Abstract
Background/Aims
The accurate preoperative prediction of the risk of malignancy of gastrointestinal stromal tumors (GISTs) is difficult. The aim of this study was to determine whether tumor size and endoscopic ultrasonography (EUS) features can preoperatively predict the risk of malignancy of medium-sized gastric GISTs.
Methods
Surgically resected, 2 to 5 cm gastric GIST patients were enrolled and retrospectively reviewed. EUS features, such as heterogeneity, hyperechoic foci, calcification, cystic change, hypoechoic foci, lobulation, and ulceration, were evaluated. Tumors were grouped in 1 cm intervals. The correlations of tumor size or EUS features with the risk of malignancy were evaluated.
Results
A total of 75 patients were enrolled. The mean tumor size was 3.43±0.92 cm. Regarding the risk of malignancy, 51 tumors (68%) had a very low risk, and 24 tumors (32%) had a moderate risk. When the tumors were divided into three groups in 1 cm intervals, the proportions of tumors with a moderate risk were not different between the groups. The preoperative EUS features also did not differ between the very low risk and the moderate risk groups.
Conclusions
Tumor size and EUS features cannot be used to preoperatively predict the risk of malignancy of medium-sized gastric GISTs. A preoperative diagnostic modality for predicting risk of malignancy is necessary to prevent the overtreatment of GISTs with a low risk of malignancy.
Keywords: Gastrointestinal stromal tumors, Endosonography, Tumor size, Malignant risk
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms in the gastrointestinal (GI) tract which are thought to originate from the interstitial cells of Cajal. GISTs occur throughout the GI tract and are most commonly found in the stomach (60%), small bowel (30%), duodenum (5%), colorectum (4%), and rarely the esophagus and appendix.1,2 GISTs consist of spindle cell, epithelioid cell, or mixed cell types. Recent immunohistochemistry has shown that up to 95% of GISTs express c-kit protein and 60% to 70% of GISTs express CD34. The c-kit protein is now recognized as a highly sensitive and specific marker for GISTs. Therefore, the GIST is now considered a completely separate entity from other smooth muscle tumors (true leiomyomas and leiomyosarcomas) and schwannomas.3
In large population-based studies, approximately 70% of patients with GISTs were clinically symptomatic. The most common presenting symptom was gastrointestinal bleeding, and the others were abdominal pain or the presence of a palpable mass.4 Small-sized GISTs are often asymptomatic, and incidentally discovered on endoscopy. In Korea, incidental detection of small-sized GIST has been increasing by biannual endoscopy for normal population over 40 years of age in national cancer screening program.
As GIST is usually located in muscularis propria which cannot be obtained by routine endoscopic biopsy, endoscopic ultrasonography (EUS) is useful for further information. In EUS, gastric GIST typically appears as well demarcated, round, hypoechoic mass arising from fourth layer of the stomach. Although many malignant features of GIST in EUS have been introduced, the sensitivity and specificity have not been promising,5-7 and definitive diagnosis has been usually made by immunohistochemical analysis after resection. EUS-guided fine needle aspiration (EUS-FNA) or biopsy can offer histological diagnosis, but it is difficult for even EUS-guided biopsy to obtain enough tissue in small-sized GIST for evaluation of mitotic index.8,9
Malignant risk of GIST can be determined based on mitotic index, size, and location of the lesion. According to clinical malignant risk, GISTs are stratified into very low, low, moderate, or high risk.3,10 Although EUS can provide tumor size as a predictor of malignant potential, mitotic index cannot be usually evaluated before surgical resection. Furthermore, small-sized gastric GIST may be overtreated despite high possibility of very low malignant risk.
The aim of this study was to evaluate whether EUS features can predict malignant risk of gastric GIST less than 5 cm in size preoperatively, and the malignant risk increases in proportion to tumor size in 2 to 5 cm.
MATERIALS AND METHODS
1. Patients
A total of 181 patients with gastric GIST underwent curative surgical resection in Seoul National University Hospital between 2000 and 2009. Of these, 75 patients with GIST in 2 to 5 cm in size were retrospectively reviewed. Recurred GISTs, cases with noncurative resection were excluded. This study was approved by the Institutional Review Board of Seoul National University Hospital.
2. EUS characteristics
All EUS staging was performed by an expert endoscopist (S.G.K.), using a radial array echoendoscope (GF-UM-2000; Olympus, Tokyo, Japan) or miniature ultrasound probe (miniprobe, Olympus UM-2R, 12MHz; Olympus). The following EUS features were recorded for all the lesions; heterogeneity, hyperechoic foci, calcification, cystic change, hypoechoic foci, lobulation, and ulceration.
3. Surgical resection
Surgical resection was performed for the patients with gastric GIST in 2 to 5 cm in size at the following situations: increasing tumor size, symptomatic patients, the physician's opinion, the patient's preference, or presence of EUS features of high malignant risk. EUS features suggestive of a risk of malignancy were defined as large size, irregular border, heterogeneous echogenecity, cystic spaces, or hyperechoic foci.5,7,11
4. Risk stratification
The malignant risk of GIST in 2 to 5 cm in size was stratified into very low (mitotic count <5 per 50 high power field [HPF]) or moderate risk (mitotic count ≥5 per 50 HPF) according to tumor size and mitotic index identified on surgical histology (Table 1).3,10 To investigate whether tumor size or EUS features correlate with clinical malignant risk in gastric GIST in 2 to 5 cm in size, the patients were grouped into three groups by tumor size at 1 cm interval: 1) 2.00 to 3.00 cm; 2) 3.01 to 4.00 cm; 3) 4.01 to 5.00 cm.
Table 1.
Risk Stratification of Primary Gastrointestinal Stromal Tumors*
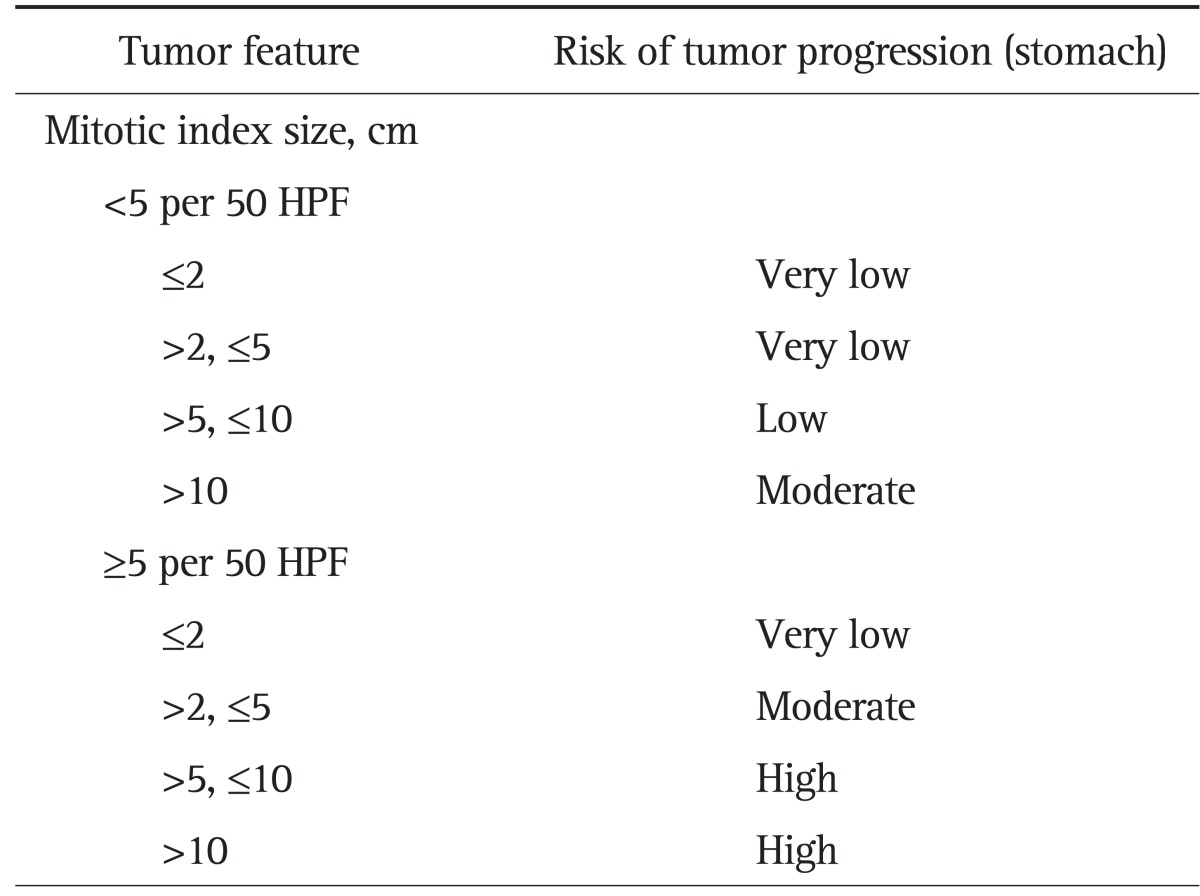
HPF, high power field.
*Modified from Miettinen and Lasota.
5. Statistical analysis
Statistical analysis was conducted using the Fischer exact test and the chi-square test. Univariate logistic regression analyses were performed to identify the EUS features that could predict malignant risk. Multivariate logistic regression analyses were performed to identify the independent predictors of malignant risk. The odds ratio, together with 95% confidence interval calculated, and p-values <0.05 were considered statistically significant. All statistics were analyzed using the SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics
Among a total of 181 patients with gastric GIST, 75 patients had a tumor with 2 to 5 cm in size, and preoperative EUS evaluation was performed in 55 cases.
The mean age was 63.3 years and the male to female ratio was 1.14:1. Thirty-one patients (41.4%) were symptomatic, and the most common symptom was abdominal pain (13 patients). Ten patients had abdominal discomfort, five patients had GI bleeding, and two patients had reflux symptom. Thirty-two of 75 tumors (42.7%) were located on fundus, 27 (35.6%) on body, 10 (13.3%) on antrum, six (8.0%) on angle (Table 2).
Table 2.
Baseline Characteristics of Surgically Resected Gastric Gastrointestinal Stromal Tumors (n=75)
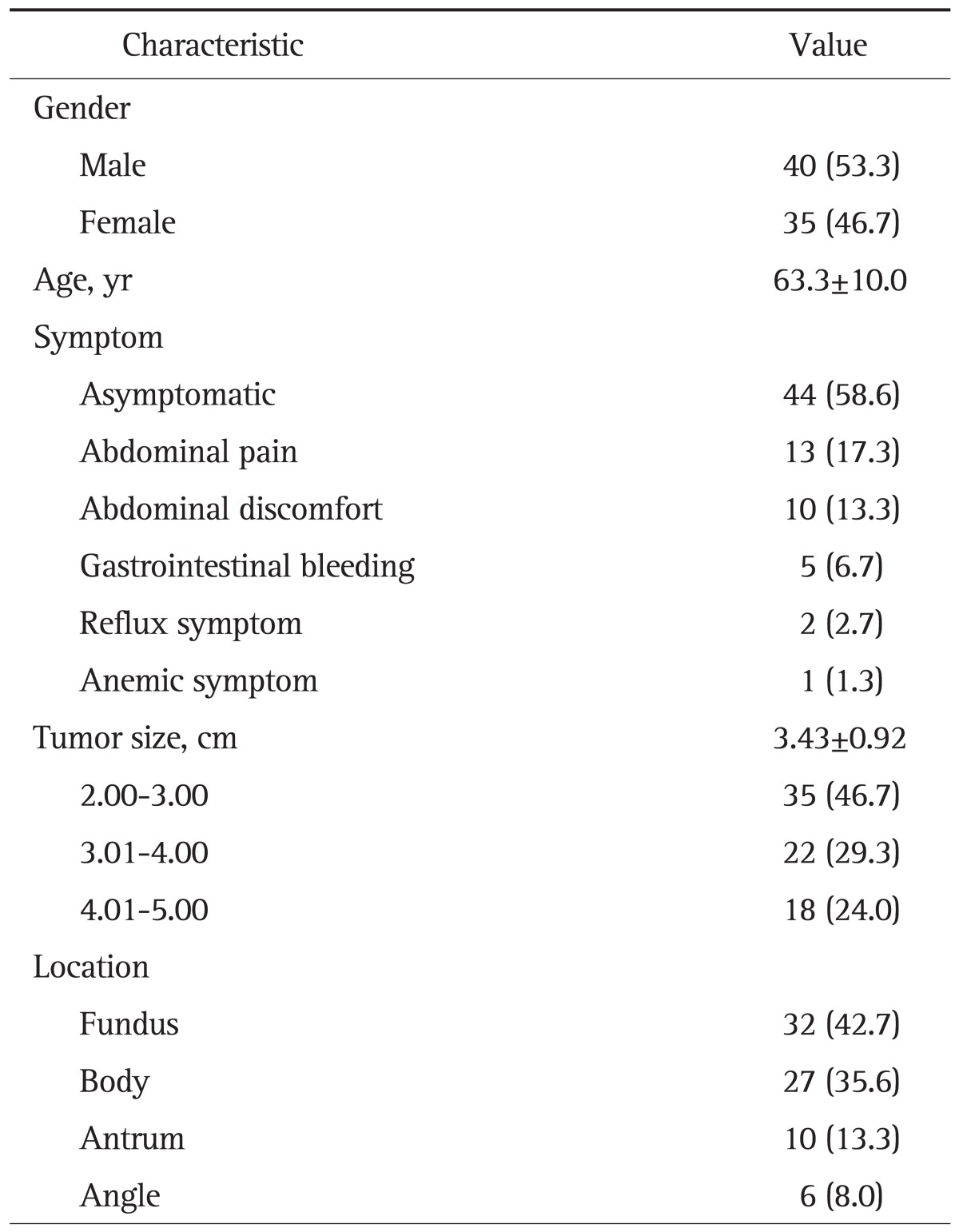
Data are presented as number (%) or mean±SD.
2. Malignant risk according to tumor size and mitotic count
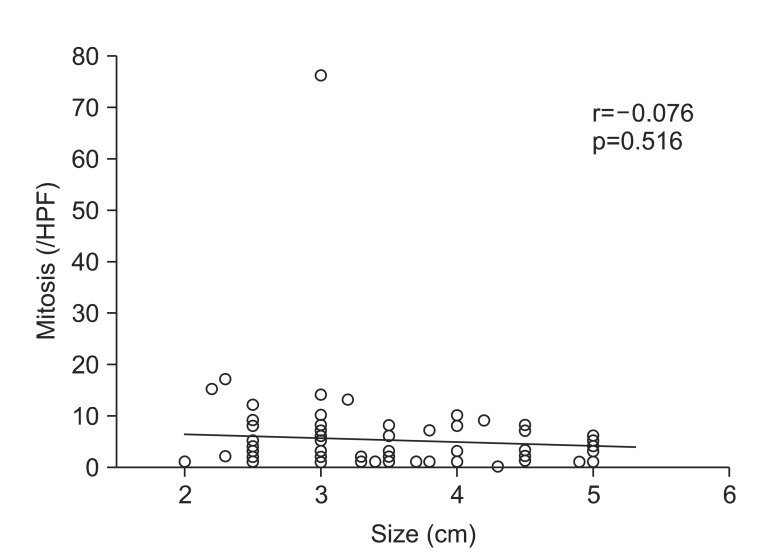
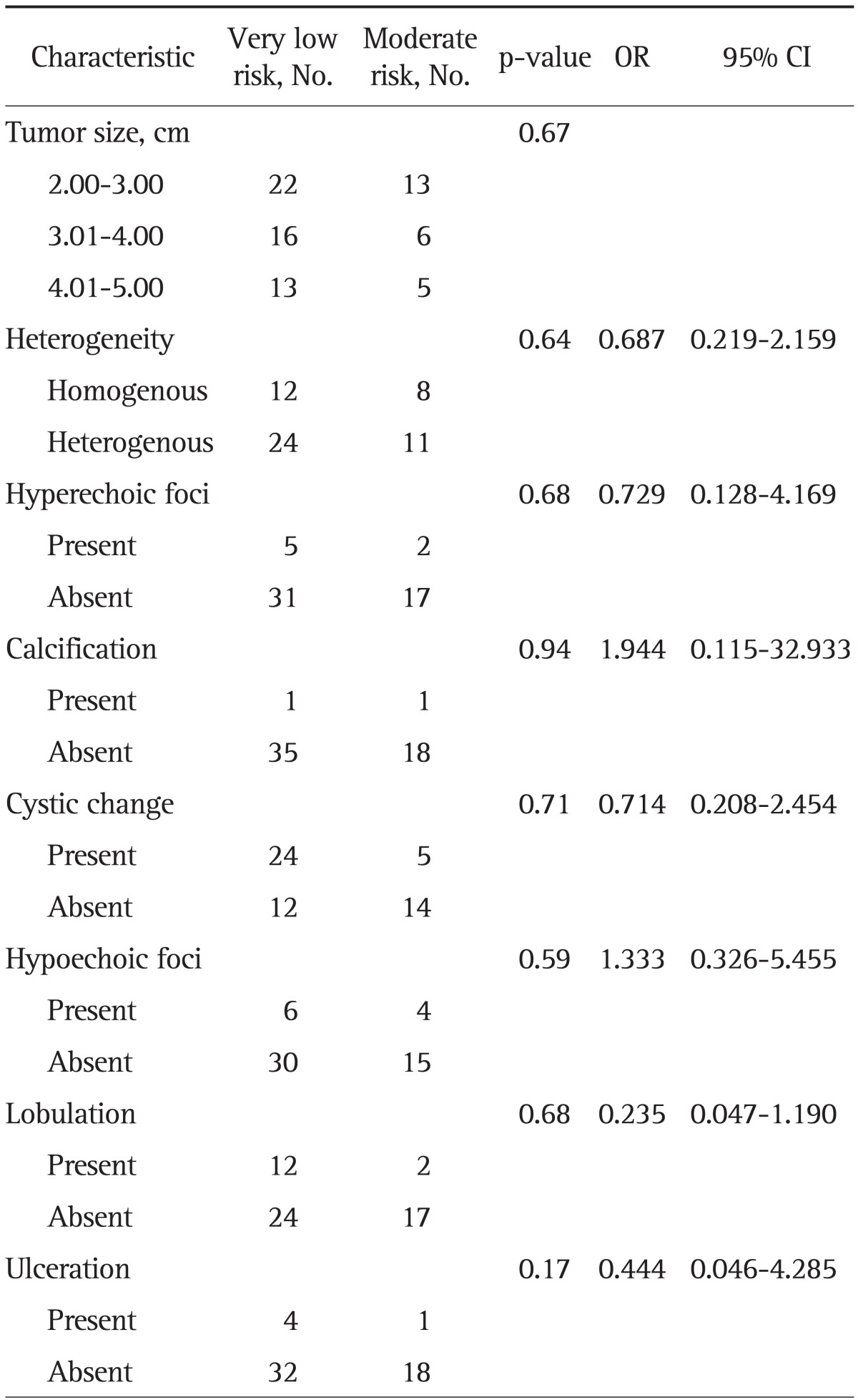
The mean size of lesions was 3.43±0.92 cm. With regard to clinical malignant risk, 51 tumors (68%) had very low risk, and 24 tumors (32%) had moderate risk. When the tumors were divided into 1 cm interval in size, 13 tumors (37.1%) had moderate risk in group of size between 2.00 and 3.00 cm, six (27.3%) between 3.01 and 4.00 cm, five (27.8%) between 4.01 and 5.00 cm, which were not statistically different among the groups (p=0.67) (Table 3). In the same way, the mitotic count was not significantly different with the size increment of tumor (Fig. 1).
Table 3.
Risk Stratification according to Tumor Size and Endoscopic Ultrasonography Characteristics
OR, odds ratio; CI, confidence interval.
Fig. 1.
Relationship between mitotic count and tumor size.
HPF, high power field.
3. EUS characteristics
Among a total of 55 patients who had preoperative EUS evaluation, 36 patients had the tumors with very low risk and 19 patients with moderate risk.
There was no statistical significance in the characteristics of heterogeneity, hyperechoic foci, calcification, cystic change, hypoechoic foci, lobulation, and ulceration between very low risk group and moderate risk group. The proportion with a hypoechoic foci within a mass was 16.6% in very low risk group, and 21.0% in moderate group (Fig. 2). The proportion with cystic space was higher in very low risk group (66.6%) than moderate risk group (26.3%). In univariate and multivariate logistic regression analysis, the EUS features were not helpful in predicting the malignant potential of GISTs (Table 3).
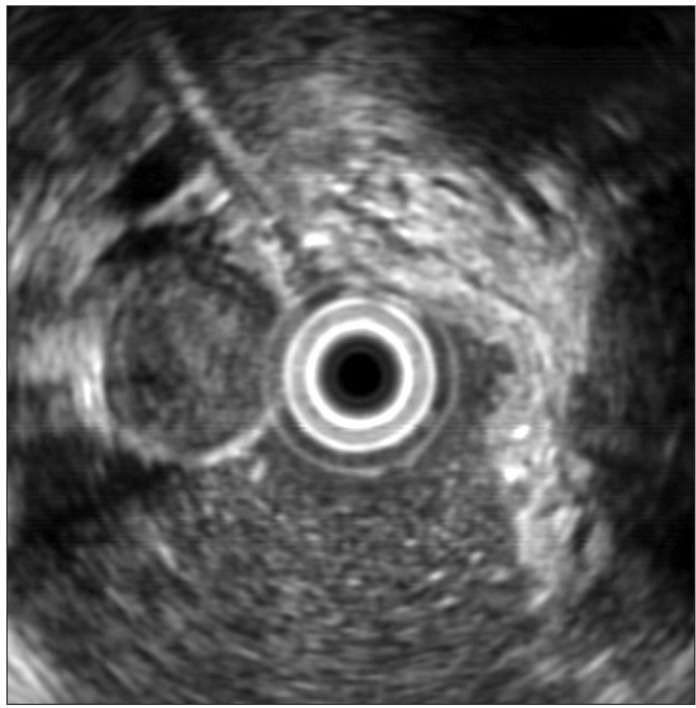
Fig. 2.
Hypoechoic foci in gastric gastrointestinal stromal tumor (GIST) (endoscopic ultrasonography shows a 2.6-cm hypoechoic mass with a relatively hypoechoic portion in the center of a gastric GIST).
DISCUSSION
GISTs are the most common subepithelial mass in the upper GI tract. Malignant behavior is detected in approximately 40% to 50% of GISTs in the small intestine and in about 20% to 25% of gastric GISTs.10 GISTs arise most commonly from the muscularis propria and are usually asymptomatic until the tumor becomes large or ulcerates, resulting in bleeding.
EUS is a useful tool for accurate diagnosis of GIST and differentiation from other subepithelial lesions, and it has been known that EUS features can be used to assist in predicting malignant potential. However, it is uncertain whether specific EUS features can help define malignant risk. It was reported that stromal cell tumors with small size less than 4 cm without EUS features of irregular extraluminal border, cystic spaces, or echogenic foci could be regarded as benign lesion.12 Predictive factors of malignant risk in EUS features include presence of an irregular extraluminal margin, cystic spaces, and lymph nodes with a malignant pattern, and when any two of the three following criteria were met: heterogenous echogenecity, tumor size >3 cm, and irregular margin, the diagnostic sensitivity and specificity for malignant risk was 80% and 77%, respectively.5,7 However, these findings which were proposed from retrospective studies with small sample size have not been validated in prospective studies. Additionally, significant portion of cases in previous studies included leiomyosarcoma or leiomyoma before the era of immunohistochemical diagnosis for c-kit.
Clinical risk of malignancy is determined by tumor size, location, and mitotic index.3,10 Although the preoperative estimate of risk can be made from tumor size and location, mitotic index cannot be known by conventional endoscopic forcep biopsy before surgical resection. As incidental discovery of small-sized GISTs on routine screening endoscopy has increased, prediction of malignant risk is important for clinicians because the resection of all incidental small-sized GISTs with low risk of malignancy may not be needed.
EUS-FNA has emerged recently as an important method for the diagnosis of GISTs. The use of EUS-FNA has been proven to provide enough cytologic material for diagnosis, however, it has limitations of inability to determine malignant potential on cytological specimens and inadequate tissue yield in up to 33.3% of samples.13 The sensitivity of EUS-FNA cytology for the diagnosis of GIST was 78.4% and was influenced by size, location, shape, and layer of origin.9
EUS-guided tissue core biopsy (TCB) using Trucut needle has been proposed to overcome the diagnostic limitations of EUS-FNA. The use of EUS-TCB yields a core tissue specimen in which mitotic activity and other histopathologic features can be more consistently identified. EUS-TCB for the diagnosis of suspected GISTs demonstrated diagnostic histology in 79% and diagnostic immunohistochemical stain in 97% of the cases.14 However, there still remain many disadvantages of EUS-TCB. Given that it relies on triggering a spring-loaded cutting sheath, its use is limited in the distal antrum because of the echoendoscope angulation interfering with its deployment. Moreover, EUS-TCB cannot be applied for small GIST less than 3 cm in size which is shorter than the length of the needle, and the final mitotic index by surgical resection did not correlate with that by EUS-TCB in a study.15 In diagnostic accuracy of GIST, EUS-TCB was not superior to EUS-FNA because of the high rate of technical failure and small size of specimen to assess the mitotic index.16 Therefore, the mitotic index can be reliably calculated only by surgical resection rather than EUS-FNA or TCB.
As gastric GIST larger than 5 cm has high risk malignant potential, complete surgical resection is recommended.17 On the other side, endoscopic surveillance is sufficient for a subset of patients with small-sized gastric GIST less than 2 cm with no EUS features of malignancy. However, treatment algorithm for medium-sized gastric GIST between 2 and 5 cm in size may be controversial, and the usefulness of EUS to predict malignant risk in 2 to 5 cm-sized gastric GIST remains unestablished.18
In this study, the mitotic count and malignant risk of 2 to 5 cm-sized gastric GISTs did not differ significantly among the groups by 1 cm interval in tumor size. EUS features could not also differentiate very low risk group from moderate risk group in multivariate analysis.
The present results showed that 68% of 2 to 5 cm-sized gastric GISTs had very low risk. In a previous large-scaled study, 16% of patients with moderate risk showed disease progression during follow-up, but only 1.9% of patients with very low risk showed disease progression during follow-up.19 Therefore, significant portion of 2 to 5 cm-sized gastric GIST in this study had performed unnecessary surgical resection despite very low risk of malignancy.
Recently, treatment guidelines of GIST have been published by the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO). A biopsy or excision was recommended for gastric GIST over 2 cm in size in ESMO guideline, and NCCN guideline recommended that all GISTs in size of 2 cm or larger should be resected.20,21 In these guidelines, significant portion of gastric GISTs with 2 to 5 cm in size may undergo unnecessary surgical resection despite very low risk of malignancy because mitotic index cannot be accurately evaluated preoperatively. However, there are no established effective methods for predicting risk stratification before surgical resection.
The present results suggest that it is very difficult for conventional EUS features and tumor size to predict the malignant risk of gastric GIST before surgical resection. In a previous study, contrast-enhanced harmonic EUS (CEH-EUS) was reported to have an advantage in visualization of the intratumoral microvascularity, which was an important factor in determining the malignant risk of GISTs, especially for small lesions.22 However, the main disadvantage of CEH-EUS was that it could not distinguish other spindle cell neoplasms (leiomyomas or schwannomas) from GISTs. Therefore, future development of new diagnostic modality rather than conventional methods is needed to predict accurate malignant risk of GIST.
This study has a few limitations as a retrospective analysis. The sample size was too small to analyze the risk of malignancy among the tumor size, and the patients were enrolled after surgical resection to evaluate accurately the risk of malignancy. Therefore, there is a possibility that the patients with low risk of malignancy may be excluded from enrollment without surgical resection.
In conclusion, tumor size and EUS features could not predict malignant risk in medium-sized gastric GISTs. Preoperative diagnostic modality to predict malignant risk is necessary to prevent overtreatment of GIST with low risk of malignancy.
ACKNOWLEDGEMENTS
This study was supported by grant no. 04-2009-4659 from the Seoul National University Hospital research fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477–489. doi: 10.1097/00000478-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 3.Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007;38:679–687. doi: 10.1016/j.humpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Tryggvason G, Kristmundsson T, Orvar K, Jónasson JG, Magnússon MK, Gíslason HG. Clinical study on gastrointestinal stromal tumors (GIST) in Iceland, 1990-2003. Dig Dis Sci. 2007;52:2249–2253. doi: 10.1007/s10620-006-9248-4. [DOI] [PubMed] [Google Scholar]
- 5.Brand B, Oesterhelweg L, Binmoeller KF, et al. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis. 2002;34:290–297. doi: 10.1016/s1590-8658(02)80150-5. [DOI] [PubMed] [Google Scholar]
- 6.Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004;126:301–307. doi: 10.1053/j.gastro.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 7.Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–919. doi: 10.1016/j.gie.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 9.Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254–261. doi: 10.1016/j.gie.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Lok KH, Lai L, Yiu HL, Szeto ML, Leung SK. Endosonographic surveillance of small gastrointestinal tumors originating from muscularis propria. J Gastrointestin Liver Dis. 2009;18:177–180. [PubMed] [Google Scholar]
- 12.Chak A, Canto MI, Rösch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468–473. doi: 10.1016/s0016-5107(97)70175-5. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez SA, Faigel DO. Endoscopic diagnosis of gastrointestinal stromal cell tumors. Curr Opin Gastroenterol. 2007;23:539–543. doi: 10.1097/MOG.0b013e32829fb39f. [DOI] [PubMed] [Google Scholar]
- 14.DeWitt J, Emerson RE, Sherman S, et al. Endoscopic ultrasound-guided Trucut biopsy of gastrointestinal mesenchymal tumor. Surg Endosc. 2011;25:2192–2202. doi: 10.1007/s00464-010-1522-z. [DOI] [PubMed] [Google Scholar]
- 15.Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy. 2009;41:329–334. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy. 2010;42:292–299. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 17.Langer C, Gunawan B, Schüler P, Huber W, Füzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg. 2003;90:332–339. doi: 10.1002/bjs.4046. [DOI] [PubMed] [Google Scholar]
- 18.Akahoshi K, Oya M. Gastrointestinal stromal tumor of the stomach: how to manage? World J Gastrointest Endosc. 2010;2:271–277. doi: 10.4253/wjge.v2.i8.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 20.Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY ESMO Guidelines Working Group. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):64–67. doi: 10.1093/annonc/mdp131. [DOI] [PubMed] [Google Scholar]
- 21.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(Suppl 2):S1–S41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–237. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]