Abstract
Background/Aims
The aims of this study were to evaluate whether doctors and nurses in a single hospital were at an increased risk of acquiring Helicobacter pylori infection in 2011 and to identify risk factors for H. pylori seroprevalence.
Methods
Nurses (n=362), doctors (n=110), health personnel without patient contact (medical control, n=179), and nonhospital controls (n=359) responded to a questionnaire during a health check-up, which included questions on socioeconomic status, education level, working years, and occupation in 2011. The prevalence of H. pylori was measured by serology.
Results
The seroprevalence rate was 29.8% (nurses), 34.5% (doctors), 30.7% (medical control), and 52.9% (nonhospital control). Among younger subjects (<40 years of age), the nonhospital control had a higher seropositivity rate (48.1%) than nurses (29.2%), doctors (29.8%), and the medical control (24.8%), which was not observable in subjects ≥40 years of age. The risk factors for H. pylori seroprevalence were not different for health and nonhealth personnel. A multivariate analysis indicated that seropositivity significantly increased with age, the province of residence, and a gastroscopic finding of a peptic ulcer.
Conclusions
The medical occupation was not associated with H. pylori infection. The seroprevalence of H. pylori in one hospital in 2011 was found to be 38.7%, most likely due to the improvement in socioeconomic status and hospital hygiene policy in Korea.
Keywords: Helicobacter pylori, Serology, Prevalence, Health personnel
INTRODUCTION
Helicobacter pylori is a spiral-shaped, gram-negative bacterium that colonizes the gastric epithelium and causes three important upper gastrointestinal diseases: duodenal or gastric ulcers, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma.1 H. pylori infection is classified as a group I human carcinogen for gastric cancer by World Health Organization in 1994.2
H. pylori is a fascinating enteric pathogen which is disappearing from many developed countries in the absence of any therapeutic or environmental strategy to reduce the prevalence of infection. While the prevalence of H. pylori remains high in the developing world but its incidence is decreasing in these countries.3 As in the developed countries, some studies have shown that H. pylori is declining in Asia, especially in Japan,4 South Korea,5 and China.6 The seroprevalence of H. pylori in Korea was 66.9% in 1998, dropping to 59.6% in 2005 in two consecutive cross-sectional studies. The main effect of decrease in H. pylori seroprevalence was found to be associated with social and economic developments.
It is still uncertain how H. pylori is acquired and transmitted although feco-oral, oro-oral and gastro-oral transmission have been proposed. The likelihood of infection increases with low social status. Most H. pylori infection is acquired in childhood but it is possible that health personnel who are frequently exposed to patients have a higher risk of acquiring this infection than the general population. An increased occupational risk of H. pylori infection has been noted among gastroenterologists and their assistants,7 with an odds ratio (OR) of 1.6 for H. pylori infection in doctors and 1.4 in assistants.
The aims of this study were to determine whether different groups in a single hospital are at increased risk of acquiring H. pylori infection and it was compared to that of health check-up population as a nonhospital control group in 2011, and to find risk factors for H. pylori seroprevalence.
MATERIALS AND METHODS
1. Study population
Between September and December 2011, a cross-sectional study was carried out in 651 health personnel aged ≥20 years. H. pylori serology testing and gastroscopy were performed during their annual health check-up program. In the same period, 359 health check-up subjects were enrolled from a health screening center at Seoul National University Bundang Hospital as nonhospital control group who received the serology test of H. pylori as well as gastroscopy. The subjects who had history of H. pylori eradication or gastric operation were excluded from this study. All subjects (n=1,010) were asked to answer a questionnaire under the supervision of a well-trained interviewer before gastroscopy. The questionnaire included questions regarding demographic data (age, gender, and residence), socioeconomic data (monthly income, education level), working years, smoking, alcohol drinking, gastrointestinal symptoms that persisted for at least 1 month within 3 years. Each subject was categorized according to their residence at the time of examination. Subjects were categorized into three education levels: low (middle school graduates; education duration ≤9 years), middle (high school graduates or university dropouts; education duration 10 to 12 years), and high (university graduates or graduates of a postgraduate course; education duration ≥13 years) and into 4 working years of employment: <1 year, 1 to 5 years, 5 to 10 years, and ≥10 years. Monthly family income was categorized into three groups: low income (<3,000 US dollars per month), middle income (3,000 to 10,000 US dollars per month), and high income (>10,000 US dollars per month). Gastrointestinal symptoms consisted of indigestion, bloating, epigastric soreness, regurgitation, and heartburn. Standard gastroscopy was performed by an endoscopy expert who is blinded to the questionnaire. In case of health personnel endoscopy was optional. The study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital, and all of the participants gave their consent to participate in this study.
2. Serology examination
Serum was collected at entry and was assigned a serial number and stored at -70℃ until measurement of anti-H. pylori immunoglobulin G (IgG). Anti-H. pylori IgG was determined qualitatively using an enzyme-linked immunosorbent assay (ELISA) (Genedia H. pylori ELISA; Green Cross Medical Science Corp., Eumseong, Korea). The Genedia kits use H. pylori antigens obtained from Korean H. pylori strains, and have sensitivity and specificity of 97.8% and 92.0% for Korean adults.8 The cutoff optical density (450 nm) used for H. pylori IgG was the mean value of a negative control plus 0.400, as described by the manufacturer. This test method was previously used in a nationwide Korean seroepidemiologic study of H. pylori infection in asymptomatic subjects.5,9
3. Statistical analysis
Data analyses were performed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). The Pearson chi-square test was used to examine the association between H. pylori infection and variables of interest, and p<0.05 was considered statistically significant. Multiple logistic regression modeling included H. pylori seropositivity as the dependent variable and all the other variables in this study as independent variables. The OR and 95% confidence interval (CI) were presented for potential risk factors.
RESULTS
1. Seroprevalence of H. pylori infection
This study included 651 health check-up health personnel (aged ≥20 years) and 359 health check-up subjects (nonhospital control) from health screening center. Of 1,010 subjects, the risk factors for H. pylori infection were investigated (Table 1).
Table 1.
Baseline Characteristics
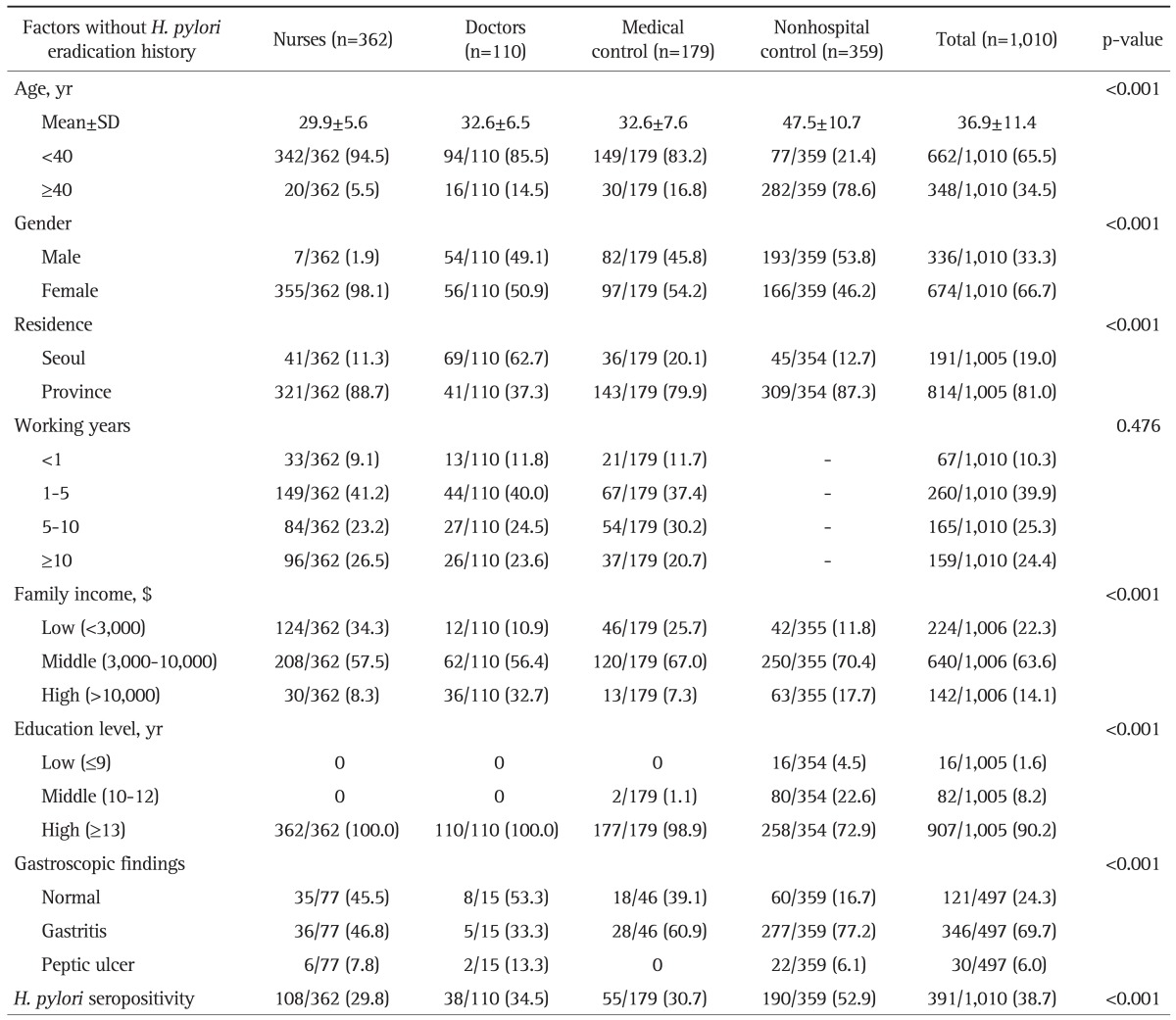
Data are presented as number (%).
SD, standard deviation; H. pylori, Helicobacter pylori.
A 651 health personnel included three kinds of jobs: nurses (n=362; persons working in the wards, outpatient clinic, and special examination units), doctors (n=110; interns, residents, fellows, and professors), and medical control (n=179; office, administration, technical, and pharmacy part without patient contact).
The overall seroprevalence of H. pylori was 38.7% (391 of 1,010 subjects). The mean age of the total 1,010 subjects was 36.9±11.4 (mean±SD) years. In case of 391 H. pylori-positive subjects it was 40.6±12.1 years, which was significantly higher than that of H. pylori-negative, 34.5±10.2 years (p<0.001). Age differed among four groups; nurses (29.9±5.6 years), doctors (32.6±6.5 years), medical control (32.6±7.6 years), and nonhospital control (47.5±10.7 years). In addition to age there were many differences among these four groups such as gender, residence, family income, education levels, gastroscopic findings and H. pylori seropositivity (Table 1). Among younger subjects (<40 years), nonhospital control had a higher seropositivity rates (48.1%) than those of nurses (29.2%), doctors (29.8%), medical control (24.8%). However, among older subjects (≥40 years), the difference disappeared.
Among 651 subjects who were health personnel, there were significant differences in gender, residence, family income. However, seroprevalence of H. pylori in nurses and doctors did not differ from that in medical control subjects (29.8%, 34.5% vs 30.7%).
2. Risk factors for H. pylori infection
The risk factors of H. pylori seropositivity were investigated using univariate and multivariate analysis for all variables in total of 1,010 subjects (Table 2). Socioeconomic status (family monthly income, education level), demographic data (age, gender, and residence), gastroscopic findings were found to be risk factors for seropositivity by univariate analysis (Table 2). Logistic regression modeling of all subjects revealed significant associations with age (age ≥40 years) OR, 1.98 (95% CI, 1.47 to 2.66); residence (province) OR, 1.42 (95% CI, 1.09 to 2.00); and gastroscopic findings (peptic ulcer group) OR, 2.05 (95% CI, 1.04 to 4.06) (Table 2).
Table 2.
Risk Factors of Helicobacter pylori Seropositivity in 1,010 Subjects without a History of H. pylori Eradication
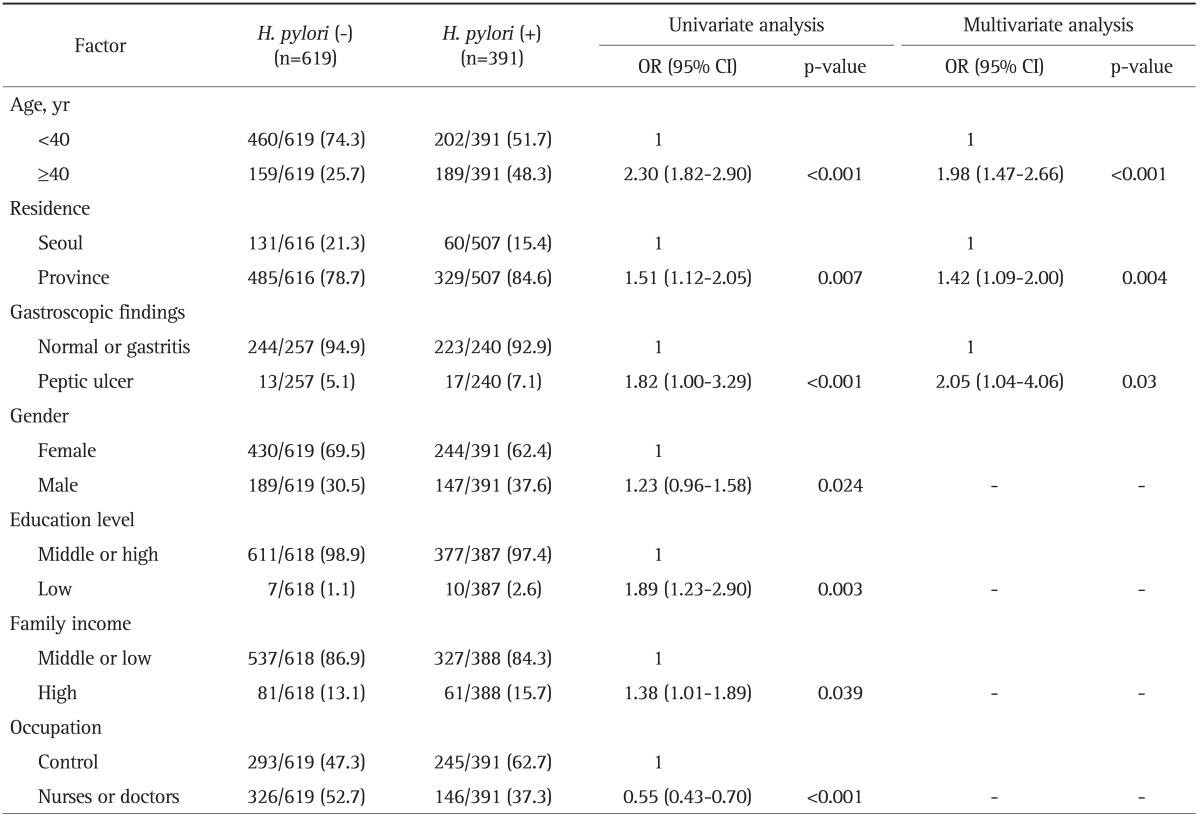
Data are presented as number (%). Missing values were not included.
OR, odds ratio; CI, confidence interval.
Logistic regression analysis for all variables in 651 health personnel showed similar results (Table 3). According to gastroscopic findings, the risk for H. pylori infection was higher in the peptic ulcer group OR, 5.85 (95% CI, 1.86 to 18.32) than in normal or gastritis group. H. pylori seroprevalence was significantly higher among hospital working years ≥10 years subjects OR, 1.95 (95% CI, 1.29 to 2.95).
Table 3.
Risk Factors of Helicobacter pylori Seropositivity in 651 Health Personnel
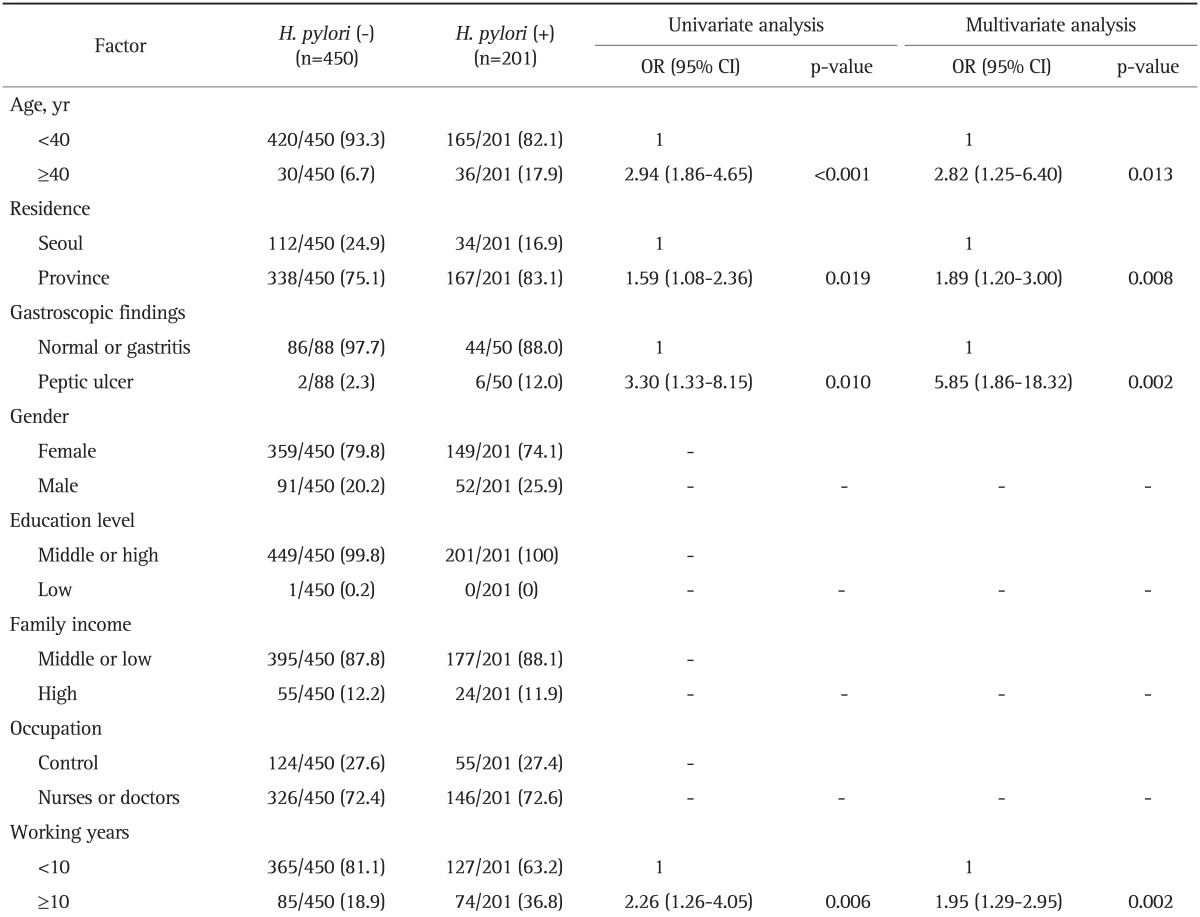
Data are presented as number (%). Missing values were not included.
OR, odds ratio; CI, confidence interval.
DISCUSSION
The present cross-sectional analysis of H. pylori infection at a single institution shows the seroprevalence and the risk factors for H. pylori infection of health personnel and Korean adult population who received survey during annual health check-up. Unexpectedly the seroprevalence to H. pylori did not differ significantly within health personnel. Neither the patient contact affected the risk of infection with H. pylori.
Age is strongly related to H. pylori infection10 and the prevalence of infection in this study also increased with age. The association between age and H. pylori seropositivity may be caused by two different mechanisms: an age effect (a longer life increases the chance to acquire infection) or a cohort effect (the risk for infection was higher in childhood).11 The likeliest explanation is a birth cohort effect.12,13 By age 29 years, the seroprevalence was 25.9%, and this low prevalence in early life is more typical of developed countries than developing countries. The younger age category (<40 years) showed the higher seroprevalence (48.1%) in nonhospital control compared with those of health personnel: nurses 29.2%, doctors 29.8%, and medical control 24.8%, while the older age category (≥40 years) showed the similar higher seroprevalence (54.3%) in nonhospital control; nurses 40.0%, doctors 62.5%, medical control 60.0%. These results seems to be that patient contact might not affect on the seroprevalence of H. pylori, which is different from the previous report.7
The importance of low socioeconomic status on the H. pylori seropositivity has been demonstrated in a few population-based studies.14-16 A recent study from Australia also showed that H. pylori infection is more common among those living in socioeconomically disadvantaged areas or who were born overseas.16 In another Korean study, white collar workers had a lower risk of H. pylori infection. In a study in 588 employees of the Subway Corporation in Seoul, white collar workers were 44.6% (n=203) in H. pylori-positive compared with 57.9% (n=77) in the H. pylori-negative group.17
Conflicting data have been reported on occupational risk in health personnel for H. pylori infection.7,18,19 One longitudinal study in Greece found that nurses had a significantly higher risk of infection compared with doctors and medical controls.19 Peters et al.7 also reported the occupational risk of H. pylori infection among gastroenterologists and their assistants. When the studies were stratified by nonhospital controls, analysis showed significant risks for doctors (OR, 1.39) and for nurses (OR, 1.37), whereas medical controls were not at elevated risk. That is, the risk of H. pylori infection was different within the health personnel in that those who are exposed to gastric secretions are at highest risk unless universal precautions are used. The risk should also have been related to how long one has been in a potential high risk area. Our cross-sectional study evaluated a 651 Korean health personnel, predominantly of young age category (<40 years) in 2011. In contrast to the previous reports, our cross sectional study did not show risk of patient contact; that is the seroprevalences among doctors (34.5%), nurses (29.8%), and medical controls (30.7%) were similar. This similarity among occupations might have been originated from the recent regulation of complete protection from patient and probably related with the increase of disposable of many medical equipments such as gloves, nasogastric tubes, masks when endoscopy procedures are performed, etc.
The interesting result in the present study is that there was a difference between health personnel and nonhospital subjects. That is, among younger subjects (<40 years), nonhospital control had a significant higher seropositivity rates (52.9%) than did nurses (29.8%), doctors (34.5%), medical control (30.7%). However, among older subjects (≥40 years), the difference disappeared. This difference in seropositivity between health personnel and health check-up nonhospital controls may be due to several factors. First, the representative control group may be important, because the selection of a poorly matched control group can alter outcomes. We used subjects not involved in patient contact working in the same institution as medical control group. Second, our hospital hygiene policy within 10 years provide the strict regulation and regular monitoring such as washing hands, facial masks, wearing gowns, which help to block the major pathway in H. pylori infection via oro-oral route. These practices may be effective tools in preventing H. pylori infection by regurged gastric secretions and may explain why the H. pylori seroprevalence in subjects involved in patient contact was not different from that in subjects without patient contact. Third, H. pylori seropositivity increased with age in all study groups, the number of years working in hospital was not a risk factor. Thus, higher seropositivity rates among the older age category (>40 years) may reflect childhood infection, but not affected by patient contact. That is, higher seroprevalences in older age category reflects higher infection rates due to poor hygiene during childhoods rather than de novo infections resulting from occupational exposure. Fourth, nonhospital group may have possibility of health problems such as peptic ulcer disease or gastroesophageal reflux disease compared with general population. In Korea, young people do not usually receive a health check-up without any symptoms.
The H. pylori prevalence rates of the general population in Korea (59.6%) corroborate the findings in our nonhospital controls.5 The prevalence of H. pylori infection observed in this study (38.7%), which was only from one institution and in 2011, looks like lower than that observed for other nation-wide Korean study in 2005 which was 59.6%. Changes over time in seropositivity rates occur during a period of improved socioeconomic status and changed life style. In addition, the frequency of eradication therapy for H. pylori has increased from 1991 in Korea. The seroprevalence in our health personnel is similar to that in developed countries such as the United States (32%).20 Several studies have shown that H. pylori prevalence is declining to 39.3% in Japan,4 58% in China,21 54.5% in Taiwan,22 and 31% in Singapore.23 Lower H. pylori seroprevalence in younger age groups is related to socioeconomic improvement, especially in early childhood and primary school age,14,24 concurrent with Korea's progress to a developed country.25 In fact, the Korean Gross National Income increased from 7,355 US dollars in 1998 to 20,759 US dollars in 2010 (according to reports from the Bureau of Statistics, Korea), leading to community awareness of sanitation and ultimately the decrease in the prevalence of H. pylori infection.
Inclusion of medical and nonhospital subjects raises generalizability of our study, and broad differences across these groups were enlightening. Strength of current study is adequate selection of control group including medical and nonhospital subjects. The composition of the control group was found to have a considerable impact. The inclusion of controls was from the general population (nonhospital control) to health personnel (medical control). Second, the precise categorization of health personnel according to the patient contact allowed us to examine possible occupational risk of H. pylori transmission. However, the main limitation of this study is its cross-sectional design, which makes a part of explanation for the controversy. Previous studies concerning the rate of H. pylori infection in health personnel produced conflicting results. A prospective study would be a better model in the current era when H. pylori prevalence is in decline. Second, imbalance of subject population can affect the results of this study.
In conclusion, medical occupation was not associated with H. pylori infection. The seroprevalence of H. pylori in one hospital in 2011 was found to be 38.7%, probably due to the improvement of socioeconomic status and hospital hygiene policy in Korea.
ACKNOWLEDGEMENTS
This work was supported by grant no. B-1106/130-006 from the Seoul National University Bundang Hospital Research Fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 3.Sýkora J, Rowland M. Helicobacter pylori in pediatrics. Helicobacter. 2011;16(Suppl 1):59–64. doi: 10.1111/j.1523-5378.2011.00882.x. [DOI] [PubMed] [Google Scholar]
- 4.Fujisawa T, Kumagai T, Akamatsu T, Kiyosawa K, Matsunaga Y. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol. 1999;94:2094–2099. doi: 10.1111/j.1572-0241.1999.01283.x. [DOI] [PubMed] [Google Scholar]
- 5.Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Bu XL, Wang QY, Hu PJ, Chen MH. Decreasing seroprevalence of Helicobacter pylori infection during 1993-2003 in Guangzhou, southern China. Helicobacter. 2007;12:164–169. doi: 10.1111/j.1523-5378.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters C, Schablon A, Harling M, Wohlert C, Costa JT, Nienhaus A. The occupational risk of Helicobacter pylori infection among gastroenterologists and their assistants. BMC Infect Dis. 2011;11:154. doi: 10.1186/1471-2334-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SY, Ahn JS, Ha YJ, et al. Serodiagnosis of Helicobacter pylori infection in Korean patients using enzyme-linked immunosorbent assay. J Immunoassay. 1998;19:251–270. doi: 10.1080/01971529808005485. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim HY, Kim NY, et al. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001;16:969–975. doi: 10.1046/j.1440-1746.2001.02568.x. [DOI] [PubMed] [Google Scholar]
- 10.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9(Suppl 2):33–39. [PubMed] [Google Scholar]
- 11.Cullen DJ, Collins BJ, Christiansen KJ, et al. When is Helicobacter pylori infection acquired? Gut. 1993;34:1681–1682. doi: 10.1136/gut.34.12.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haruma K, Okamoto S, Kawaguchi H, et al. Reduced incidence of Helicobacter pylori infection in young Japanese persons between the 1970s and the 1990s. J Clin Gastroenterol. 1997;25:583–586. doi: 10.1097/00004836-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Roosendaal R, Kuipers EJ, Buitenwerf J, et al. Helicobacter pylori and the birth cohort effect: evidence of a continuous decrease of infection rates in childhood. Am J Gastroenterol. 1997;92:1480–1482. [PubMed] [Google Scholar]
- 14.Ford AC, Axon AT. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2010;15(Suppl 1):1–6. doi: 10.1111/j.1523-5378.2010.00779.x. [DOI] [PubMed] [Google Scholar]
- 15.Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16(Suppl 1):1–9. doi: 10.1111/j.1523-5378.2011.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandeya N, Whiteman DC Australian Cancer Study. Prevalence and determinants of Helicobacter pylori sero-positivity in the Australian adult community. J Gastroenterol Hepatol. 2011;26:1283–1289. doi: 10.1111/j.1440-1746.2011.06726.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim N, Lim SH, Lee KH, et al. Seroconversion of Helicobacter pylori in Korean male employees. Scand J Gastroenterol. 2005;40:1021–1027. doi: 10.1080/00365520510015917. [DOI] [PubMed] [Google Scholar]
- 18.Rosenstock SJ, Andersen LP, Rosenstock CV, Bonnevie O, Jørgensen T. Socioeconomic factors in Helicobacter pylori infection among Danish adults. Am J Public Health. 1996;86:1539–1544. doi: 10.2105/ajph.86.11.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triantafillidis JK, Gikas A, Hyphantis T, et al. Helicobacter pylori infection in hospital workers over a 5-year period: correlation with demographic and clinical parameters. J Gastroenterol. 2002;37:1005–1013. doi: 10.1007/s005350200170. [DOI] [PubMed] [Google Scholar]
- 20.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359–1363. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 21.Wang KJ, Wang RT. Meta-analysis on the epidemiology of Helicobacter pylori infection in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:443–446. [PubMed] [Google Scholar]
- 22.Teh BH, Lin JT, Pan WH, et al. Seroprevalence and associated risk factors of Helicobacter pylori infection in Taiwan. Anticancer Res. 1994;14(3B):1389–1392. [PubMed] [Google Scholar]
- 23.Fock KM. Helicobacter pylori infection: current status in Singapore. Ann Acad Med Singapore. 1997;26:637–641. [PubMed] [Google Scholar]
- 24.Magalhães Queiroz DM, Luzza F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2006;11(Suppl 1):1–5. doi: 10.1111/j.1478-405X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 25.Lindkvist P, Asrat D, Nilsson I, et al. Age at acquisition of Helicobacter pylori infection: comparison of a high and a low prevalence country. Scand J Infect Dis. 1996;28:181–184. doi: 10.3109/00365549609049072. [DOI] [PubMed] [Google Scholar]


