Abstract
Background/Aims
Stent migration occurs frequently, but the prevention of complications resulting from covered self-expandable metal stents (C-SEMSs) remains unresolved. We prospectively assessed a newly developed C-SEMS, a modified covered Zeo stent (m-CZS), in terms of its antimigration effect.
Methods
Between February 2010 and January 2011, an m-CZS was inserted into 42 patients (31 initial drainage cases and 11 reintervention cases) at a tertiary referral center and three affiliated hospitals. The laser-cut stent was flared for 1.5 cm at both ends, with a 1 cm raised bank located 1 cm in from each flared end. The main outcome of this study was the rate of stent migration, and secondary outcomes were the rate of recurrent biliary obstruction (RBO), the time to RBO, the frequencies of complications, and overall survival.
Results
Of the 31 patients with initial drainage, stent migration occurred in four (12.9%, 95% confidence interval, 5.1% to 29.0%), with a mean time of 131 days. RBO occurred in 18 (58%), with a median time to RBO of 107 days. Following previous C-SEMS migration, seven of 10 patients (70%) did not experience m-CZS migration until death.
Conclusions
m-CZSs with antimigration properties effectively, although not completely, prevented stent migration after stent insertion.
Keywords: Cholestasis, extrahepatic; Stents; Cholangiography; Endoscopic retrograde
INTRODUCTION
Covered self-expandable metal stent (C-SEMS) placement is a widely accepted procedure for the management of distal malignant biliary obstruction.1 C-SEMSs can prevent tumor ingrowth while maintaining stent patency. However, current C-SEMSs, particularly braided SEMSs, display high shortening rates, making it difficult to achieve precise length and placement. Laser-cut nitinol stents display no such shortening.
Despite the extended patency of C-SEMSs, the high rate of complications compared to uncovered SEMSs (U-SEMSs) is a major concern.2 Stent migration remains an unresolved complication of C-SEMSs. C-SEMSs and their antimigration properties have been evaluated in patients with benign pancreatobiliary strictures and malignant biliary obstruction. An improved prognosis in accordance with the recent development of chemotherapy will necessitate C-SEMS with longer patency without stent migration.3,4
We developed a covered Zeo stent (CZS; ZEON Medical Inc., Kawasaki, Japan) with flare and bank structures that prevent stent migration. This is the first commercially available laser-cut type C-SEMS. We hypothesized that a C-SEMS with antimigration properties would prevent migration and prolong stent patency.
This was a prospective pilot feasibility study. The aim was to evaluate the safety and efficacy of C-SEMSs with antimigration properties in patients with nonresectable distal malignant biliary obstruction.
MATERIALS AND METHODS
1. An original CZS (stage 1)
The newly designed fully covered metal stent is made of laser-cut nitinol (nickel-titanium alloy) (Fig. 1). The covered membrane consists of polyurethane and polyolefin elastomer. The basic characteristics of this stent are those of an uncovered Zeo stent (ZEON Medical Inc.): the stent has 12 V-shaped struts with flexible wave-shaped hinges connected at three positions. This stent does not shorten after insertion. We used CZSs, 10 mm in diameter and 60 to 80 mm in length.
Fig. 1.

Original fully covered Zeo stent. Three placed stents migrated within 1 week of placement, and the stents' use was terminated.
A 9.1-Fr pull-back stent delivery system with a grasping handle connected to a water container was used (Fig. 2). When the operator grasped the handle, a small volume of water was injected into the inner lumen, towards the proximal side of the container, while pulling back the outer sheath. This water supply system, termed the pumping flush system, was developed for smooth stent deployment. Supplied water is infused into the space between the stent and the outer sheath, and the stent is cooled to prevent increased radial force (RF) due to expansion of the nitinol.
Fig. 2.
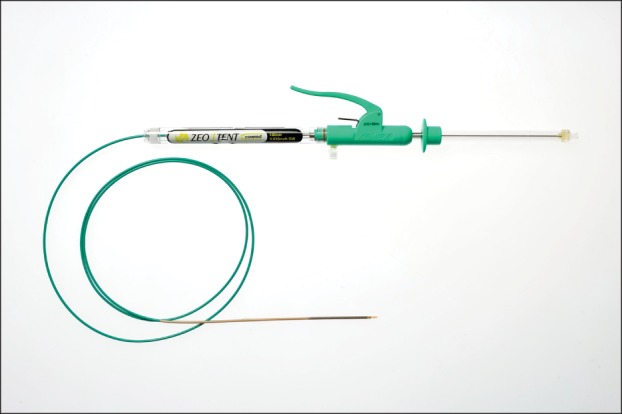
Covered Zeo stent 10-Fr delivery system. A pumping flush system: when the operator grasps the handle, a small volume of water is injected into the inner lumen, toward the proximal side of the container of the grasping handle, while pulling back the outer sheath.
Between November 2009 and December 2009, three patients with distal malignant biliary obstruction due to nonresectable pancreatic cancer underwent placement of an original CZS at the University of Tokyo Hospital. Written informed consent was obtained from all patients. Our local ethical committee approved this study. Endoscopic stent insertion was successful in all patients and no stent-related complications were observed. Following stent placement, all three original CZSs spontaneously migrated from the bile duct and were eliminated from their bodies within a week. As a result, stage 1 of the study protocol was discontinued at this point.
2. A modified CZS with antimigration properties (stage 2)
In view of the results of stage 1, we added an antimigration property to the original CZS by using a flare and band structure. Both ends of the modified CZS (m-CZS) were flared for 1.5 cm, and a raised band was added at each end, 1 cm in from the 1.5 cm flared portion (Fig. 3). The stents were 60 or 80 mm in length, with a diameter of 10 mm. The axial force (AF) of each stent was 0.26 N (60°) and the RF was 7.1 N (φ 4 mm). The mechanical properties of the stent were measured using conditions described previously.5
Fig. 3.

Modified covered Zeo stent with antimigration properties. This stent has the flare and bank structure.
3. Study design
Stage 2 of the study protocol was conducted as a multicenter prospective consecutive feasibility study at the University of Tokyo Hospital and three affiliated hospitals (Japanese Red Cross Medical Center, Kanto Central Hospital, and Tokyo Police Hospital), and was approved by the ethical committee at each institution. Patients with nonresectable distal malignant biliary obstruction were included in this study. Each patient provided written informed consent for participation.
4. Patients
Patients with nonresectable distal malignant biliary obstruction between February 2010 and January 2011 were recruited. Patients with dysfunction in previously placed C-SEMSs were also included. Each patient underwent m-CZS insertion at the University of Tokyo Hospital or at three affiliated hospitals.
5. Endoscopic stent placement
All patients underwent m-CZS placement as an inpatient. The m-CZS was inserted using a therapeutic duodenoscope with a working channel of 4.2 mm (TJF240 or TJF260V; Olympus, Tokyo, Japan) under fluoroscopic and endoscopic guidance (Fig. 4). The length of stent was selected following measurement of the biliary stricture.6 In patients with previous SEMS placement, the stent was removed and the m-CZS was inserted in a single session. Endoscopic biliary sphincterotomy or papillary balloon dilation was performed at the discretion of the endoscopist. The distal end of the m-CZS was positioned above or below the papilla according to the position of the biliary stricture.
Fig. 4.
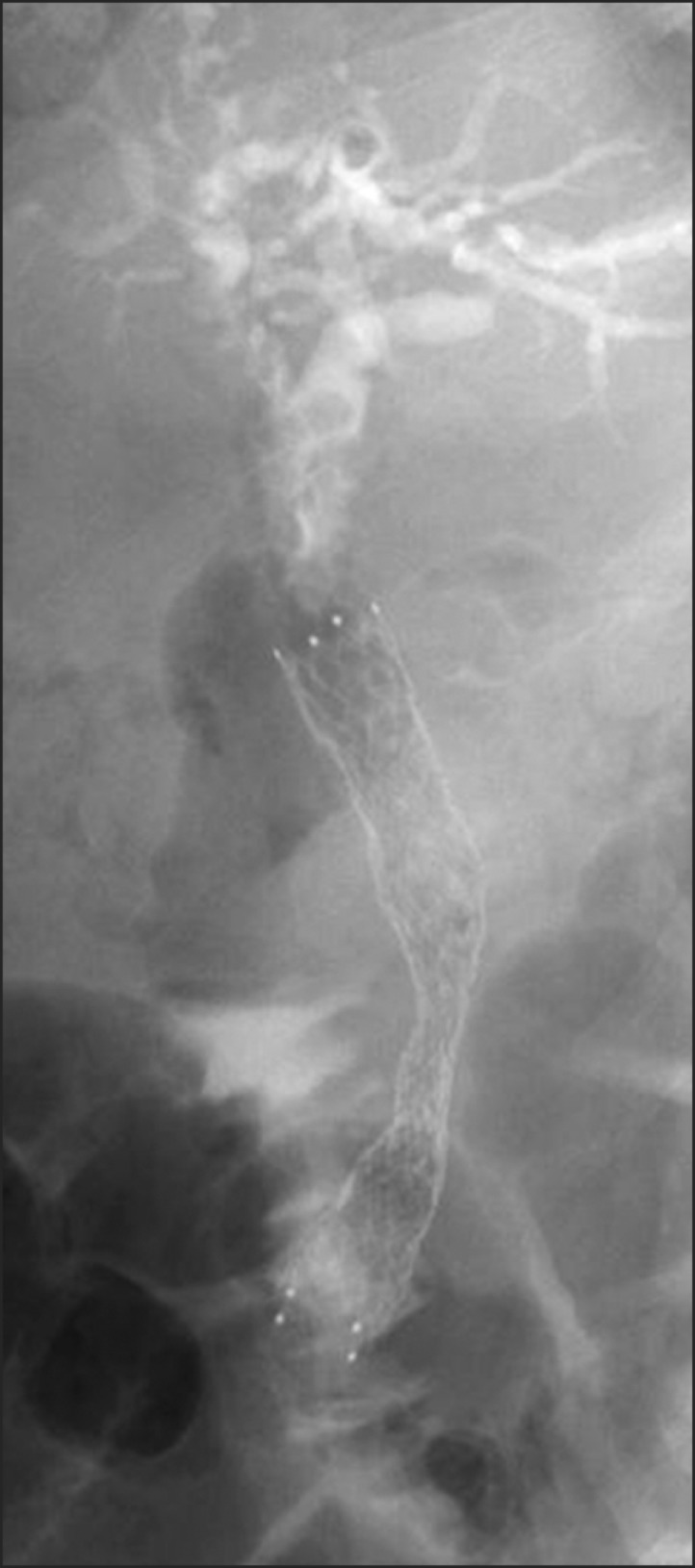
Abdominal X-rays displaying a modified covered Zeo stent that conforms to the shape of the bile duct.
6. Outcome measurements
The primary outcome of this study was the rate of stent migration. Proximal stent migration was defined as any migration of the stent into the bile duct in conjunction with stent dysfunction. Distal stent migration was defined as any stent dislodgement towards the duodenum in conjunction with stent dysfunction. Secondary outcomes were the rate of recurrent biliary obstruction (RBO), time to RBO using the Kaplan-Meier method, frequencies of complications, and overall survival after C-SEMS insertion. The definition of RBO was the recurrence of jaundice and/or cholangitis following SEM insertion, including stent migration. Tumor ingrowth and overgrowth were diagnosed by cholangiography. Cholangitis without stent occlusion was defined as cholangiography revealing no stent occlusion in cases with fever and cholestasis without jaundice. Complications were evaluated using consensus criteria. Statistical analysis was performed using JMP 9.0.1 (SAS Institute, Cary, NC, USA).
RESULTS
1. Patient characteristics
During stage 2 of the study, 42 patients received an m-CZS at a participating hospital (Table 1). Thirty-one patients underwent biliary drainage (initial drainage), while the remaining 11 exchanged previously placed C-SEMSs for an m-CZS due to RBO. The median age was 75 years, and 28 (67%) were male. The causes of biliary obstruction were pancreatic cancer (n=25), bile duct carcinoma (n=5), gallbladder carcinoma (n=3), lymph node metastasis (n=6), and ampullary carcinoma (n=3). Fourteen patients received 6 cm m-CZSs, the remainder received 8 cm m-CZSs. Endoscopic m-CZS insertion using a therapeutic duodenoscope was successful in all patients (TJF240 or 260). Endoscopic sphincterotomy (EST) and endoscopic papillary balloon dilation (EPBD) were performed in 23 and three patients, respectively. The remaining 16 patients did not undergo EST or EPBD. No stent-insertion-related complications were observed.
Table 1.
Patient Characteristics (n=42)
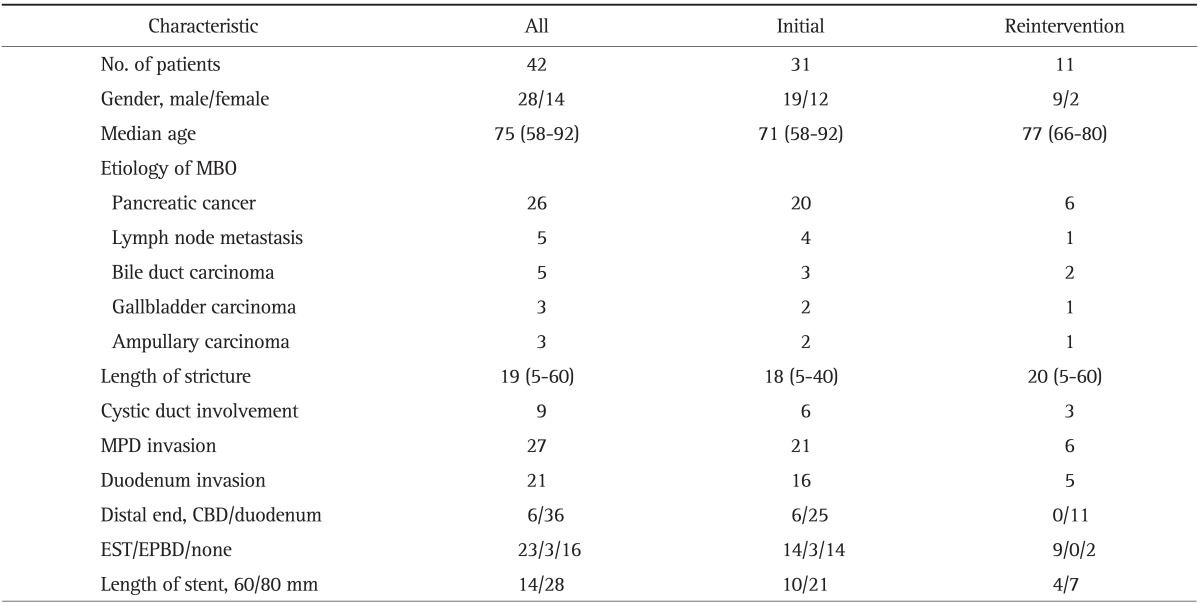
Data are presented as number or median (range).
MBO, malignant biliary obstruction; MPD, main pancreatic duct; CBD, common bile duct; EST, endoscopic sphincterotomy; EPBD, endoscopic papillary balloon dilation.
2. Outcomes
1) Initial drainage
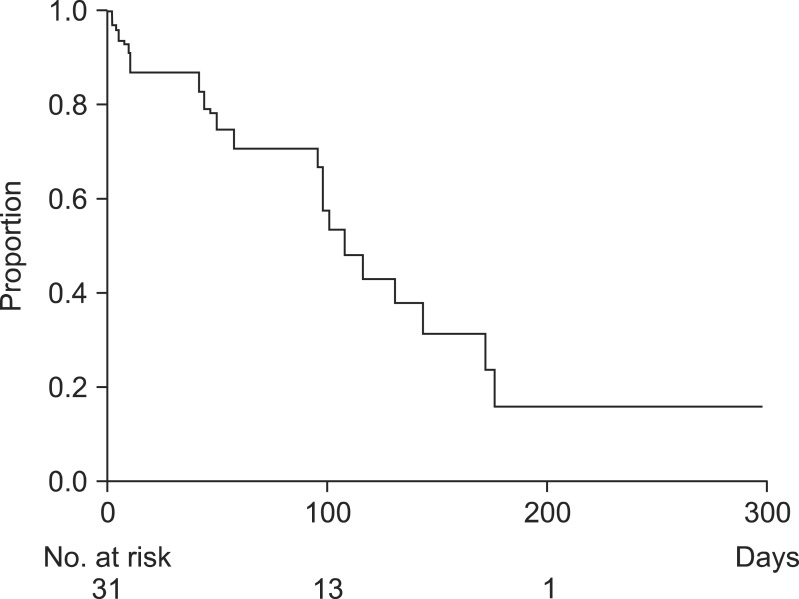
During the follow-up period, four m-CZS (12.9%, 95% confidence interval [CI], 5.1% to 29.0%) migrated, with a mean time of 131 days. Furthermore, 18 m-CZS (58%) insertions resulted in RBO. The median time to RBO was 107 days (95% CI, 56 to 171 days) (Table 2). The cumulative time to RBO curve was calculated using the Kaplan-Meier method (Fig. 5). The causes of RBO were tumor ingrowth (n=2), tumor overgrowth (n=1), biliary sludge (n=6), food impaction (n=3), stent migration (n=4), and ascending cholangitis (n=2). Twenty-two patients died during the study period and the median survival time after m-CMS placement was 127 days. Three (10%) cases (one each of pancreatic cancer, lymph node metastasis, and gallbladder cancer) of pancreatitis were observed, all of which were mild according to the consensus criteria, and resolved with conservative management. No cholecystitis following m-CZS placement was observed.
Table 2.
Clinical Outcomes of a Modified Covered Zeo Stent with Antimigratory Properties in Patients with Initial Drainage (n=31)
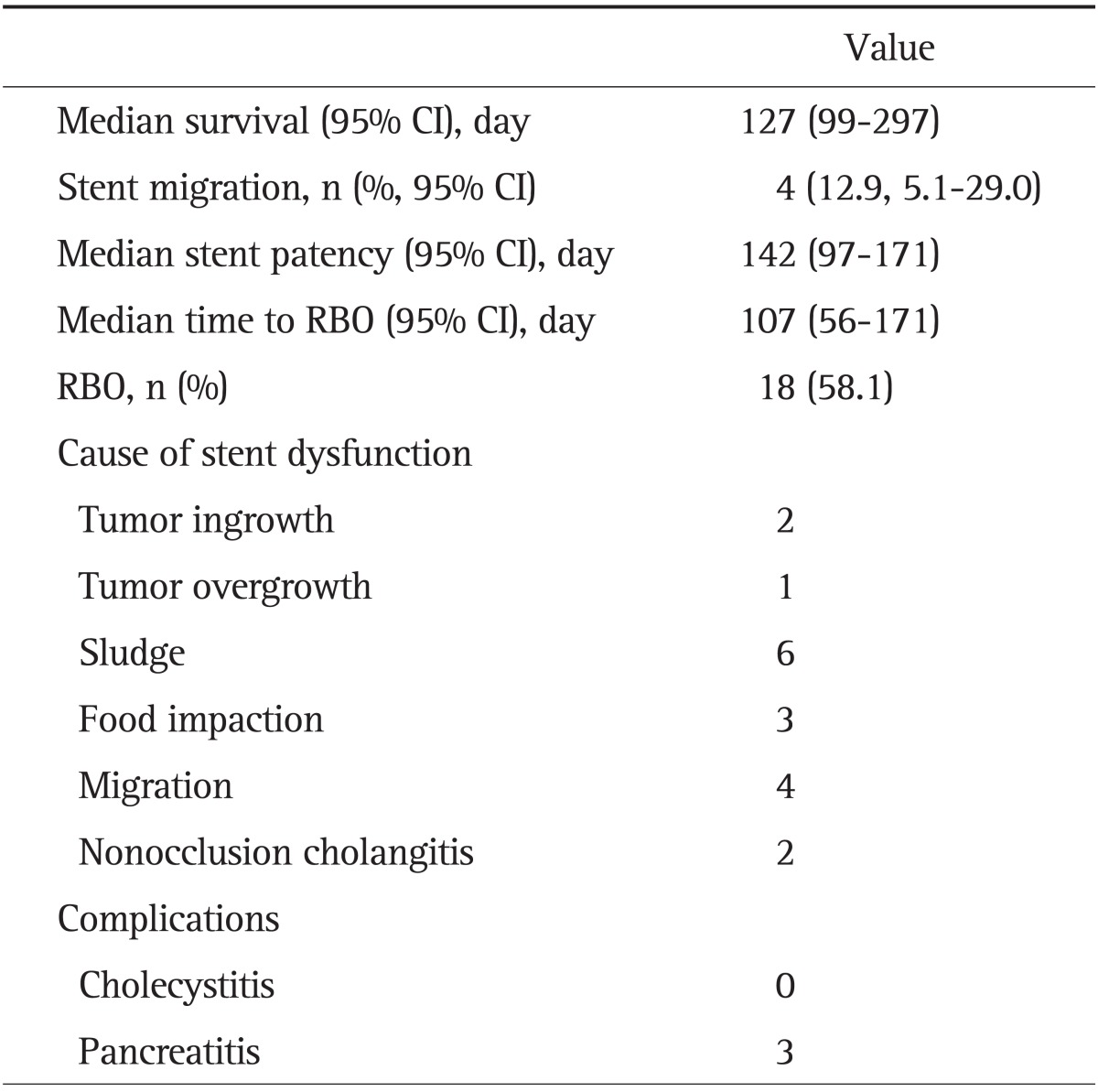
CI, confidence interval; RBO, recurrent biliary obstruction.
Fig. 5.
Kaplan-Meier graphs illustrating the time to recurrent biliary obstruction in patients with initial drainage.
2) Reintervention
Eleven patients received an m-CZS as a re-intervention due to previous C-SEMS dysfunction. Among them, the initial C-SEMS had migrated in 10. After placement of an m-CZS in these 10 patients, seven did not experience stent migration (Table 3). The rate of stent migration was 30.0% (95% CI, 10.7 to 60.6). Five m-CZS resulted in RBO, at a median time of 176 days (95% CI, 43 to 197). No stent-insertion-related complication was observed.
Table 3.
Clinical Outcomes of a Covered Zeo Stent with Antimigratory Properties as a Reintervention in Patients with Previous Self-Expandable Metal Stent Migration (n=10)
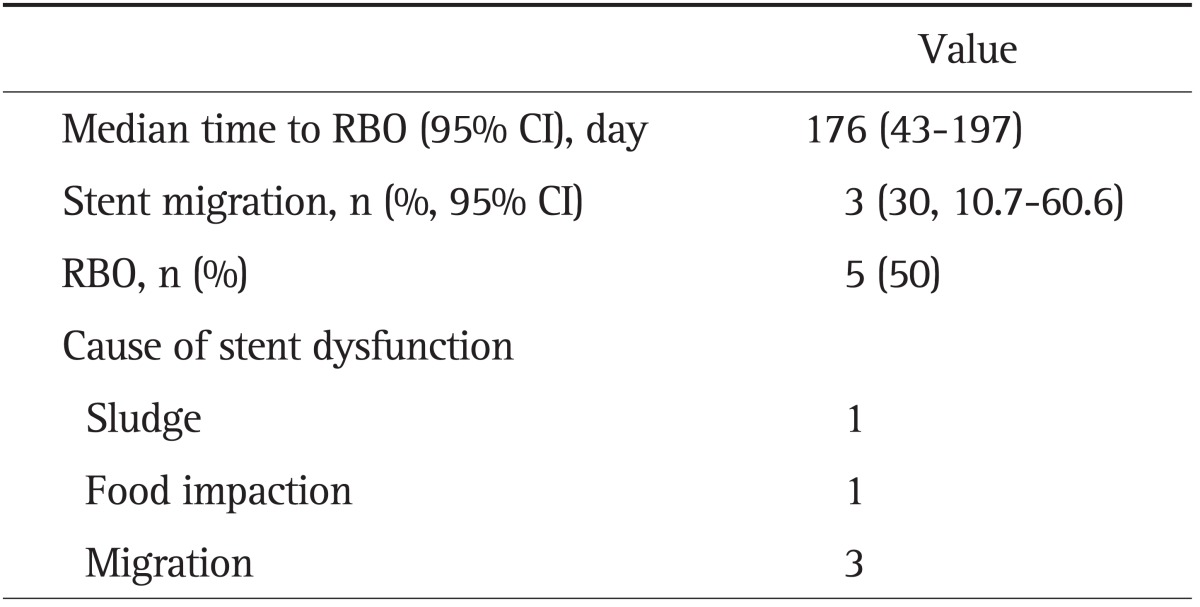
RBO, recurrent biliary obstruction; CI, confidence interval.
DISCUSSION
Stent migration remains an unresolved complication following SEMS placement. Our newly designed fully covered metal stent with antimigration properties (m-CZS) displays promising effects against stent migration. The flare and bank features contribute to preventing the migration of these metal stents. However, stent migration was not completely eliminated, so there remains a continuing need for more sophisticated methods to prevent stent migration.
The antimigration features of SEMS have been evaluated and can be divided into two principal strategies to protect against stent migration, termed the flared end and anchoring flap. Moon et al.7 reported that SEMSs with flared ends and an irregular cell size can prevent stent migration for benign pancreatic duct strictures. Park et al.8 compared the antimigration effects of two types of SEMSs and concluded that the anchoring design was superior to the flared-end design. The antimigration properties of the m-CZS stent are mediated by the flared ends and the two raised bands in the stent; however, these do not completely prevent stent migration. Considering that all the original CZSs migrated within one week of stent insertion, the flare and band structure effectively protected against stent migration. From this point of view, this stent might prevent migration more effectively with further improvements. Furthermore, in our study, an m-CZS was placed as a reintervention in 10 patients who had already suffered SEMS migration. The m-CZS prevented stent migration in seven (70%), displaying a similar stent function period to that seen in initial drainage patients. Further prospective studies are now necessary to evaluate the antimigration properties of m-CZSs, not only as initial drainage but also as a re-intervention for patients who experience stent migration.
Two cases of tumor ingrowth were observed as a stent dysfunction in this study. In general, the C-SEMS was developed to overcome tumor ingrowth in U-SEMSs.1 Several types of membrane materials are present in C-SEMSs, i.e., expanded polytetrafluoroethylene, polyurethane or silicone, and any C-SEMS can prevent tumor ingrowth, according to our previous studies.1,9-11 However, cases of tumor ingrowth with C-SEMS using silicone and polycarbonate-polyurethane have been reported recently.12,13 In these studies, the covering membrane consisted of two layers of polyurethane and polyolefin elastomer. Degradation of the covered membrane represents a possible mechanism of tumor ingrowth,14 meaning that a more durable membrane is required. This is particularly so in the case of laser-cut nitinol stents, since the sharp edges between the cells can lead to membrane damage.
The SEMS design is divided into the braided and laser-cut types. The CZS was the first commercially available C-SEMS with a laser-cut structure. The major advantage of the laser-cut type is the ease of stent placement compared to the braided type, as stent shortening does not occur. However, removal difficulties due to the sharp edges between the cells are a major drawback of the laser-cut stent. In contrast, braided-stent removal is both safe and simple.15 In this study, stent removal was attempted, and a stent fracture occurred in one patient. The antimigration and removability properties of stents tend to conflict, but the development of a stent that possesses both is mandatory for improved management.
The characteristics of an SEMS are dependent on its mechanical properties, such as AF and RF.5 Placement of SEMSs with a high AF has been reported to be significantly related to the development of pancreatitis, stent patency, and cholecystitis.16-19 We measured the AF and RF of m-CZSs using methods described previously.5 The AF 20 mm from the bending point was 0.26 N and the RF at an SEMS diameter of 4 mm was 7.10 N. The AF of m-CZSs was classified as low, meaning the stents conform to the shape of the bile duct, with no obvious kinking (Fig. 4). Furthermore, this may contribute to the previously reported low complication rate.17,18 Since the stent patency was short compared to previous studies, further revisions are necessary to improve SEMS performance.
The limitations of this study include the small number of patients in a single arm. Many confounding factors can affect stent migration, so the antimigratory properties of the stent were not confirmed in this study. It is unclear whether an m-CZS can prolong stent patency and the time to RBO based on this small study group alone. However, as it showed no shortening after placement, the ease of placement may make this stent preferable. Further randomized, controlled trials comparing m-CZSs with ordinary SEMSs are necessary to confirm the benefits of the antimigration properties of the m-CZS.
In conclusion, our data suggest the feasibility of developing a SEMS with antimigration properties using 'flare and bank' structures. The development of further sophisticated SEMSs with antimigration properties is mandatory for prevention of stent migration.
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance of Dr. Ryou Nakata of the Japanese Red Cross Medical Center, Dr. Keiji Ogura of Tokyo Police Hospital, and Dr. Tateo Kawase of Kanto Central Hospital.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of "covered" versus "uncovered" diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729–734. doi: 10.1136/gut.2003.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321–327. doi: 10.1016/j.gie.2011.03.1249. [DOI] [PubMed] [Google Scholar]
- 3.Tada M, Nakai Y, Sasaki T, et al. Recent progress and limitations of chemotherapy for pancreatic and biliary tract cancers. World J Clin Oncol. 2011;2:158–163. doi: 10.5306/wjco.v2.i3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakai Y, Isayama H, Kawabe T, et al. Efficacy and safety of metallic stents in patients with unresectable pancreatic cancer receiving gemcitabine. Pancreas. 2008;37:405–410. doi: 10.1097/MPA.0b013e3181706d93. [DOI] [PubMed] [Google Scholar]
- 5.Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointest Endosc. 2009;70:37–44. doi: 10.1016/j.gie.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Nakai Y, Isayama H, Togawa O, et al. New method of covered wallstents for distal malignant biliary obstruction to reduce early stent-related complications based on characteristics. Dig Endosc. 2011;23:49–55. doi: 10.1111/j.1443-1661.2010.01043.x. [DOI] [PubMed] [Google Scholar]
- 7.Moon SH, Kim MH, Park do H, et al. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010;72:86–91. doi: 10.1016/j.gie.2010.01.063. [DOI] [PubMed] [Google Scholar]
- 8.Park do H, Lee SS, Lee TH, et al. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos) Gastrointest Endosc. 2011;73:64–70. doi: 10.1016/j.gie.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Isayama H, Komatsu Y, Tsujino T, et al. Polyurethane-covered metal stent for management of distal malignant biliary obstruction. Gastrointest Endosc. 2002;55:366–370. doi: 10.1067/mge.2002.121876. [DOI] [PubMed] [Google Scholar]
- 10.Nakai Y, Isayama H, Komatsu Y, et al. Efficacy and safety of the covered Wallstent in patients with distal malignant biliary obstruction. Gastrointest Endosc. 2005;62:742–748. doi: 10.1016/j.gie.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Isayama H, Kawabe T, Nakai Y, et al. Management of distal malignant biliary obstruction with the ComVi stent, a new covered metallic stent. Surg Endosc. 2010;24:131–137. doi: 10.1007/s00464-009-0537-9. [DOI] [PubMed] [Google Scholar]
- 12.Telford JJ, Carr-Locke DL, Baron TH, et al. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010;72:907–914. doi: 10.1016/j.gie.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Kullman E, Frozanpor F, Söderlund C, et al. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010;72:915–923. doi: 10.1016/j.gie.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 14.Isayama H, Nakai Y, Tsujino T, Togawa O, Kogure H, Koike K. Covered biliary metal stent: which are worse: the concepts, current models, or insertion methods? Gastrointest Endosc. 2011;73:1329–1330. doi: 10.1016/j.gie.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Kasher JA, Corasanti JG, Tarnasky PR, McHenry L, Fogel E, Cunningham J. A multicenter analysis of safety and outcome of removal of a fully covered self-expandable metal stent during ERCP. Gastrointest Endosc. 2011;73:1292–1297. doi: 10.1016/j.gie.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu S, Naitoh I, Nakazawa T, et al. Predictive factors for pancreatitis and cholecystitis in endoscopic covered metal stenting for distal malignant biliary obstruction. J Gastroenterol Hepatol. 2013;28:68–72. doi: 10.1111/j.1440-1746.2012.07283.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawakubo K, Isayama H, Nakai Y, et al. Risk factors for pancreatitis following transpapillary self-expandable metal stent placement. Surg Endosc. 2012;26:771–776. doi: 10.1007/s00464-011-1950-4. [DOI] [PubMed] [Google Scholar]
- 18.Isayama H, Kawakubo K, Nakai Y, et al. Self-expandable metallic stent with high axial force is the risk factor of cholecystitis. Gastrointest Endosc. 2012;75(4 Suppl):AB380. [Google Scholar]
- 19.Mukai T, Yasuda I, Isayama H, et al. Comparison of axial force and cell width of self-expandable metallic stents: which type of stent is better suited for hilar biliary strictures? J Hepatobiliary Pancreat Sci. 2011;18:646–652. doi: 10.1007/s00534-011-0406-5. [DOI] [PubMed] [Google Scholar]