ETV therapy
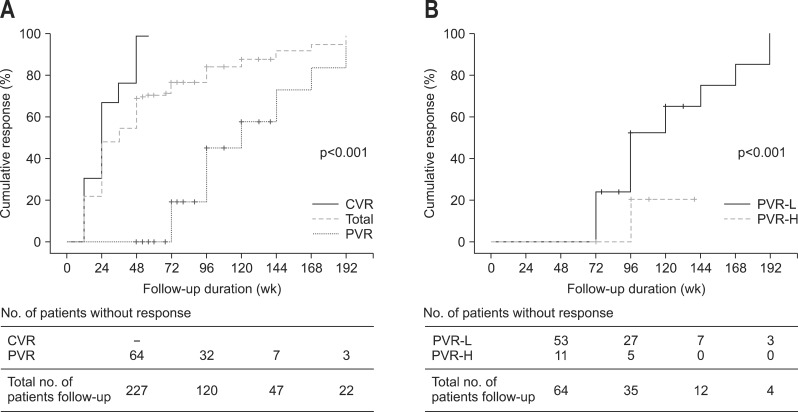
The overall cumulative rates of virologic response at week 96 and 144 were 82.4%, and 91.6%, respectively (Fig. 1A). Among patients with PVR, the cumulative rates of virologic response at week 96 and 144 were 45.2% and 73.8%, respectively (Fig. 1A). We analyzed the virologic response during long-term ETV therapy according to the serum HBV DNA level at week 48. In the subgroup analysis, the cumulative rates of virologic response at week 96 and 144 were significantly higher in patients with PVR and low viral load at week 48 (serum HBV DNA level 20 to 2,000 IU/mL) compared to those with PVR and high viral load at week 48 (serum HBV DNA level ≥2,000 IU/mL) (51.9% and 74.8% vs 20% and 20%, respectively; p<0.001) (Fig. 1B).
Fig. 1.
Cumulative rates of virologic response (serum hepatitis B virus [HBV] DNA level <20 IU/mL). (A) Cumulative rates of virologic response between patients with a partial virologic response (PVR) and patients with a complete virologic response (CVR) at week 48. (B) Cumulative rates of virologic response between patients with a PVR and high viral load (serum HBV DNA ≥2,000 IU/mL) and patients with a PVR and low viral load (serum HBV DNA 20 to 2,000 IU/mL) at week 48.
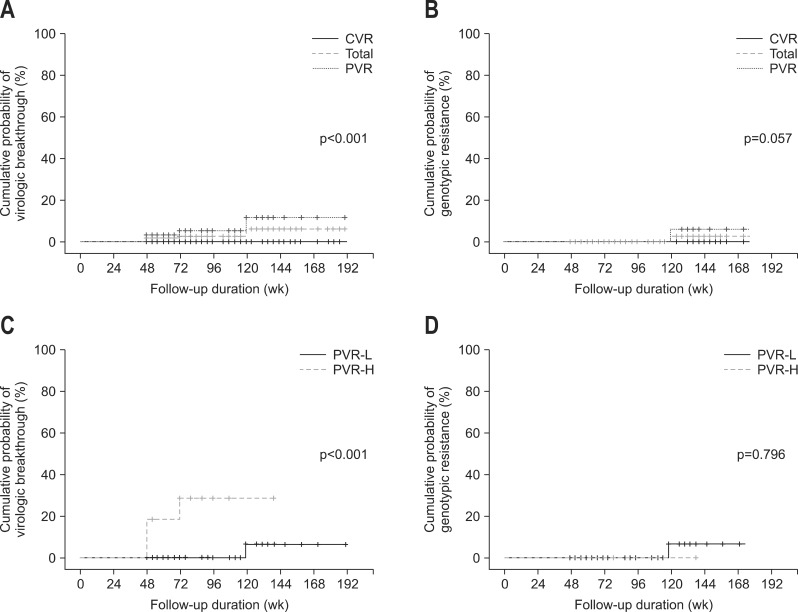
The overall cumulative rates of virologic breakthrough at week 96 and 144 were 2%, and 6%, respectively (Fig. 2A). The cumulative rates of virologic breakthrough at week 96 and 144 were significantly higher in patients with PVR compared to those with CVR (4% and 11.2% vs 0% and 0%, respectively; p<0.001) (Fig. 2A). The overall cumulative rates of genotypic resistance at week 96, and 144 were 0%, and 3%, respectively (Fig. 2B). The cumulative rates of genotypic resistance at week 96 and 144 were slightly higher in patients with PVR compared to those with CVR, but these differences were not statistically significant (0% and 6.2% vs 0% and 0%, respectively; p=0.057) (Fig. 2B). In subgroup analysis, the cumulative rates of virologic breakthrough at week 96 and 144 were significantly higher in patients with PVR and high viral load at week 48 (serum HBV DNA ≥2,000 IU/mL) compared to patients with PVR and low viral load at week 48 (serum HBV DNA 20 to 2,000 IU/mL) (28.4% and 28.4% vs 0% and 6.7%, respectively; p<0.001) (Fig. 2C). However, the cumulative rates of genotypic resistance at week 96 and 144 were not statistically different between patients with PVR and high viral load at week 48 (serum HBV DNA ≥2,000 IU/mL) and those with PVR and low viral load at week 48 (serum HBV DNA 20 to 2,000 IU/mL) (0% and 0% vs 0% and 6.7%, respectively; p=0.796) (Fig. 2D).
Fig. 2.
Cumulative rates of secondary treatment failure during long-term continuous entecavir therapy. (A) Cumulative rates of virologic breakthrough between patients with a partial virologic response (PVR) and patients with a complete virologic response (CVR). (B) Cumulative rates of genotypic resistance between patients with a PVR and patients with a CVR. (C) Cumulative rates of virologic breakthrough between patients with a PVR and high viral load and patients with a PVR and low viral load. (D) Cumulative rates of genotypic resistance between patients with a PVR and high viral load and patients with a PVR and low viral load.
DISCUSSION
PVR indicates the failure to reach undetectable serum HBV DNA levels which corresponds to a minimum risk of antiviral resistance during long-term NA therapy.3 Therefore, early treatment adaptation with switching to more potent NAs is recommended for patients with PVR. This strategy works well in patients receiving LAM, LdT, or ADV.12,13 However, there is very limited data for patients with PVR to a more potent, high genetic barrier drug, ETV. This study showed that long-term continuous ETV monotherapy in naïve patients showing PVR could achieve further virologic response without a significant increase in genotypic resistance. However, the rate of virologic breakthrough was higher in patients with PVR compared to those with CVR. Furthermore, patients with PVR and high viral load (≥2,000 IU/mL) at week 48 had a lower rate of virologic response and higher rate of virologic breakthrough during long-term ETV therapy compared to those with PVR and low viral load (20 to 2,000 IU/mL) at week 48.
The time point for defining PVR according to the 2009 EASL HBV Clinical Practice Guidelines is at week 24 for LAM and LdT or at week 48 for ADV, ETV, and tenofovir (TDF) depending on the antiviral potency and genetic barrier to resistance.14 PVR at week 24 for LAM and LdT and at week 48 for ADV have been well documented to predict the risk of subsequent resistance.5,6 In contrast, the time point of PVR for a more potent, high genetic barrier NA such as ETV or TDF, has not been fully established. Recently, Chon et al.15 suggested that an HBV DNA level >35 IU/mL at week 48 is the optimal PVR criteria for predicting nonvirologic response at week 96 in treatment-naïve CHB patients who are receiving ETV. In our study, we used the criteria of PVR suggested by a recent EASL guideline, namely a decrease of 1 log10 IU/mL but still detectable HBV DNA by real-time PCR (≥20 IU/mL) at week 48. There have been differences in the prevalence of PVR during ETV therapy among independent studies. Our study showed that the rate of ETV PVR was 64 of 227 (28.2%) naïve CHB patients and significantly higher among HBeAg-positive patients compared to HBeAg-negative patients (38.7% vs 8.8%; p<0.001). Previous studies reported that high baseline HBV DNA and HBeAg positivity were the only independent risk factors for PVR.15,16 In this study, we found that a high baseline serum HBV DNA level ≥8 log10 IU/mL, serum HBV DNA at week 12 ≥2,000 IU/mL and detectable serum HBV DNA (≥20 IU/mL) at week 24 were significantly associated with PVR. In addition, a previous study demonstrated that the presence of LAM-resistant mutations at baseline and a previous history of LAM resistance were also significantly associated with a reduced probability of achieving virologic response.16 Therefore, it is important to ascertain the previous history of LAM experience in patients with PVR during ETV therapy.
We found that further virologic response (45.2% at week 96 and 73.8% at week 144) could be achieved with continued extended ETV monotherapy in patients who had a PVR. The probability of further virologic response was high among patients with PVR and low serum HBV DNA levels (20 to 2,000 IU/mL) at week 48 (51.9% and 74.8% at week 96 and 144, respectively). These findings were in agreement with the results of two other recent studies.16,17 Zoutendijk et al.16 reported that among 36 of 175 NA-naïve patients (21%) having a PVR at week 48, 29 patients (81%) achieved virologic response during prolonged ETV monotherapy without development of ETV resistance. In addition, the cumulative rate of achieving a virologic response beyond week 48 was higher for patients with HBV DNA <1,000 IU/mL at week 48 compared to those with HBV DNA ≥1,000 IU/mL. Another study by Ko et al.17 also reported that 18 of 128 naïve CHB patients (14.0%) showed PVR (serum HBV DNA ≥20 IU/mL) up to week 48 of ETV therapy. Among 13 patients who showed PVR and were followed up for over 24 months, nine patients (69.2%) achieved CVR during prolonged ETV therapy and only one patient with poor compliance developed genotypic resistance to ETV. Meanwhile, our study showed that the rates of further virologic response were relatively low among patients with PVR and high serum HBV DNA levels (≥2,000 IU/mL) at week 48 (20% and 20% at week 96 and 144, respectively). Recently, Pan et al.18 suggested that switching to TDF is safe and very effective in the management of HBV patients with a suboptimal response to ETV (failure to achieve >1 log10 HBV DNA reduction during the last 24 weeks of ETV treatment). Thus further studies are required to evaluate the treatment efficacy of switching to TDF-based regimen in patients with PVR and a high viral load at week 48.
The association between PVR and secondary treatment failure during long-term ETV therapy has not been fully established. In this study, we found that the rate of virologic breakthrough was significantly higher in patients with PVR compared to those with CVR at week 48 (11.2% vs 0% at week 144; p<0.001). The rate of virologic breakthrough was also significantly increased in patients with PVR and high viral load at week 48 (serum HBV DNA ≥2,000 IU/mL) compared to patients with PVR and low viral load at week 48 (serum HBV DNA 20 to 2,000 IU/mL) (28.4% vs 6.7% at week 144; p<0.001). Virologic breakthrough typically results from the emergence of genotypic resistance or poor medical adherence.3 In our study, the emergence of genotypic resistance to ETV was infrequent and there was no significant increase even in patients with PVR at week 48 (6.2% at week 144). On the other hand, 15.6% of patients with virologic breakthrough were associated with poor medical adherence (medication <90%). Genotypic resistance occurred in only one patient in this study, but medical nonadherence significantly impacts the rate of viral suppression and persistent, low level viremia increases the risk of selecting resistance. Thus it is important to provide support and verify medical adherence in patients with PVR to improve antiviral treatment efficacy.
We note that our study had several limitations. First, because our study was an observational cohort study, we could not determine whether switching to another potent drug, TDF, may have improved treatment efficacy to maximize virologic response and minimize the risk of subsequent risk of emergence of resistance. Second, the number of patients who developed virologic breakthrough or genotypic resistance to ETV was very small, thus, we could not define the factors associated with secondary treatment failure during long-term continuous ETV therapy.
In conclusion, long-term continuous ETV monotherapy in naïve patients with PVR achieved further virologic response without a significant increase in genotypic resistance. However, there was a substantial increase of virologic breakthrough during extended ETV therapy in patients with PVR, especially for those with a high viral load at week 48. Considering that the risk of development of genotypic resistance may increase in patients with persistent detectable viremia, an additional large prospective study is needed to determine whether treatment adaptation to another potent drug is a better treatment strategy for patients with ETV PVR, especially for those with a high viral load at week 48.
ACKNOWLEDGEMENTS
This study was supported by research funds of Research Institute of Clinical Medicine, Chonbuk National University Hospital.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.European Association For The Study of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Korean Association for the Study of the Liver. KASL Clinical Practice Guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2012;18:109–162. doi: 10.3350/cmh.2012.18.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoulim F, Locarnini S. Management of treatment failure in chronic hepatitis B. J Hepatol. 2012;56(Suppl 1):S112–S122. doi: 10.1016/S0168-8278(12)60012-9. [DOI] [PubMed] [Google Scholar]
- 4.Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34(4 Pt 1):785–791. doi: 10.1053/jhep.2001.27563. [DOI] [PubMed] [Google Scholar]
- 5.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486–495. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 8.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 9.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 10.Osborn M. Safety and efficacy of entecavir for the treatment of chronic hepatitis B. Infect Drug Resist. 2011;4:55–64. doi: 10.2147/IDR.S4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han KH, Hong SP, Choi SH, et al. Comparison of multiplex restriction fragment mass polymorphism and sequencing analyses for detecting entecavir resistance in chronic hepatitis B. Antivir Ther. 2011;16:77–87. doi: 10.3851/IMP1702. [DOI] [PubMed] [Google Scholar]
- 12.Heo J, Park JY, Lee HJ, et al. A 96-week randomized trial of switching to entecavir in chronic hepatitis B patients with a partial virological response to lamivudine. Antivir Ther. 2012;17:1563–1570. doi: 10.3851/IMP2277. [DOI] [PubMed] [Google Scholar]
- 13.Reijnders JG, Deterding K, Petersen J, et al. Antiviral effect of entecavir in chronic hepatitis B: influence of prior exposure to nucleos(t)ide analogues. J Hepatol. 2010;52:493–500. doi: 10.1016/j.jhep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 14.European Association For The Study of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Chon YE, Kim SU, Lee CK, et al. Partial virological response to entecavir in treatment-naive patients with chronic hepatitis B. Antivir Ther. 2011;16:469–477. doi: 10.3851/IMP1772. [DOI] [PubMed] [Google Scholar]
- 16.Zoutendijk R, Reijnders JG, Brown A, et al. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naive patients with a partial virological response. Hepatology. 2011;54:443–451. doi: 10.1002/hep.24406. [DOI] [PubMed] [Google Scholar]
- 17.Ko SY, Choe WH, Kwon SY, et al. Long-term impact of entecavir monotherapy in chronic hepatitis B patients with a partial virologic response to entecavir therapy. Scand J Gastroenterol. 2012;47:1362–1367. doi: 10.3109/00365521.2012.719927. [DOI] [PubMed] [Google Scholar]
- 18.Pan CQ, Hu KQ, Yu AS, Chen W, Bunchorntavakul C, Reddy KR. Response to tenofovir monotherapy in chronic hepatitis B patients with prior suboptimal response to entecavir. J Viral Hepat. 2012;19:213–219. doi: 10.1111/j.1365-2893.2011.01533.x. [DOI] [PubMed] [Google Scholar]