Abstract
Background/Aims
Heat shock protein (HSP) 70 is constitutively overexpressed in pancreatic cancer cells (PCCs) and appears to confer protection against chemotherapeutics. We investigated whether modulating HSP 70 increases chemoresponsiveness to gemcitabine in PCCs.
Methods
Varying concentrations of quercetin and gemcitabine, either alone or in combination, were added to PCCs (Panc-1 and MiaPaCa-2). MTT assay was performed to analyze cell viability. HSP 70 expression was assessed by Western blot analysis. Apoptosis was determined by measuring caspase-3 activity. Western blot for the LC3-II protein detected the presence of autophagy.
Results
HSP 70 levels were not affected by the incubation of Panc-1 and MiaPaCa-2 cells with gemcitabine, whereas with quercetin, the levels were reduced in both cell lines. The viability of both Panc-1 and MiaPaCa-2 cells significantly decreased with gemcitabine treatment but not with quercetin. A combination of gemcitabine and quercetin decreased the viability of both cell lines in a dose-dependent manner, which was more pronounced than gemcitabine treatment alone. Treatment with either gemcitabine or quercetin augmented caspase-3 activity in both cell lines, and a combination of these compounds further potentiated caspase-3 activity. LC3-II protein expression was negligible with gemcitabine treatment but marked with quercetin. The addition of gemcitabine to quercetin did not potentiate LC3-II protein expression.
Conclusions
Modulation of HSP 70 expression with quercetin enhanced the chemoresponsiveness of PCCs to gemcitabine. The mechanism of cell death was both apoptosis and autophagy.
Keywords: Gemcitabine, Heat shock proteins, Pancreatic neoplasms, Quercetin
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer-related death across the world1 and the fifth most common cause of cancer-related mortality in Korea.2,3 The prognosis of pancreatic cancer is dismal with 5-year survival rate being approximately 5% for all stages combined.4 Although resection with curative intent is the treatment modality that can somewhat increase survival in these patients, up to 25% to 30% at best, only about 15% to 20% of patients can benefit from pancreatectomy,5 and the majority of patients have unresectable disease at clinical presentation or diagnosis.6 Therefore, many efforts have been put into improving the survival of patients with both resectable and unresectable pancreatic cancer. Although some progress has been made, current chemotherapy and radiotherapy either alone or in combination are only modestly effective5,7 and thus, the results of intensive research and therapeutic achievements are still far from satisfactory.
Gemcitabine, which is a currently available representative chemotherapeutic agent against pancreatic cancer, has been shown to provide little benefit with regards to prolonging the survival and to moderately improve the quality of life of the patients suffering from this devastating disease.8,9 Even with the combination of erlotinib, improvement in the survival of patients with advanced pancreatic cancer was only small, albeit statistically significant.10 Gemcitabine is known to exert its effect in pancreatic cancer by causing apoptosis of malignant cells,11,12 but the presence and overexpression of heat shock protein (HSP) in these cells has been shown to confer resistance to gemcitabine.13
HSP is an intracellular protein that is widely present in many types of cells and plays an important role in protecting cells from various harmful stimuli.14,15 HSP is present in pancreatic cells and HSP 70, a stress induced HSP, has been shown in animal models to protect pancreatic acinar cells from injury and cell necrosis due to acute pancreatitis.15-17 This HSP 70 is also found in pancreatic cancer cells (PCCs), and overexpression of HSP 70 is believed to contribute to carcinogenesis by inhibiting apoptosis.18-20 Since a natural flavonoid quercetin is known to reduce the expression of HSP 70 by inactivating the heat shock transcription factor,18 it can be expected that quercetin would decrease the resistance and increase the efficacy of gemcitabine against PCCs by acting as a chemosensitizer. Therefore, the study was conducted to see whether reducing the HSP 70 expression in PCCs would modulate chemoresponsiveness to gemcitabine, thereby enhancing its efficacy, and to investigate if there is additional mechanism involved in PCC death other than apoptosis.
MATERIALS AND METHODS
1. Materials
Quercetin was purchased from Sigma-Aldrich (St Louis, MO, USA). Gemcitabine was a kind donation from Dong-A Pharmaceutical (Seoul, Korea). The following antibodies were purchased from Abcam (Cambridge, UK): mouse monoclonal anti-HSP 70 (ab2787), rabbit anti-β-actin (ab8227), and rabbit anti-β-tubulin (ab6046). The following antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA): goat antimouse immunoglobulin G (IgG)-horseradish peroxidase (HRP) (sc-2005) and goat antirabbit IgG-HRP (sc-2004). Rabbit anti-LC3B polyclonal antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). PCCs (Panc-1 and MiaPaCa-2) were from Korean Cell Line Bank (Seoul National University, Seoul, Korea). Dulbecco's modified eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from HyClone (Logan, UT, USA). Penicillin-streptomycin was acquired from WelGENE Inc. (Daegu, Korea). Culture dishes and plastic labware were from BD Falcon (Franklin Lakes, NJ, USA). Caspase-Glo assay kit was purchased from Promega (Madison, WI, USA). All other reagents were from Sigma-Aldrich.
2. Cell culture and treatment
PCCs (Panc-1 and MiaPaCa-2) were cultured in DMEM containing 10% heat-inactivated FBS and 1% penicillin-streptomycin at 37℃ in a humidified atmosphere with 5% CO2. Cells were seeded into 96-well plates using 1×104 cells per well for viability measurements. Cells were plated in six-well plates at a density of 2.5×105 cells per well and were then incubated overnight at 37℃ for analysis of protein and apoptosis. Various concentrations of quercetin (10, 50, 100, and 200 µM) and gemcitabine (0.01, 0.1, 1, and 10 µg/mL) were dissolved in DMSO and added to cells at the indicated concentrations in serum-free medium, and incubated for varying times at 37℃.
3. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide salt (MTT) assay
Cell viability was determined using a colorimetric assay based on the uptake of MTT by viable cells. Cells were seeded into a 96-well plate at 1×104 cells per well and allowed to adhere overnight. After treatment with quercetin and gemcitabine either alone or in combination at various concentrations for 24 hours, 10 µL of MTT (0.5 mg/mL) was added to each well of the plate followed by incubation at 37℃ to form blue formazan crystals. After 2 hours, the residual MTT was carefully removed and the crystals were dissolved by incubation with 150 µL of DMSO for 30 minutes. The plates were then shaken for 1 hour and the absorbance was measured at 570 nm with spectrophotometry. All experiments were done in quadruplicate and repeated three independent times.
4. Western blot analysis
Cultured cells were solubilized in lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100) for 20 minutes on ice using a homogenizer. It was then cleared by centrifugation for 15 minutes at 13,000 ×g. Supernatants of the lysates were used to determine the protein concentration by Pierce bicinchoninic acid assay. An equal amount of lysates was run onto 10% SDS-polyacrylamide gels, and blotted to the nitrocellulose membrane (Millipore, MA, USA). After confirming equal protein loading by staining with Ponceau S, the membranes were blocked with 5% skim milk for 1 hour at room temperature, and incubated with mouse monoclonal anti-HSP 70, rabbit anti-β-actin, and rabbit anti-β-tubulin for measurement of HSP 70 levels; to measure LC3-II protein levels, rabbit anti-LC3B polyclonal antibody was used. After two times washes in Tris-Buffered Saline Tween-20, the blots were incubated with goat antimouse IgG-HRP and goat antirabbit IgG-HRP. After extensive washing, the immune complexes were detected using the ECL system (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
5. Caspase-3 activity analysis
Detection of apoptosis was performed by measuring caspase-3 activity with Caspase-Glo assay kit according to the manufacturer's instructions. Cells were seeded into a 96-well white opaque plate and a corresponding optically clear 96-well plate at 1×104 cells per well and then allowed to adhere overnight. The following day, cells were treated with varying concentrations of quercetin and gemcitabine either alone or in combination for 24 hours. At the end of the incubation time, 100 µL of Caspase-Glo reagent was added to each well. Plates were gently mixed and incubated at room temperature for 2 hours. The luminescence of each sample was then read in a luminometer.
6. Statistical analysis
Statistical analysis of the samples was performed using the Predictive Analytics Software Statistics (PASW version 18.0; IBM Co., Armonk, NY, USA). Data are expressed as the mean±standard error. Unpaired Student t-test was used to analyze the significance of the difference between the control and each experimental test condition. A value of p<0.05 was considered statistically significant.
RESULTS
1. Reduction of HSP 70 enhances chemoresponsiveness to gemcitabine
1) HSP 70 Western blot analysis
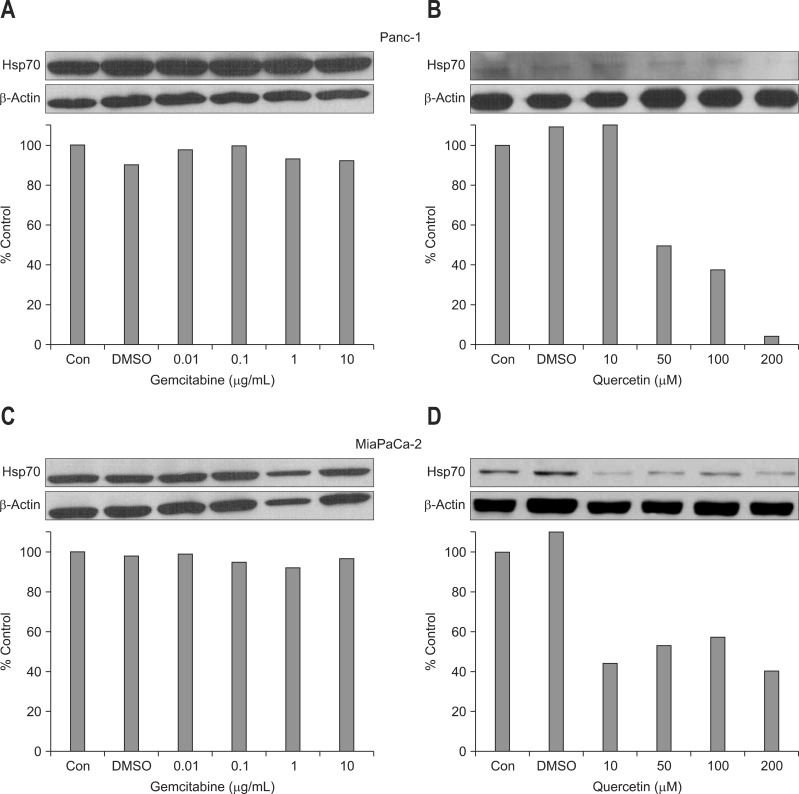
Incubation of Panc-1 and MiaPaCa-2 cells with gemcitabine did not significantly change the level of HSP 70 at concentrations 0.01, 0.1, 1, and 10 µg/mL (Fig. 1A and C). However, treatment with quercetin inhibited the expression of HSP 70 in a rather dose-dependent manner for Panc-1 cells. The expression of HSP 70 in MiaPaCa-2 cells was also inhibited by quercetin treatment but was not dose-dependent (Fig. 1B and D).
Fig. 1.
Effect of gemcitabine and quercetin on heat shock protein (HSP) 70 expression in (A, B) Panc-1 and (C, D) MiaPaCa-2 cells. Western blot performed to assess the expression of HSP 70 shows that gemcitabine treatment did not significantly affect HSP 70 expression in both (A) Panc-1 and (C) MiaPaCa-2 cells at all concentrations. However, (B) quercetin treatment reduced HSP 70 expression in a dose-dependent manner in Panc-1 cells, and (D) significantly inhibited HSP 70 expression at all concentrations in MiaPaCa-2 cells.
2) MTT assay
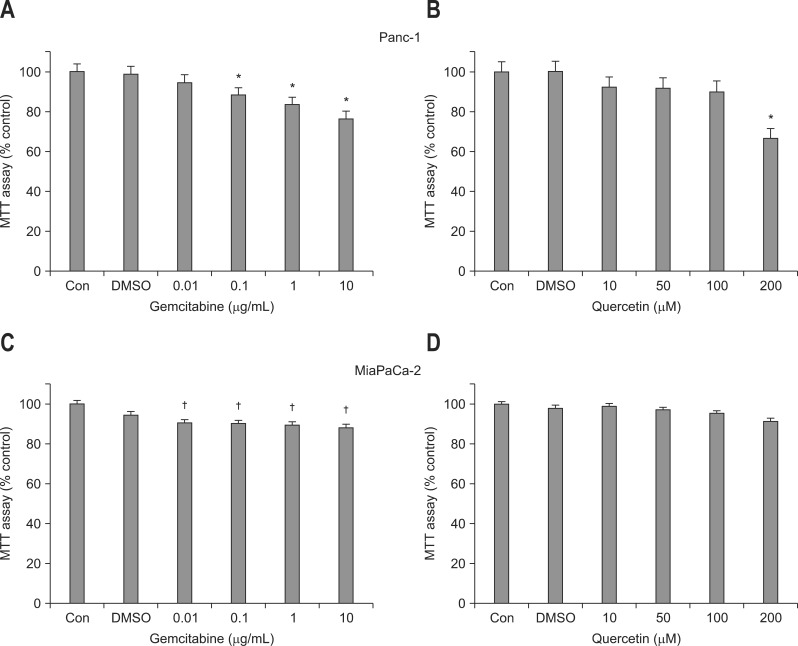
Gemcitabine decreased the number of both Panc-1 and MiaPaCa-2 cells in a dose-dependent manner compared to the control (Fig. 2A and C). The reduction in cell viability of Panc-1 and MiaPaCa-2 with gemcitabine treatment became significant beginning at a concentration of 0.1 and 0.01 µg/mL, respectively. As for the effect of quercetin on cell viability, the number of Panc-1 cells significantly decreased only at a concentration of 200 µM. Although quercetin treatment showed tendency to slightly reduce cell viability of MiaPaCa-2 cells, it was not significant at all concentrations (Fig. 2B and D).
Fig. 2.
Effects of gemcitabine and quercetin on the viability of (A, B) Panc-1 and (C, D) MiaPaCa-2 cells. The cell viability measured using MTT assay demonstrates that the presence of gemcitabine reduced the viability of (A) Panc-1 and (C) MiaPaCa-2 cells in a relatively dose-dependent manner, which became significant above concentrations of 0.1 and 0.01 µg/mL, respectively. (B) The viability of Panc-1 cells significantly decreased only when incubated with 200 µM quercetin, (D) whereas quercetin treatment had no significant effect on the viability of MiaPaCa-2 cells, regardless of the concentration. Each bar represents the number of cells as a percentage compared with the control.
DMSO, dimethyl sulfoxide.
*p<0.01 vs control; †p<0.05.
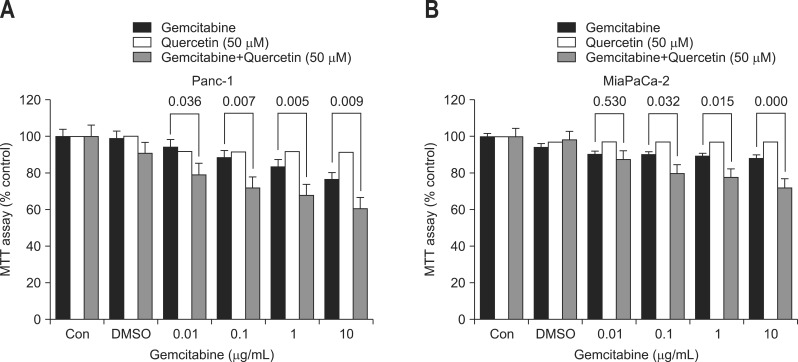
To see the additive effect of gemcitabine and quercetin, Panc-1 and MiaPaCa-2 cells were treated both with a fixed dose of quercetin (50 µM), which did not significantly affect the viability of cells, and varying concentrations of gemcitabine. When Panc-1 and MiaPaCa-2 cells were treated with both compounds, the cell viability significantly decreased at all concentrations in a dose-dependent manner (Fig. 3). This decrease was more pronounced compared to when only gemcitabine was added to the cells.
Fig. 3.
Additive effect of gemcitabine and quercetin on the viability of (A) Panc-1 and (B) MiaPaCa-2 cells. Incubation of Panc-1 and MiaPaCa-2 cells with various concentrations of gemcitabine and a fixed dose of quercetin (50 µM) led to a significant dose-dependent reduction in cell viability at all concentrations in Panc-1 cells and above 0.1 µg/mL in MiaPaCa-2 cells. This reduction was more pronounced compared with the effect of gemcitabine treatment alone. Each bar represents the number of cells as a percentage compared with the control.
2. Mechanism of PCC death is apoptosis supplemented with autophagy
1) Effect of gemcitabine and quercetin either alone or in combination on caspase-3 activation
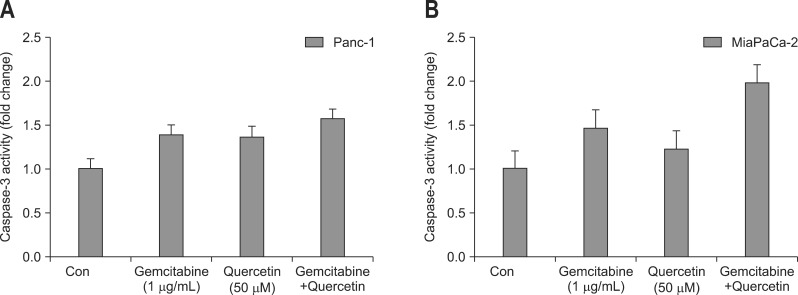
Addition of 1 µg/mL gemcitabine increased caspase-3 activity in both Panc-1 and MiaPaCa-2 cells by 1.38 and 1.46 folds, respectively. Treatment of Panc-1 and MiaPaCa-2 cells with 50 µM quercetin for 24 hours resulted in a 1.36-fold and 1.22-fold increase in caspase-3 activity compared with control, respectively. Incubation of Panc-1 and MiaPaCa-2 cells with both gemcitabine and quercetin led to a 1.56-fold and 1.98-fold increase in caspase-3 activity, respectively (Fig. 4).
Fig. 4.
Effect of gemcitabine and quercetin either alone or in combination on caspase-3 activation. The presence of either gemcitabine or quercetin increased caspase-3 activity in both (A) Panc-1 and (B) MiaPaCa-2 cells. Caspase-3 activity further increased when Panc-1 and MiaPaCa-2 cells were treated with both gemcitabine and quercetin.
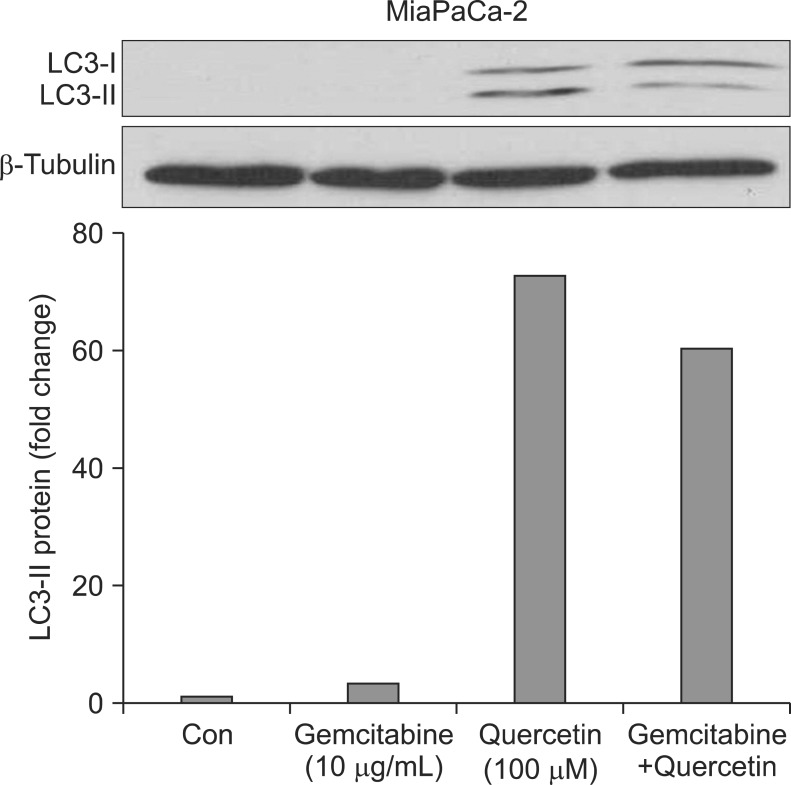
2) Induction of autophagy with gemcitabine and quercetin in MiaPaCa-2
To further investigate the cell death mechanism, LC3-II western blot was performed to analyze the presence of autophagy in MiaPaCa-2 cells. Induction of LC3-II protein expression was negligible with 10 µg/mL gemcitabine (3.1-fold) but was remarkable with 100 µM quercetin (72.9-fold). Combination of gemcitabine and quercetin resulted in 60.6-fold increase in LC3-II protein expression (Fig. 5).
Fig. 5.
Induction of autophagy in MiaPaCa-2 cells with gemcitabine and quercetin. MiaPaCa-2 cells treated with gemcitabine showed a negligible increase in LC3-II protein expression, whereas quercetin dramatically increased the protein's expression. A combination of gemcitabine and quercetin did not further augment LC3-II protein expression.
DISCUSSION
The result of our study shows that reducing the HSP 70 expression in PCCs (Panc-1 and MiaPaCa-2) enhances chemoresponsiveness to gemcitabine, and that the underlying mechanism of cell death is apoptosis which is supplemented with autophagy by quercetin. Evidence that HSP 70 expression reduction enhances chemoresponsiveness to gemcitabine is that addition of quercetin to increasing concentration of gemcitabine in PCCs significantly decreased the cell viability at all concentrations when compared to the PCC treated with gemcitabine alone (Fig. 3). Evidence that the mechanisms through which cell death occur are apoptosis and autophagy is that caspase-3 activity was enhanced in the presence of the combination of quercetin and gemcitabine compared with when the cancer cells were treated with either quercetin or gemcitabine alone (Fig. 4), and that LC3-II protein expression was evident with quercetin treatment, respectively (Fig. 5).
A previous report has shown that HSP 70 was constitutively elevated in PCC lines (Panc-1 and MiaPaCa-2).18 We could see that similar to their findings with Panc-1 cells, HSP 70 expression assessed by Western blot showed a reduction of more than 50% of control starting from 50 µM quercetin and above. However, unlike their study, cell viability was not significantly affected up until the addition of 200 µM quercetin. As for the MiaPaCa-2 cells, HSP 70 expression was reduced to almost 50% of control with 50 µM quercetin, but this reduction was not dose-dependent. In addition, different from the previous study,18 various doses of quercetin used in our experiment did not significantly affect cell viability in MiaPaCa-2 cells. Therefore, to see the inhibitory effect of HSP 70 on the efficacy of gemcitabine in cancer cell death, the quercetin concentration of 50 µM was chosen since at this concentration, HSP 70 expression significantly decreased to about 50% but did not affect cell viability. As expected, inhibition of HSP 70 expression with quercetin augmented the effect of gemcitabine on cancer cell death at all concentrations in Panc-1 cells and at concentrations above 0.1 µg/mL in MiaPaCa-2 cells, suggesting the potential role of HSP 70 in modulating chemoresponsiveness in pancreatic cancer.
Possibility of the beneficial role of modulating HSP 70 with quercetin to sensitize cancer cells during chemotherapy has been demonstrated by Sliutz et al.13 using fibrosarcoma WEHI-S cell line that was transfected to overexpress HSP 70. In their experiment, they found that the concentration necessary for antineoplastic drugs (gemcitabine and topotecan) to exert cytotoxic activity became markedly lower when these cells were pretreated with quercetin to inhibit the expression of HSP 70. Although quercetin was not pretreated but was added simultaneously with gemcitabine in the present experiment, similar result could be observed as mentioned above. Since treatment of gemcitabine alone did not affect HSP 70 expression in both Panc-1 and MiaPaCa-2 cells in our study, the enhanced cytotoxic activity could be attributed to the down-regulating effect of quercetin on HSP 70 expression.
The mechanism of cell death by gemcitabine is known to occur through apoptosis,11,12 and HSP 70 has been demonstrated to interact with and inhibit multiple steps, perhaps simultaneously, along both the intrinsic and extrinsic pathways of caspase-dependant apoptosis.15 Studies that looked into the role of HSP 70 in inhibiting intrinsic apoptosis pathway have shown that HSP 70 decreased cytosolic calcium level,20 stabilized lysosomes thereby reducing the release of cathepsins,20,21 prevented Bax translocation that resulted in inhibition of pore formation in the mitochondria which led to impairment of cytochrome c release,22,23 interfered with the formation of apoptosome by directly associating with Apaf-1,24 etc. In addition to the aforementioned studies on intrinsic apoptosis pathway, HSP 70 has also been shown to inhibit extrinsic apoptosis pathway by binding to the death receptors DR4 and DR5 leading to the inhibition of tumor necrosis factor-related apoptosis-induced ligand assembly and resultant inhibition of death-inducing signaling complex activation.25 Based on the role of HSP 70 in preventing apoptosis, inhibition of HSP 70 has been evaluated and was shown to induce apoptosis in PCCs.18-20 In this regard, it could be speculated that inhibition of HSP 70 expression would further enhance the efficacy of gemcitabine now that protection against apoptosis is suppressed. In line with this speculation, our result showed that induction of apoptosis by gemcitabine was further augmented by addition of quercetin. However, this induction of apoptosis was not so profound and could only marginally explain the enhanced efficacy of gemcitabine in the presence of decreased HSP 70 expression. Therefore, another mechanism of death, i.e., autophagy, was investigated.
Autophagy is a lysosomal degradation pathway in which injured or long-lived intracellular organelles and excessive or unnecessary proteins are eliminated to maintain homeostasis, thus protecting the cells during stress conditions such as nutrient starvation.26 However, the observation that autophagy occurs in dying cells during cancer therapy led the researchers to consider autophagy as an alternative way to cell death.27 Although gemcitabine had negligible effect on inducing autophagy in our experiment, the impact of quercetin on autophagy was quite pronounced. This remarkable increase in autophagy by quercetin could be attributed to the inhibition of HSP 70, but two contradictory points mandate explanation. First, our result shows that cell viability of MiaPaCa-2 cells was not so much affected with quercetin treatment despite considerable increase in autophagy. To detect the presence of autophagy, we have used LC3 as its marker. LC3 is known to exist in two forms: LC3-I which is cytosolic and LC3-II which is membrane-bound. During autophagy, LC3-I converts to LC3-II. Since LC3 associates with the membrane of autophagosomes, measuring the membrane-bound form LC3-II can be regarded as a reasonable marker of autophagy. Nevertheless, the completion of autophagy requires fusion with lysosomes to form autolysosome and thus, it might be postulated that quercetin mainly induces the formation of autophagosome and that gemcitabine is required to ultimately instigate the formation of autolysosome that leads to autophagic cell death. Second, among three types of autophagy, i.e., macroautophagy, microautophagy, and chaperon-mediated autophagy, HSP 70 has been shown to be involved in chaperon-mediated autophagy.28 Therefore, up-regulation rather than down-regulation of HSP 70 would facilitate chaperon-mediated autophagy and this also contradicts the speculation that inhibition of HSP 70 would enhance autophagy by quercetin. Thus, the question still remains whether up-regulating or down-regulating HSP 70 is beneficial in modulating chemoresponsiveness. To make matters more complicated, a study by Adachi et al.29 has interestingly demonstrated that induction of HSP 70, rather than inhibition of HSP 70, enhanced the efficacy of gemcitabine-induced cytotoxicity in PCCs.
Many studies have shown that HSP 70 is frequently and preferentially overexpressed in high-grade or malignant cells compared to their surrounding normal or low-grade cells in a variety of cancers. This overexpression has been demonstrated to correlate with poorer survival in endometrial cancer,30 high risk for disease recurrence in node-negative breast cancer,31 increase in tumor size in cervical cancer,32 poor prognosis in malignant melanoma,33 etc. However, elevated HSP 70 did not always correlate with poor prognosis and/or decreased treatment response in all cancers. In renal cell carcinoma, the proportion of cells that expressed HSP 70 was significantly higher in renal cancer cells compared to normal renal cells, but its expression was paradoxically associated with favorable prognosis.34 In osteosarcoma, HSP 70 was significantly overexpressed in osteosarcoma cells compared with its normal counterpart, but the overexpression of HSP 70 correlated with good response to neoadjuvant chemotherapy.35 Therefore, it is conceivable to think that the correlation between expression of HSP 70 and response to therapy or prognosis could be cancer specific.
There are some limitations to this study. First, only LC3-II protein expression was analyzed to assess the presence of autophagy. LC3-II protein is expressed on the membrane of autophagosome, but it might not necessarily mean that this results in the formation of autolysosome. Therefore, further study would be necessary to actually observe the occurrence of autophagy and to discern between the formation of autophagosome and autolysosome. Second, nuclear factor-κB (NF-κB) was not analyzed. External stimuli, such as gemcitabine, activate the apoptotic pathway but at the same time initiate the antiapoptotic pathway. To which side the balance would be tilted depends on many factors but the relationship between HSP 70 and NF-κB seems to be fundamental in getting a better insight regarding its interaction with HSP 70 in PCCs. This would be crucial in unraveling the seeming contradictory role of HSP 70 in apoptotic cell death as has been demonstrated by Adachi et al.29
Many studies have shown that HSP 70 is overexpressed in many types of cancer cells that lead to unfavorable outcome, and pancreatic cancer does not seems to be an exception. Our study has shown that inhibition of HSP 70 increased the efficacy of gemcitabine, and that the mechanism of death was apoptosis, albeit marginal, with gemcitabine having negligible effect on autophagy. Although quercetin notably suppressed HSP 70 expression and remarkably induced autophagy, it did not significantly affect cell survival. Thus, appreciable cytotoxic effect cannot be expected with quercetin alone. Therefore, it could be concluded that HSP 70 expression is related to chemoresistance in PCCs and that manipulating HSP 70 could favorably contribute to enhancing the efficacy of currently available chemotherapeutic agents, such as gemcitabine, against pancreatic cancer.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10:58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010;25:1113–1121. doi: 10.3346/jkms.2010.25.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 9.Rothenberg ML. New developments in chemotherapy for patients with advanced pancreatic cancer. Oncology (Williston Park) 1996;10(9 Suppl):18–22. [PubMed] [Google Scholar]
- 10.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 11.Bold RJ, Chandra J, McConkey DJ. Gemcitabine-induced programmed cell death (apoptosis) of human pancreatic carcinoma is determined by Bcl-2 content. Ann Surg Oncol. 1999;6:279–285. doi: 10.1007/s10434-999-0279-x. [DOI] [PubMed] [Google Scholar]
- 12.Huang P, Plunkett W. Induction of apoptosis by gemcitabine. Semin Oncol. 1995;22(4 Suppl 11):19–25. [PubMed] [Google Scholar]
- 13.Sliutz G, Karlseder J, Tempfer C, Orel L, Holzer G, Simon MM. Drug resistance against gemcitabine and topotecan mediated by constitutive hsp70 overexpression in vitro: implication of quercetin as sensitiser in chemotherapy. Br J Cancer. 1996;74:172–177. doi: 10.1038/bjc.1996.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong HR. Heat shock proteins. Facts, thoughts, and dreams. A. De Maio. Shock. 1999;12:323–325. doi: 10.1097/00024382-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Dudeja V, Vickers SM, Saluja AK. The role of heat shock proteins in gastrointestinal diseases. Gut. 2009;58:1000–1009. doi: 10.1136/gut.2007.140194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng JY, Li YY. Alteration and role of heat shock proteins in acute pancreatitis. J Dig Dis. 2010;11:277–283. doi: 10.1111/j.1751-2980.2010.00450.x. [DOI] [PubMed] [Google Scholar]
- 17.Ethridge RT, Ehlers RA, Hellmich MR, Rajaraman S, Evers BM. Acute pancreatitis results in induction of heat shock proteins 70 and 27 and heat shock factor-1. Pancreas. 2000;21:248–256. doi: 10.1097/00006676-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Aghdassi A, Phillips P, Dudeja V, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 19.Phillips PA, Dudeja V, McCarroll JA, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 20.Dudeja V, Mujumdar N, Phillips P, et al. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009;136:1772–1782. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nylandsted J, Gyrd-Hansen M, Danielewicz A, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosser DD, Caron AW, Bourget L, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 24.Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 25.Guo F, Sigua C, Bali P, et al. Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood. 2005;105:1246–1255. doi: 10.1182/blood-2004-05-2041. [DOI] [PubMed] [Google Scholar]
- 26.Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–1908. doi: 10.1053/j.gastro.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2:85–90. doi: 10.4161/auto.2.2.2463. [DOI] [PubMed] [Google Scholar]
- 28.Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Adachi S, Kokura S, Okayama T, et al. Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int J Hyperthermia. 2009;25:210–219. doi: 10.1080/02656730802657036. [DOI] [PubMed] [Google Scholar]
- 30.Nanbu K, Konishi I, Mandai M, et al. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect Prev. 1998;22:549–555. doi: 10.1046/j.1525-1500.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- 31.Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- 32.Ralhan R, Kaur J. Differential expression of Mr 70,000 heat shock protein in normal, premalignant, and malignant human uterine cervix. Clin Cancer Res. 1995;1:1217–1222. [PubMed] [Google Scholar]
- 33.Kalogeraki A, Garbagnati F, Darivianaki K, et al. HSP-70, C-myc and HLA-DR expression in patients with cutaneous malignant melanoma metastatic in lymph nodes. Anticancer Res. 2006;26(5A):3551–3554. [PubMed] [Google Scholar]
- 34.Santarosa M, Favaro D, Quaia M, Galligioni E. Expression of heat shock protein 72 in renal cell carcinoma: possible role and prognostic implications in cancer patients. Eur J Cancer. 1997;33:873–877. doi: 10.1016/s0959-8049(97)00002-6. [DOI] [PubMed] [Google Scholar]
- 35.Trieb K, Lechleitner T, Lang S, Windhager R, Kotz R, Dirnhofer S. Heat shock protein 72 expression in osteosarcomas correlates with good response to neoadjuvant chemotherapy. Hum Pathol. 1998;29:1050–1055. doi: 10.1016/s0046-8177(98)90412-9. [DOI] [PubMed] [Google Scholar]